Abstract
The insulin-like growth factor II (IGF-II)/mannose-6-phosphate (M-6-P) receptor is known to participate in endocytosis as well as sorting of lysosomal enzymes and is involved in membrane trafficking through rapid cycling between cytosolic membrane compartments and the plasma membrane. Here we demonstrate that IGF-II, acting through the IGF-II/M-6-P receptor, promotes exocytosis of insulin in the pancreatic β cell. The effect of IGF-II was evoked at nonstimulatory concentrations of glucose, was mediated by a pertussis toxin sensitive GTP-binding protein, was dependent on protein kinase C-induced phosphorylation, and was independent of changes in cytoplasmic free Ca2+ concentration. Since the applied concentration of IGF-II is within the range normally found free in circulation in humans, this novel signaling pathway for the IGF-II/M-6-P receptor is likely to be involved in modulation of insulin exocytosis under physiological conditions.
Insulin-like growth factor II (IGF-II) belongs to a family of hormonally active polypeptides, also comprising IGF-I, insulin, the Leydig cell-specific insulin-like peptide (1), and relaxin (2). IGF-II circulates bound to specific IGF-binding proteins (IGFBPs) (3). IGF-II binds to the cation-independent mannose-6-phosphate (M-6-P) receptor and crossreacts, although with a lower affinity, with the insulin and the IGF-I receptors (4, 5). The IGF-II/M-6-P receptor only binds IGF-II and M-6-P, whereas IGF-I and insulin do not crossreact (6). The IGF-II/M-6-P receptor is a 250-kDa protein with a monomeric structure that has repetitive homologous noncytosolic elements and a short cytosolic domain comprising possible sites for phosphorylation on Tyr and Ser residues (4, 7, 8), and is a substrate for tyrosine kinases (9) and protein kinase C (PKC)- or cAMP-dependent protein kinases (5). The receptor binds M-6-P and IGF-II at different sites of the molecule (10, 11). IGF-II binding has not been shown to induce phosphorylation of its receptor, but it has been suggested that IGF-II causes IGF-II/M-6-P receptor redistribution by a mechanism involving okadaic acid-sensitive Ser/Thr protein phosphatases (12). The fact that the cytoplasmic tail of the IGF-II/M-6-P receptor lacks a kinase domain has prompted the suggestion that this receptor mediates its signaling through heterotrimeric GTP-binding proteins (13, 14). By using the nonhydrolyzable GTP analogue GTP-γ-S, it was possible to show that cytosolic recycling of IGF-II/M-6-P receptors to the trans-Golgi network depends on GTP hydrolysis (15). Insulin exocytosis from the pancreatic β cell can serve as an example of highly regulated membrane trafficking, and in the present study, we investigated a possible role for the IGF-II/M-6-P receptor in this process.
MATERIALS AND METHODS
Pancreatic islets, known to contain >90% β cells (16), were isolated by collagenase and DNAse digestion from a local colony of obese (ob/ob) mice, 12 months of age. Islets were dispersed into single β cells (16), and receptor binding studies were carried out as described (17). Cell suspensions (100 μl) were incubated overnight at 4°C with 125I-labeled peptides, in the presence or absence of the corresponding unlabeled peptides (with a final incubation volume of 300 μl). In the binding experiments with the polyclonal anti-IGF-II/M-6-P receptor antibody, cells were preincubated with the antibody at a dilution of 1:100 for 2 hr at room temperature before addition of 125I-IGF-II in the presence (100 nM) or absence of unlabeled IGF-II. The cells were further incubated for 45 min. Mean values ± SD for an experiment performed in triplicate are shown. Affinity crosslinking was performed as described (18). Pancreatic β cells were washed with ice-cold phosphate buffer (50 mM; pH 7.4), resuspended in the same type of phosphate buffer containing 1% BSA, and incubated with 125I-IGF-II or 125I-IGF-I for 45 min at room temperature in the presence or absence of unlabeled peptides. Affinity crosslinking was carried out in phosphate buffer without BSA on ice for 15 min with use of disuccinimidyl suberate. After quenching the reaction with 0.5 M Tris⋅HCl, the affinity-labeled cells were solubilized and heated to 95°C in sample buffer in the presence of 10 mM 1.4-dithio-dl-threitol and applied to SDS/PAGE with a 10% separating gel (19). The gel was dried after gel electrophoresis and autoradiographed at −70°C. Western ligand blotting of medium conditioned by β cells was performed as described (20). The conditioned medium was collected and applied to SDS/polyacrylamide gels under nonreducing conditions. The proteins in the gels were thereafter electroblotted onto a nitrocellulose sheet. The nitrocellulose sheet was blocked, followed by incubation with 125I-IGF-II or 125I-IGF-I at 4°C overnight. The protein-containing sheet was washed with PBS and visualized by autoradiography.
Insulin release was investigated in column-perifused pancreatic β cells as described (21). Insulin content in the fractions collected from perifusion was assayed at a 10- to 50-fold dilution. Insulin release was also performed in 24-well plates at 3 or 20 mM glucose in the presence or absence of IGF-II (50 ng/ml). Insulin in the medium was measured after 6 min of incubation at 37°C. In the latter case, β cells were preincubated with or without anti-IGF-II/M-6-P receptor antibodies (1:100) for 2 hr at 37°C. Insulin release from electropermeabilized β cells (16) was investigated in a 12-well plate at 37°C in a potassium glutamate buffer containing 140 mM potassium glutamate, 5 mM NaCl, 1 mM MgCl2, 25 mM Hepes, 2 mM creatine phosphate, 4 mM Mg-ATP, 10 mM EGTA, 10 units/ml creatine phosphokinase, and 1 mg/ml BSA at an ambient free Ca2+ concentration of 10−7 M. The Ca2+ concentration in the buffer was measured by a Ca2+-selective minielectrode and related to standard solutions calibrated with regard to Ca2+ concentrations (ranging from 0.1 to 1.0 μM and adjusted by addition of Ca2+ and EGTA). After 20 min of incubation, the cell suspension was collected from each well and centrifuged, the supernatants being used for insulin radioimmunoassay. Bound and free insulin were separated by activated charcoal (22). The insulin antibodies used were raised in guinea pigs against porcine insulin, in our laboratory. Rat insulin, a mixture of rat insulins I and II, was used as standard.
In the amperometric experiments, carbon fiber microelectrodes were prepared by use of previously described techniques (23–26). In the single cell measurements, a Krebs buffer containing 118 mM NaCl, 5.8 mM KCl, 2.4 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 3 mM glucose (pH 7.4) was used at 37°C. To perform measurements, the sensing tip of a microelectrode was positioned ≈1 μm from an isolated cell adhered to the bottom of the Petri dish. Data were collected using an EI-400 potentiostat (Ensman Instrumentation, Bloomington, IN). The data were low pass filtered with a cut-off frequency of 20 Hz. Stimulation of the cell was accomplished by pressure ejecting solutions from micropipette tips (tip o.d. = 10 μm), which were positioned ≈30 μm from the cell. Flow rates through the pipettes were ≈1 nl/sec. This approach to secretion measurements allows high temporal resolution because the insulin is detected immediately after it diffuses the small distance between the electrode and the cell.
Phosphorylation of intracellular proteins was carried out according to ref. 27, with modification. β cells were incubated with (for down-regulation of PKC activity) or without 1 μM 12–0-tetradecanoylphorbol-13-acetate (TPA) in RPMI medium 1640 for 3 hr before electropermeabilization. Electropermeabilized cells were incubated in the presence or absence of 50 ng/ml IGF-II for 4 min, at 37°C, in a buffer (pH 6.6), containing 140 mM potassium glutamate, 15 mM Hepes, 2.3 mM MgSO4, 0.3 mM ATP, 0.5 mM EGTA, 10−4 mM Ca2+, and 3 mM glucose. Phosphorylation was initiated by addition of [γ-32P]ATP (20 μCi/100 μl; 1 Ci = 37 GBq). After 1 min of incubation at 37°C, the cell suspension was briefly centrifuged, and the pelleted cells were solubilized in SDS/PAGE sample buffer. The radiolabeled products were separated by SDS/PAGE, under reducing conditions, and visualized by autoradiography. Equal amounts of protein samples were applied to the gel, as verified by silver staining of the parallel lanes (data not shown).
For statistical evaluation the Student–Newman–Keuls method for multiple comparison was used.
RESULTS AND DISCUSSION
Despite the fact that the IGF-II/M-6-P receptor has been reported to participate in endocytosis, sorting of lysosomal enzymes, and regulation of membrane trafficking (5), the extent to which this receptor affects exocytosis in general is not clear. In the present study, we have used insulin-secreting cells as a model for regulated exocytosis and then specifically evaluated a possible role for the IGF-II/M-6-P receptor in this process.
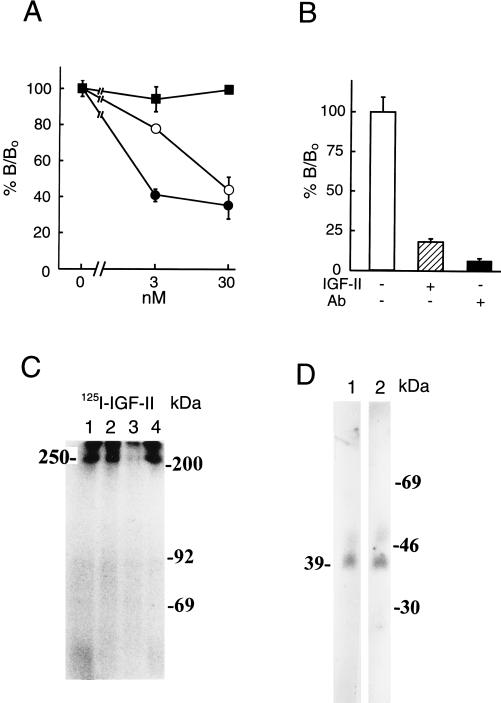
The presence of receptors for IGF-II was evaluated by specific binding as well as by affinity crosslinking of 125I-IGF-II to intact mouse β cells. As shown in Fig. 1 A and B, specific binding was found with 125I-IGF-II, using unlabeled IGF-II, IGF-I, and insulin as competitive inhibitors, but not with labeled insulin or IGF-I (data not shown). IGF-I, but not insulin, could compete with IGF-II with less potency (Fig. 1A). However, as discussed below, this does not reflect competition for the IGF-II receptor but rather the presence of IGFBP because the IGF-II receptor does not bind IGF-I and insulin. In the presence of anti-IGF-II/M-6-P receptor antibody, the binding of 125I-IGF-II was abolished (Fig. 1B). Affinity crosslinking using labeled IGF-II followed by SDS/PAGE, at reducing conditions, showed specific IGF-II binding to a 250-kDa protein, likely to be the IGF-II/M-6-P receptor (3, 4, 7, 8) (Fig. 1C). As evident from the results neither unlabeled IGF-I nor insulin could compete with labeled IGF-II for the 250-kDa protein. When affinity crosslinking was performed, using labeled IGF-I, no specific bands appeared (data not shown). To further substantiate the absence of receptors for IGF-I and insulin, phosphorylation of wheat-germ lectin affinity-purified proteins from mouse β cells was performed in the absence or presence of insulin and/or IGF-I, with negative results (data not shown). When serum-free medium was conditioned for 20 hr with mouse β cells and analyzed for IGFBPs, using SDS/PAGE and Western ligand blotting, it was clear that these cells release a 39-kDa IGFBP (Fig. 1D). IGFBP is to a certain extent associated with the outer surface of cells and binds both IGF-I and IGF-II. Hence, crossreactivity of unlabeled IGF-I with labeled IGF-II in conventional cell binding (Fig. 1A) was not associated with IGF-I or insulin receptors (28) but rather indicated the presence of an IGFBP.
Figure 1.
Presence of IGF-II/M-6-P-receptor in pancreatic β cells. Cell binding assays (A) were performed in triplicate using 125I-IGF-II in the presence (3 or 30 nM) or absence of unlabeled IGF-I (○), IGF-II (•), or insulin (▪). The results are presented as the means ± SD. Binding was evaluated as the percentage of IGF-II bound (B/B0; B corresponding to observed binding and B0 to total binding of labeled IGF-II). In B blocking capacity of an anti-IGF-II/M-6-P receptor antibody on IGF-II binding to the cells was evaluated. Experimental conditions were as indicated in the figure. A and B show one representative experiment out of two, respectively. For affinity crosslinking (C), cells were incubated with 125I-IGF-II, in the absence (lane 1) or presence (lane 2) of 100 nM IGF-I, IGF-II (lane 3), or insulin (lane 4) and crosslinked with disuccinimidyl suberate. Affinity-labeled proteins were separated by SDS/PAGE under reducing conditions. Positions of the protein molecular mass standards are shown (in kilodaltons). IGFBPs secreted from the cells were investigated with Western ligand blotting of conditioned media (D), using labeled IGF-I (lane 1) or IGF-II (lane 2). Positions of protein molecular mass standards are shown in kilodaltons. In C and D, a representative experiment out of three and two, respectively, is shown.
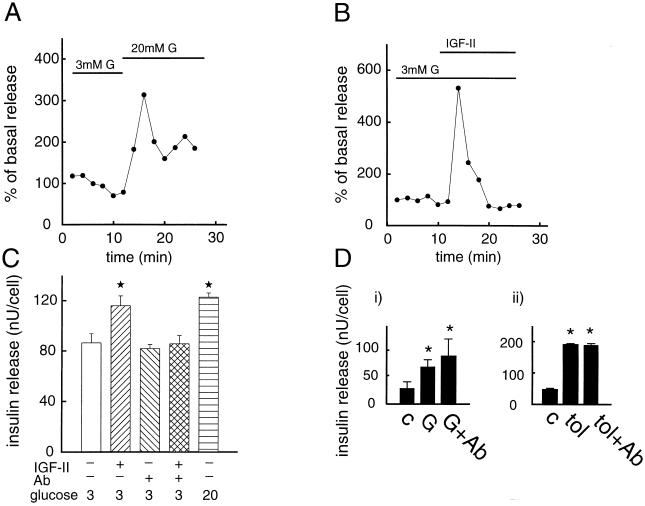
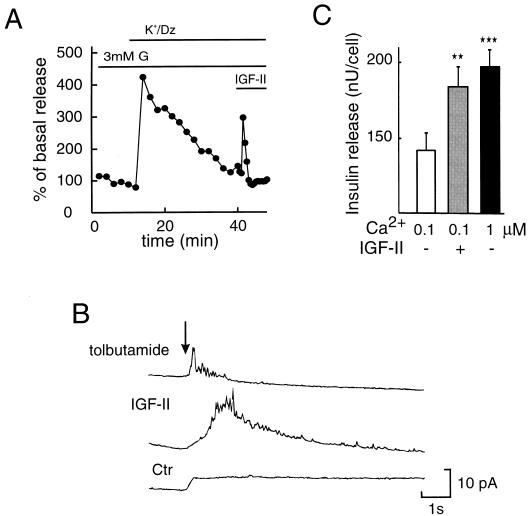
It is well known that an increase in glucose concentration from 3 to 20 mM gives rise to a pronounced stimulation of insulin release from the pancreatic β cell, as is shown in Fig. 2A. Noteworthy is that an equally pronounced release of insulin was obtained by IGF-II (50 ng/ml), at 3 mM glucose (Fig. 2B). Under similar experimental conditions, IGF-I failed to evoke insulin release (data not shown). The stimulatory effect of IGF-II on insulin release was blocked in the presence of an anti-IGF-II/M-6-P receptor antibody (Fig. 2C). This antibody had no effect of its own on insulin exocytosis, and it did not affect glucose- or sulfonylurea (SU)-induced insulin release (Fig. 2 C and D). When the plasma membrane was depolarized and the ATP-regulated K+ channels (29) were kept open (Fig. 3A), i.e., exposing the cells to high K+ and diazoxide, to prevent any possible effects on plasma membrane ion channels, IGF-II still promoted release of insulin. This suggests that IGF-II interacts directly with the molecular mechanisms regulating the exocytotic machinery. To further substantiate that IGF-II stimulates exocytosis of insulin, a highly sensitive amperometric method was applied to single pancreatic β cells (Fig. 3B). In previous studies, this method has been used to detect exocytosis from adrenal chromaffin cells, PC-12 cells, mast cells, and individual pancreatic β cells (23, 30–34). The anodic current resulting from a secreted substance appears as a series of spikes that indicate concentration pulses occurring at the electrode surface. The concentration pulses are due to secretion of small packets of molecules associated with exocytosis. As shown in Fig. 3B, the current spikes measured by amperometry after IGF-II stimulation of single β cells are consistent with exocytosis. Under the same experimental conditions, the SU tolbutamide also promoted exocytosis of insulin. SU drugs are well known stimulators of insulin release and are used in the treatment of diabetes. We have recently shown that SU, in addition to closing the ATP-regulated K+ channels, directly activates the exocytotic machinery in the pancreatic β cell (35). IGF-II also had a stimulatory effect on insulin release (Fig. 3C), similar to that of 10−6 M Ca2+, in electropermeabilized β cells at an ambient free Ca2+ concentration of 10−7 M, further supporting a direct interaction with the exocytotic machinery. That some previous studies (36–38) have not been able to document a stimulatory effect of IGF-II on insulin exocytosis is likely to be explained by both different experimental conditions and the fact that the insulin-secreting cells were obtained from other sources than the ob/ob mice. In this context, it should be noted that the ob/ob β cell does not contain detectable receptors for either IGF-I or insulin, implying that in our study crossreactivity and thereby signaling through these receptors will not disturb signaling by IGF-II through the IGF-II/M-6-P receptor.
Figure 2.
Effect of IGF-II on exocytosis of insulin. β cells were perifused in a buffer containing 3 mM glucose (G) for 10 min. The perifusion was continued in the presence of 3 (B) or 20 mM (A) glucose. Time of addition of IGF-II (50 ng/ml) (B) is indicated. Because glucose is the normal insulin secretagogue under physiological conditions, we used stimulation with a 20 mM concentration of the sugar as a positive control (A). Insulin release in A and B is expressed as percentage of basal secretion, defined as the average insulin release obtained at 3 mM glucose during the first 10 min of perifusion. Representative experiments out of three are shown. (C) Effect of IGF-II (50 ng/ml) on insulin release in cells that were preincubated in the absence or presence of an anti-IGF-II/M-6-P receptor antibody. Insulin is expressed as nanounits per cell. Insulin release was investigated in batch incubations. Mean values ± SD for one representative experiment in quadruples out of three are shown. (D) Influence of an anti-IGF-II/M-6-P receptor antibody on glucose-stimulated (20 mM) (i) and tolbutamide-stimulated (100 μM) insulin release (ii). The figure shows a representative experiment, out of three, in quadruples for glucose and tolbutamide, respectively. ∗, P < 0.05.
Figure 3.
Direct effect of IGF-II on insulin exocytosis. (A) β cells were perifused in the presence of 25 mM K+/100 μM diazoxide (Dz), to induce membrane depolarization and to ensure that the ATP-regulated K+ channels are kept open. IGF-II (50 ng/ml) was added 30 min after the addition of K+/diazoxide. Insulin release is expressed as the percentage of basal secretion. Representative experiments out of three are shown. (B) Comparison of amperometric current recordings in single β cells after stimulation with 200 μM tolbutamide (5 sec; top trace) and 100 ng/ml IGF-II (10 sec; middle trace). In this case, the SU tolbutamide is used as a positive control. The arrow indicates the initiation of stimulation for all recordings. The spikes elicited are of similar size and time course for the two stimulation protocols. This is expected for an exocytosis measurement because the amount released per vesicle and the amount detected in a spike will be independent of the method used to initiate exocytosis (30, 31). In both cases, the spikes are superimposed over a broad envelope, although the envelope is more obvious with IGF-II stimulation. The envelope is partially due to overlapping of many spikes occurring simultaneously (30, 31) and partially due to a background change associated with the IGF-II stimulation. The bottom trace shows the background shift induced by IGF-II addition and recorded in a β cell not responding to the agonist. A similar background shift is obtained when no cell is present (data not shown). Spikes were observed in 41% (7 out of 17) of the cells tested with IGF-II. Trains of 10 or more spikes were observed in 18% (3 out of 17) of the cells tested. The response is given in picoamperes per second. (C) Effect of IGF-II (50 ng/ml) on exocytosis of insulin in electropermeabilized β cells, incubated in the presence of ATP and an ATP regenerating system at a concentration of 10−7 M ambient free Ca2+. Results for insulin release at 10−7 M Ca2+ in the absence (□) or presence ( ) of IGF-II as well as release induced by 10−6 M Ca2+ (▪) (as control) are given as mean values ± SD (nanounits per cell) for one representative experiment in quadruples, out of four. ∗∗, P < 0.01; ∗∗∗, P < 0.001.
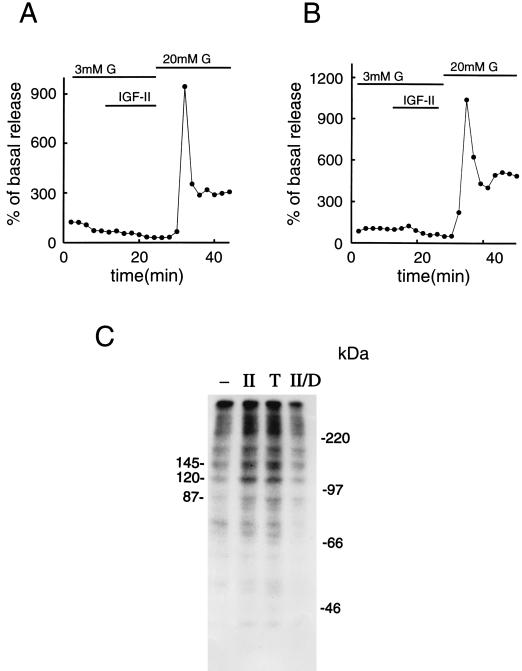
The stimulatory effect of IGF-II on exocytosis of insulin was not associated with any changes in cytoplasmic free Ca2+ concentration (data not shown). β cells that had been either preincubated in the presence of pertussis toxin, to suppress GTP-binding protein activity, or with high concentrations of the phorbol ester TPA, to down-regulate PKC activity (39, 40), failed to respond to IGF-II with insulin release (Fig. 4 A and B). The lack of a stimulatory effect did not reflect absence of a releasable pool of the hormone because glucose was still able to promote pronounced insulin release (Fig. 4 A and B). The involvement of PKC-induced phosphorylation in IGF-II/M-6-P receptor signaling was further investigated by applying electropermeabilized cells and studying phosphorylation of intracellular proteins separated on SDS/PAGE gels. At 3 mM glucose, IGF-II induced specific phosphorylation of at least three prominent proteins, with approximate molecular masses of 145, 120, and 87 kDa (Fig. 4C). A similar pattern of protein phosphorylation was obtained when acutely activating PKC with 25 nM of the phorbol ester TPA (Fig. 4C). After down-regulation of PKC activity, IGF-II failed to induce phosphorylation of these proteins. The 87-kDa protein band is likely to represent a protein belonging to the family of PKC substrates previously described and generally termed myristoylated Ala-rich C kinase substrate, or MARCKS, proteins (41). It is not clear whether the 145-kDa protein has any resemblance to the previously described 145-kDa cytosolic protein that has been shown to reconstitute PKC-dependent exocytosis in various secretory cells, including the clonal insulin-secreting cell line RINm5F (42–44).
Figure 4.
Role of GTP binding proteins, PKC, and phosphorylation in mediating the effect of IGF-II on insulin release. (A) β cells were incubated in RPMI medium 1640 supplemented with 50 ng/ml pertussis toxin for 8 hr. Thereafter, they were subjected to column perifusion and stimulation with 50 ng/ml IGF-II for 14 min at a basal glucose concentration of 3 mM. (B) A similar type of experiment was performed with β cells incubated in RPMI medium 1640 supplemented with 1 μM TPA for 3 hr, to down-regulate PKC activity. In both A and B, the cells were exposed to 20 mM glucose at the end of the experiment to ensure that they were still capable of secreting normal amounts of the hormone. Insulin release is expressed as percentage of basal secretion, obtained at 3 mM glucose. Representative experiments out of three are shown. (C) Protein phosphorylation in β cells incubated for 4 min at 37°C in buffer only (−), in the presence of 50 ng/ml IGF-II (II), 25 nM TPA (T), or 50 ng/ml IGF-II, subsequent to down-regulation of PKC activity (II/D). Three prominent protein bands and protein molecular mass standards are indicated (in kilodaltons). In C, a representative experiment out of three is shown.
The molecular mechanism underlying the stimulatory effect of IGF-II on insulin release, mediated by the IGF-II/M-6-P receptor, shares some similarities with the mechanism that we have previously described for the direct effect of SU on insulin exocytosis (35). The signaling pathway activated by both SU and IGF-II does not involve changes in cytoplasmic free Ca2+ concentration but requires PKC-induced phosphorylation. The extent to which IGF-II-induced exocytosis of insulin in the pancreatic β cell is related to IGF-II-induced internalization and recycling of the IGF-II/M-6-P receptor complex between cytosolic membranes, such as the secretory granules, and the plasma membrane is not clear. The fact that activation of the receptor, by physiologically relevant concentrations of IGF-II (45), promotes insulin release at nonstimulatory glucose concentrations makes this signaling pathway an interesting candidate in the regulation of basal release of the hormone.
Acknowledgments
The antiserum against rat IGF-II/M-6-P receptor from rabbit was a generous gift from Dr. Peter Nissley (National Institutes of Health, Bethesda, MD). This work was supported by The Swedish Medical Research Council (Grants 03X-09890, 04X-09891, 19X-00034, and 19X-4224), the Swedish Diabetes Association, the Nordic Insulin Foundation Committee, the Berth von Kantzows Foundation, the Funds of the Karolinska Institute, the National Institutes of Health (Grant 1 RO1 DK 46960–01), and the National Science Foundation (Grant CHE-9357411).
ABBREVIATIONS
- IGF-II
insulin-like growth factor II
- IGFBP
IGF-binding protein
- M-6-P
mannose-6-phosphate
- ob
obese
- PKC
protein kinase C
- TPA
tetradecanoylphorbol-13-acetate
- SU
sulfonylurea
References
- 1.Burkhardt E, Adham I M, Brosig B, Gastmann A, Mattei M G, Engel W. Genomics. 1994;20:13–19. doi: 10.1006/geno.1994.1121. [DOI] [PubMed] [Google Scholar]
- 2.Dull T J, Gray A, Hayflick J S, Ullrich A. Nature (London) 1984;310:777–781. doi: 10.1038/310777a0. [DOI] [PubMed] [Google Scholar]
- 3.Sara V R, Hall K. Physiol Rev. 1990;70:591–614. doi: 10.1152/physrev.1990.70.3.591. [DOI] [PubMed] [Google Scholar]
- 4.Morgan D O, Edman J C, Standring D N, Fried V A, Smith M C, Roth R A, Rutter W J. Nature (London) 1987;329:301–307. doi: 10.1038/329301a0. [DOI] [PubMed] [Google Scholar]
- 5.Roth, R. A. & Kiess, W. (1994) Growth Regul.4, Suppl., 31–38. [PubMed]
- 6.Tally M, Enberg G, Li C H, Hall K. Biochem Biophys Res Commun. 1987;147:1206–1212. doi: 10.1016/s0006-291x(87)80198-5. [DOI] [PubMed] [Google Scholar]
- 7.Lobel P, Dahms N, Kornfeld S. J Biol Chem. 1988;263:2563–2570. [PubMed] [Google Scholar]
- 8.Kornfeld S. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 9.Corvera S, Whitehead R E, Mottola C, Czech M P. J Biol Chem. 1986;261:7675–7679. [PubMed] [Google Scholar]
- 10.Braulke T, Causin C, Waheed A, Junghans U, Hasilik A, Maly P, Humbel R E, von Figura K. Biochem Biophys Res Commun. 1988;150:1287–1293. doi: 10.1016/0006-291x(88)90769-3. [DOI] [PubMed] [Google Scholar]
- 11.Garmroudi F, MacDonald R G. J Biol Chem. 1994;269:26944–26952. [PubMed] [Google Scholar]
- 12.Braulke T, Mieskes G. J Biol Chem. 1992;267:17347–17353. [PubMed] [Google Scholar]
- 13.Okamoto T, Katada T, Murayama Y, Ui M, Ogata E, Nishimoto I. Cell. 1990;62:709–717. doi: 10.1016/0092-8674(90)90116-v. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Murayama Y, Okamoto T, Yokota T, Ikezu T, Takahashi S, Giambarella U, Ogata E, Nishimoto I. Proc Natl Acad Sci USA. 1993;90:11772–11776. doi: 10.1073/pnas.90.24.11772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goda Y, Pfeffer S R. Cell. 1988;55:309–320. doi: 10.1016/0092-8674(88)90054-2. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson T, Arkhammar P, Hallberg A, Hellman B, Berggren P-O. Biochem J. 1987;248:329–336. doi: 10.1042/bj2480329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tally M, Tang X Z, Enberg G, Hall K. Biosci Rep. 1984;4:1071–1077. doi: 10.1007/BF01116701. [DOI] [PubMed] [Google Scholar]
- 18.Pilch P G, Czech M P. J Biol Chem. 1979;254:3375–3381. [PubMed] [Google Scholar]
- 19.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Hossenlopp P, Seurin D, Segovia-Quinson B, Hardouin S, Binoux M. Anal Biochem. 1986;154:138–143. doi: 10.1016/0003-2697(86)90507-5. [DOI] [PubMed] [Google Scholar]
- 21.Kanatsuna T, Lernmark Å, Rubenstein A H, Steiner D F. Diabetes. 1981;30:231–234. doi: 10.2337/diab.30.3.231. [DOI] [PubMed] [Google Scholar]
- 22.Herbert V, Lau K S, Gottlieb C W, Bleicher S J. J Clin Endocrinol Metab. 1965;25:1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy R T, Huang L, Atkinson M A, Dush P. Anal Chem. 1993;65:1882–1887. doi: 10.1021/ac00062a012. [DOI] [PubMed] [Google Scholar]
- 24.Kelly R S, Wightman R M. Anal Chim Acta. 1986;187:79–87. [Google Scholar]
- 25.Cox J A, Kulesza P J. Anal Chem. 1984;56:1021–1025. [Google Scholar]
- 26.Cox J A, Gray T J. Anal Chem. 1989;61:2462–2464. doi: 10.1021/ac00196a027. [DOI] [PubMed] [Google Scholar]
- 27.Jones P M, Persaud S J, Howell S L. Biochem J. 1992;285:973–978. doi: 10.1042/bj2850973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tally M, Li C H, Hall K. Biochem Biophys Res Commun. 1987;147:1206–1212. doi: 10.1016/s0006-291x(87)80198-5. [DOI] [PubMed] [Google Scholar]
- 29.Berggren P-O, Larsson O. Biochem Soc Trans. 1994;22:12–18. doi: 10.1042/bst0220012. [DOI] [PubMed] [Google Scholar]
- 30.Wightman R M, Jankowski J A, Kennedy R T, Kawagoe K T, Schroeder T J, Leszczyszyn D J, Near J A, Diliberto EJ, Jr, Viveros O H. Proc Natl Acad Sci USA. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leszczyszyn D J, Jankowski J A, Viveros O H, Diliberto E J, Jr, Near J A, Wightman R M. J Biol Chem. 1990;265:14736–14737. [PubMed] [Google Scholar]
- 32.Chow R H, von Rüden L, Neher E. Nature (London) 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- 33.Chen T K, Luo G, Ewing A G. Anal Chem. 1994;66:3031–3035. doi: 10.1021/ac00091a007. [DOI] [PubMed] [Google Scholar]
- 34.de Toledo G A, Fernández-Chacón R, Fernández J M. Nature (London) 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- 35.Eliasson L, Renström E, Ämmälä C, Berggren P-O, Bertorello A M, Bokvist K, Chibalin A, Deeney J T, Flatt P R, Gäbel J, Gromada J, Larsson O, Lindström P, Rhodes C J, Rorsman P. Science. 1996;271:813–815. doi: 10.1126/science.271.5250.813. [DOI] [PubMed] [Google Scholar]
- 36.Leahy J L, Vandekerkhove K M. Endocrinology. 1990;126:1593–1598. doi: 10.1210/endo-126-3-1593. [DOI] [PubMed] [Google Scholar]
- 37.Dheen S T, Rajkumar K, Murphy L J. Diabetologia. 1996;39:1249–1254. doi: 10.1007/s001250050566. [DOI] [PubMed] [Google Scholar]
- 38.Fehmann H C, Jehle P, Markus U, Goke B. Metab Clin Exp. 1996;45:759–766. doi: 10.1016/s0026-0495(96)90143-2. [DOI] [PubMed] [Google Scholar]
- 39.Arkhammar P, Nilsson T, Welsh M, Welsh N, Berggren P-O. Biochem J. 1989;264:207–215. doi: 10.1042/bj2640207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arkhammar P, Juntti-Berggren L, Larsson O, Welsh M, Nånberg E, Sjöholm Å, Köhler M, Berggren P-O. J Biol Chem. 1994;269:2743–2749. [PubMed] [Google Scholar]
- 41.Albert K A, Walaas S I, Wang J K, Greengard P. Proc Natl Acad Sci USA. 1986;83:2822–2826. doi: 10.1073/pnas.83.9.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishizaki T, Walent J H, Kowalchyk J A, Martin T F J. J Biol Chem. 1992;267:23972–23981. [PubMed] [Google Scholar]
- 43.Walent J H, Porter B W, Martin T F J. Cell. 1992;70:765–776. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- 44.Praz G A, Halban P A, Wollheim C B, Blondel B, Strauss A J, Renold A E. Biochem J. 1983;210:345–352. doi: 10.1042/bj2100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lassarre C, Binoux M. Endocrinology. 1994;134:1254–1262. doi: 10.1210/endo.134.3.7509737. [DOI] [PubMed] [Google Scholar]