Abstract
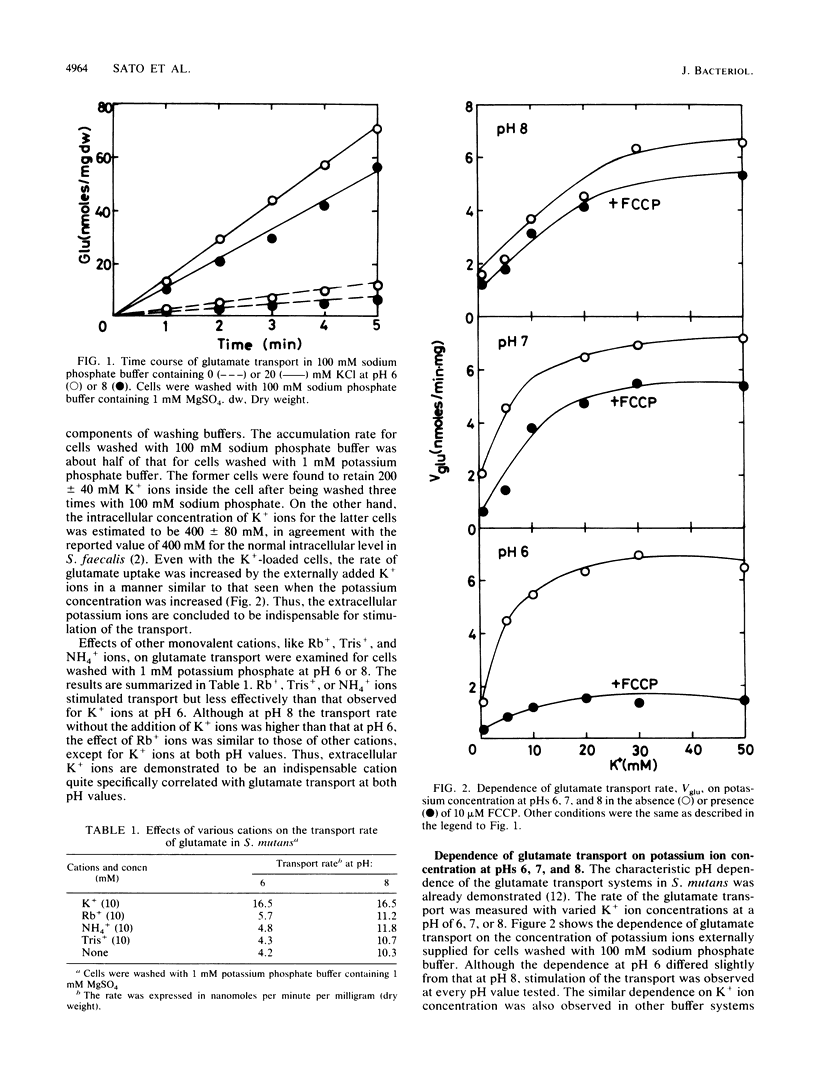
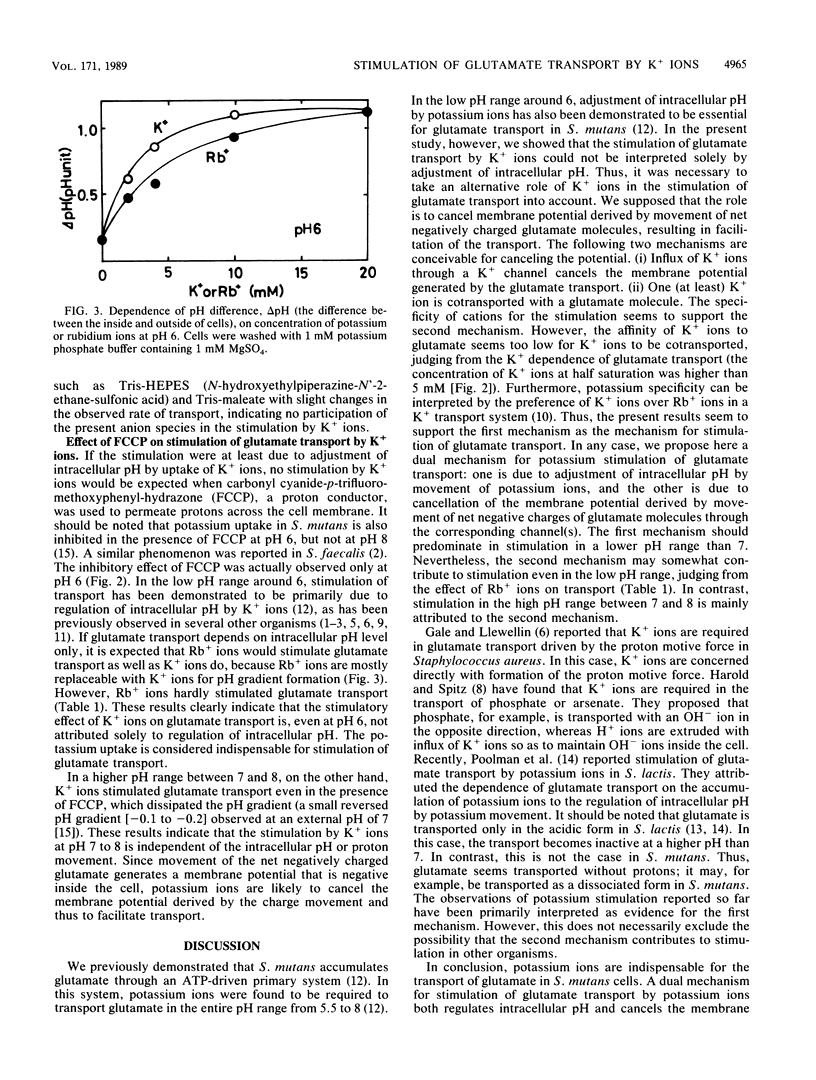
An ATP-driven primary transport system operative for L-glutamate or L-aspartate in Streptococcus mutans is, through the entire pH range from 5.5 to 8.5, specifically stimulated by extracellular potassium ions. The stimulation by potassium ions observed in the low pH range between 5.5 and 7 has been interpreted to be due to potassium ion-dependent regulation of the intracellular pH (the first mechanism). In the high pH range from 7 to 8.5, on the other hand, the present study demonstrates that potassium stimulation is essentially not associated with such intracellular pH regulation. This conclusion is based on our observation that potassium stimulation in the high pH range is insensitive to a proton conductor, carbonyl cyanide-p-trifluoromethoxy-phenyl-hydrazone. Since none of the other monovalent cations, including sodium, rubidium, ammonium, and Tris ions, could replace potassium ions in significantly stimulating glutamate transport, it is most likely that the influx of potassium ions specifically cancels the membrane potential derived by movement of glutamate with the net negative charges across a membrane and thus facilitates transport (the second mechanism). The second mechanism appears to be operative even in a low pH range, in addition to the first mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abee T., Hellingwerf K. J., Konings W. N. Effects of potassium ions on proton motive force in Rhodobacter sphaeroides. J Bacteriol. 1988 Dec;170(12):5647–5653. doi: 10.1128/jb.170.12.5647-5653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E. P., Harold F. M. Energy coupling to potassium transport in Streptococcus faecalis. Interplay of ATP and the protonmotive force. J Biol Chem. 1980 Jan 25;255(2):433–440. [PubMed] [Google Scholar]
- Bakker E. P., Mangerich W. E. Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J Bacteriol. 1981 Sep;147(3):820–826. doi: 10.1128/jb.147.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman K. S., Gibbons R. J., Nalbandian J. Localization of intracellular polysaccharide granules in Streptococcus mitis. Arch Oral Biol. 1967 Oct;12(10):1133–1138. doi: 10.1016/0003-9969(67)90061-1. [DOI] [PubMed] [Google Scholar]
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F., Llewellin J. M. The role of hydrogen and potassium ions in the transport of acidic amino acids in Staphylococcus aureus. Biochim Biophys Acta. 1972 Apr 14;266(1):182–205. doi: 10.1016/0005-2736(72)90134-4. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Spitz E. Accumulation of arsenate, phosphate, and aspartate by Sreptococcus faecalis. J Bacteriol. 1975 Apr;122(1):266–277. doi: 10.1128/jb.122.1.266-277.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Barker S. L. Effects of potassium ions on the electrical and pH gradients across the membrane of Streptococcus lactis cells. J Bacteriol. 1977 Jun;130(3):1017–1023. doi: 10.1128/jb.130.3.1017-1023.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Murakami N., Unemoto T. Regulation of the cytoplasmic pH in Streptococcus faecalis. J Biol Chem. 1982 Nov 25;257(22):13246–13252. [PubMed] [Google Scholar]
- Kobayashi H. Second system for potassium transport in Streptococcus faecalis. J Bacteriol. 1982 May;150(2):506–511. doi: 10.1128/jb.150.2.506-511.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji S., Sato Y., Suzuki R., Taniguchi S. Effect of intracellular pH and potassium ions on a primary transport system for glutamate/aspartate in Streptococcus mutans. Eur J Biochem. 1988 Aug 15;175(3):491–495. doi: 10.1111/j.1432-1033.1988.tb14221.x. [DOI] [PubMed] [Google Scholar]
- Poolman B., Driessen A. J., Konings W. N. Regulation of solute transport in streptococci by external and internal pH values. Microbiol Rev. 1987 Dec;51(4):498–508. doi: 10.1128/mr.51.4.498-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Hellingwerf K. J., Konings W. N. Regulation of the glutamate-glutamine transport system by intracellular pH in Streptococcus lactis. J Bacteriol. 1987 May;169(5):2272–2276. doi: 10.1128/jb.169.5.2272-2276.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Yamamoto Y., Suzuki R. Effects of potassium ions on lactate production and growth of Streptococcus mutans in relationship to the proton motive force. Bull Tokyo Dent Coll. 1987 Aug;28(3):99–109. [PubMed] [Google Scholar]
- Zarlengo M. H., Schultz S. G. Cation transport and metabolism in Streptococcus fecalis. Biochim Biophys Acta. 1966 Oct 10;126(2):308–320. doi: 10.1016/0926-6585(66)90068-9. [DOI] [PubMed] [Google Scholar]



