Abstract
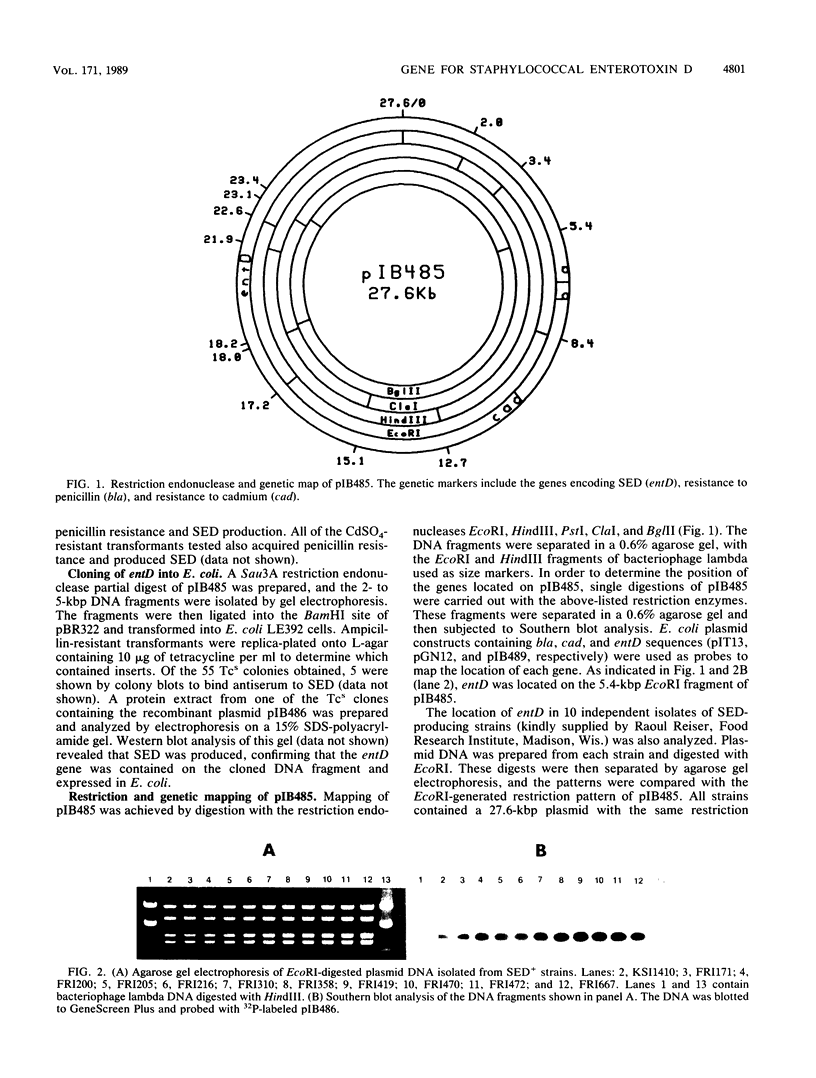
The gene (entD) encoding staphylococcal enterotoxin D (SED) has been located on a 27.6-kilobase penicillinase plasmid designated pIB485. This plasmid was present in all SED-producing strains tested. The entD gene was cloned on a 2.0-kilobase DNA fragment and was expressed in Escherichia coli. Sequence analysis of this fragment revealed an open reading frame that encoded a 258-amino-acid protein that possessed a 30-amino-acid signal peptide. The 228-amino-acid mature polypeptide had a molecular weight of 26,360 and contained a high degree of sequence similarity to the other staphylococcal enterotoxins. S1 nuclease mapping showed that transcription of entD was initiated 266 nucleotides upstream from the translation start codon. The entD gene was also shown to be activated by the staphylococcal regulatory element known as agr.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altboum Z., Hertman I., Sarid S. Penicillinase plasmid-linked genetic determinants for enterotoxins B and C1 production in Staphylococcus aureus. Infect Immun. 1985 Feb;47(2):514–521. doi: 10.1128/iai.47.2.514-521.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Betley M. J., Löfdahl S., Kreiswirth B. N., Bergdoll M. S., Novick R. P. Staphylococcal enterotoxin A gene is associated with a variable genetic element. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5179–5183. doi: 10.1073/pnas.81.16.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley M. J., Mekalanos J. J. Nucleotide sequence of the type A staphylococcal enterotoxin gene. J Bacteriol. 1988 Jan;170(1):34–41. doi: 10.1128/jb.170.1.34-41.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley M. J., Mekalanos J. J. Staphylococcal enterotoxin A is encoded by phage. Science. 1985 Jul 12;229(4709):185–187. doi: 10.1126/science.3160112. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Schlievert P. M. Nucleotide sequence of the staphylococcal enterotoxin C1 gene and relatedness to other pyrogenic toxins. Mol Gen Genet. 1987 Aug;209(1):15–20. doi: 10.1007/BF00329830. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brown D. R., Pattee P. A. Identification of a chromosomal determinant of alpha-toxin production in Staphylococcus aureus. Infect Immun. 1980 Oct;30(1):36–42. doi: 10.1128/iai.30.1.36-42.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. C., Bergdoll M. S. Purification and some physicochemical properties of staphylococcal enterotoxin D. Biochemistry. 1979 May 15;18(10):1937–1942. doi: 10.1021/bi00577a013. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Couch J. L., Soltis M. T., Betley M. J. Cloning and nucleotide sequence of the type E staphylococcal enterotoxin gene. J Bacteriol. 1988 Jul;170(7):2954–2960. doi: 10.1128/jb.170.7.2954-2960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dyer D. W., Iandolo J. J. Plasmid-chromosomal transition of genes important in staphylococcal enterotoxin B expression. Infect Immun. 1981 Aug;33(2):450–458. doi: 10.1128/iai.33.2.450-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gaskill M. E., Khan S. A. Regulation of the enterotoxin B gene in Staphylococcus aureus. J Biol Chem. 1988 May 5;263(13):6276–6280. [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Johns M. B., Jr, Khan S. A. Staphylococcal enterotoxin B gene is associated with a discrete genetic element. J Bacteriol. 1988 Sep;170(9):4033–4039. doi: 10.1128/jb.170.9.4033-4039.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. L., Khan S. A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986 Apr;166(1):29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Iandolo J. J. Integration of staphylococcal phage L54a occurs by site-specific recombination: structural analysis of the attachment sites. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5474–5478. doi: 10.1073/pnas.83.15.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Y., Iandolo J. J. Lysogenic conversion of staphylococcal lipase is caused by insertion of the bacteriophage L54a genome into the lipase structural gene. J Bacteriol. 1986 May;166(2):385–391. doi: 10.1128/jb.166.2.385-391.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Y., Schmidt J. J., Johnson-Winegar A. D., Spero L., Iandolo J. J. Sequence determination and comparison of the exfoliative toxin A and toxin B genes from Staphylococcus aureus. J Bacteriol. 1987 Sep;169(9):3904–3909. doi: 10.1128/jb.169.9.3904-3909.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallonee D. H., Glatz B. A., Pattee P. A. Chromosomal mapping of a gene affecting enterotoxin A production in Staphylococcus aureus. Appl Environ Microbiol. 1982 Feb;43(2):397–402. doi: 10.1128/aem.43.2.397-402.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morfeldt E., Janzon L., Arvidson S., Löfdahl S. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol Gen Genet. 1988 Mar;211(3):435–440. doi: 10.1007/BF00425697. [DOI] [PubMed] [Google Scholar]
- O'Reilly M., de Azavedo J. C., Kennedy S., Foster T. J. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its haemolysins. Microb Pathog. 1986 Apr;1(2):125–138. doi: 10.1016/0882-4010(86)90015-x. [DOI] [PubMed] [Google Scholar]
- O'Toole P. W., Foster T. J. Molecular cloning and expression of the epidermolytic toxin A gene of Staphylococcus aureus. Microb Pathog. 1986 Dec;1(6):583–594. doi: 10.1016/0882-4010(86)90043-4. [DOI] [PubMed] [Google Scholar]
- O'Toole P. W., Foster T. J. Nucleotide sequence of the epidermolytic toxin A gene of Staphylococcus aureus. J Bacteriol. 1987 Sep;169(9):3910–3915. doi: 10.1128/jb.169.9.3910-3915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H. L., Novick R. P., Kreiswirth B., Kornblum J., Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988 Sep;170(9):4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunkett G., 3rd, Echols H. Retroregulation of the bacteriophage lambda int gene: limited secondary degradation of the RNase III-processed transcript. J Bacteriol. 1989 Jan;171(1):588–592. doi: 10.1128/jb.171.1.588-592.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recsei P., Kreiswirth B., O'Reilly M., Schlievert P., Gruss A., Novick R. P. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol Gen Genet. 1986 Jan;202(1):58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Iandolo J. J. Genetics of staphylococcal enterotoxin B in methicillin-resistant isolates of Staphylococcus aureus. Infect Immun. 1979 Sep;25(3):902–911. doi: 10.1128/iai.25.3.902-911.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D. A genetic system for analysis of staphylococcal nuclease. Gene. 1983 May-Jun;22(2-3):181–189. doi: 10.1016/0378-1119(83)90102-6. [DOI] [PubMed] [Google Scholar]
- Warren R., Rogolsky M., Wiley B. B., Glasgow L. A. Isolation of extrachromosomal deoxyribonucleic acid for exfoliative toxin production from phage group II Staphylococcus aureus. J Bacteriol. 1975 Apr;122(1):99–105. doi: 10.1128/jb.122.1.99-105.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks C. R., Ferretti J. J. Nucleotide sequence of the type A streptococcal exotoxin (erythrogenic toxin) gene from Streptococcus pyogenes bacteriophage T12. Infect Immun. 1986 Apr;52(1):144–150. doi: 10.1128/iai.52.1.144-150.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]