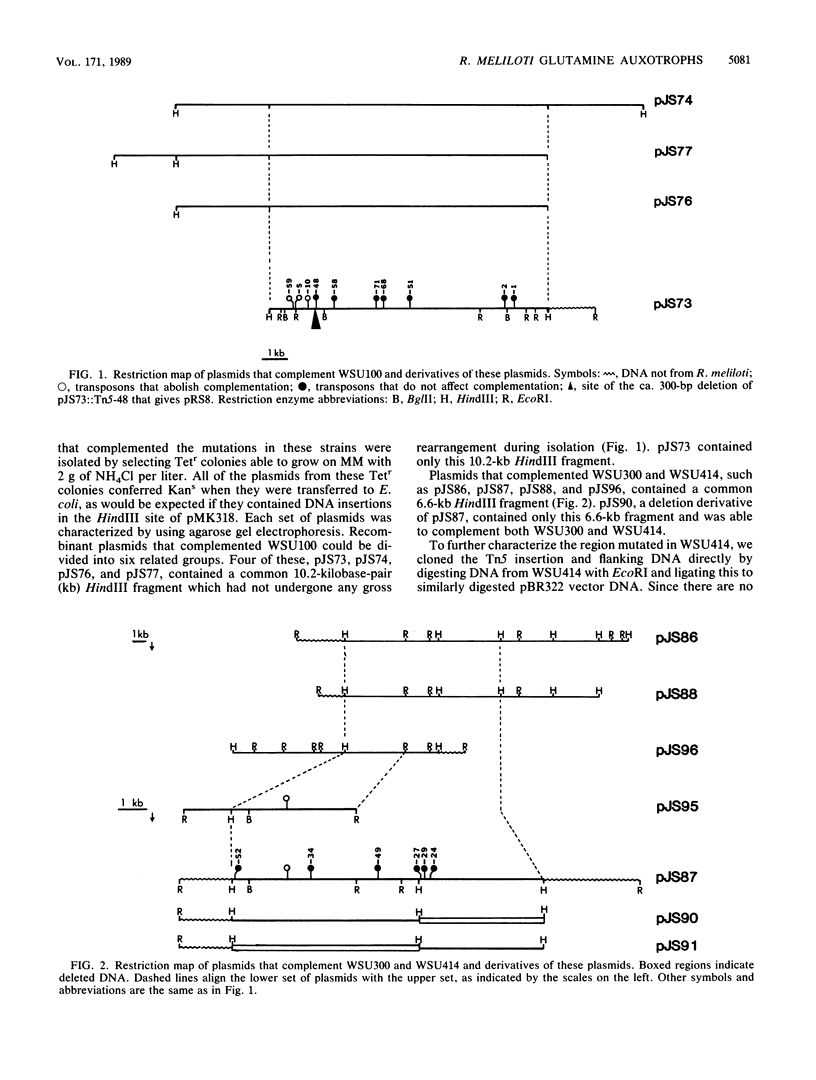
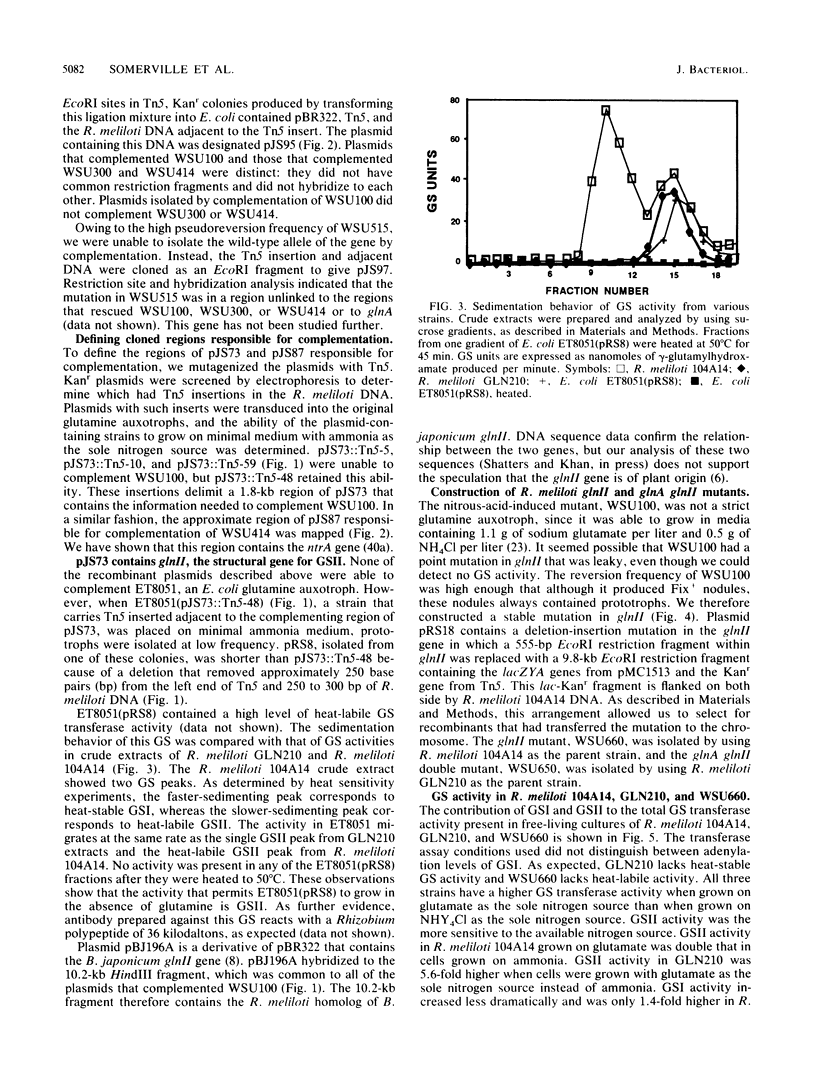
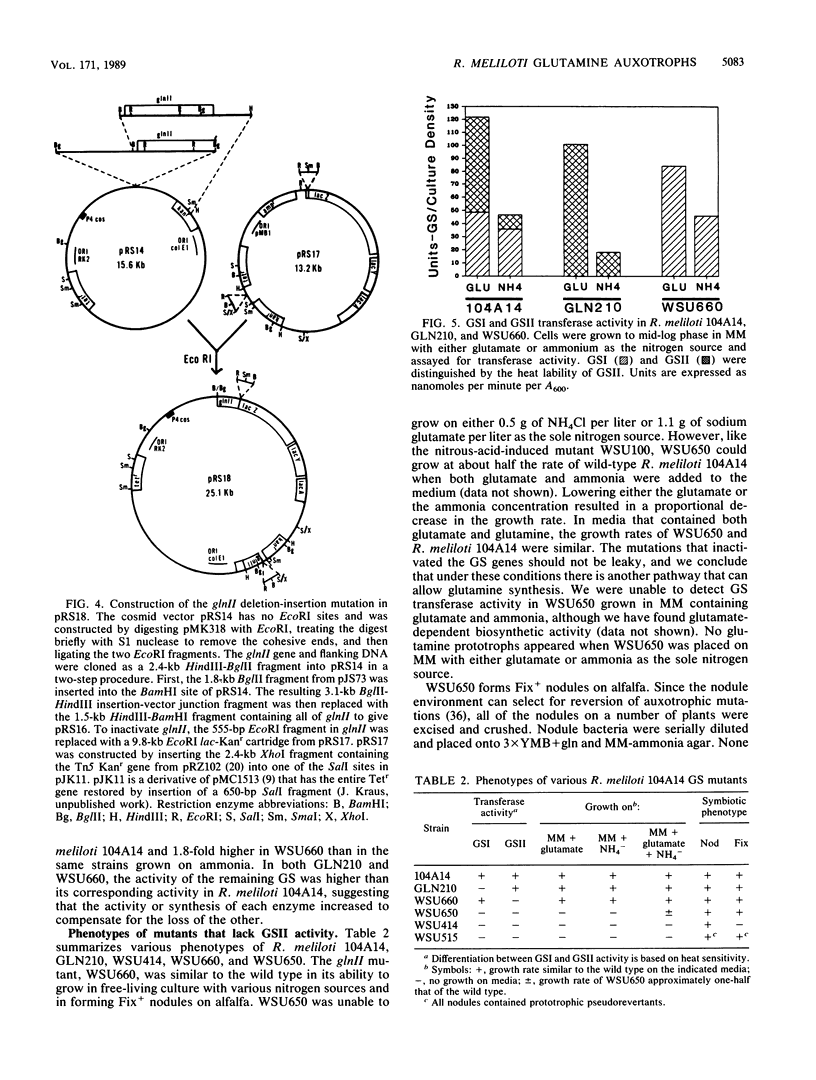
Abstract
The glutamine synthetase (GS)-glutamate synthase pathway is the primary route used by members of the family Rhizobiaceae to assimilate ammonia. Two forms of glutamine synthetase, GSI and GSII, are found in Rhizobium and Bradyrhizobium species. These are encoded by the glnA and glnII genes, respectively. Starting with a Rhizobium meliloti glnA mutant as the parent strain, we isolated mutants unable to grow on minimal medium with ammonia as the sole nitrogen source. For two auxotrophs that lacked any detectable GS activity, R. meliloti DNA of the mutated region was cloned and partially characterized. Lack of cross-hybridization indicated that the cloned regions were not closely linked to each other or to glnA; they therefore contain two independent genes needed for GSII synthesis or activity. One of the cloned regions was identified as glnII. An R. meliloti glnII mutant and an R. meliloti glnA glnII double mutant were constructed. Both formed effective nodules on alfalfa. This is unlike the B. japonicum-soybean symbiosis, in which at least one of these GS enzymes must be present for nitrogen-fixing nodules to develop. However, the R. meliloti double mutant was not a strict glutamine auxotroph, since it could grow on media that contained glutamate and ammonia, an observation that suggests that a third GS may be active in this species.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali H., Niel C., Guillaume J. B. The pathways of ammonium assimilation in Rhizobium meliloti. Arch Microbiol. 1981 Jul;129(5):391–394. doi: 10.1007/BF00406469. [DOI] [PubMed] [Google Scholar]
- Bergersen J. F., Turner G. L. Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim Biophys Acta. 1967 Aug 29;141(3):507–515. doi: 10.1016/0304-4165(67)90179-1. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bravo A., Mora J. Ammonium assimilation in Rhizobium phaseoli by the glutamine synthetase-glutamate synthase pathway. J Bacteriol. 1988 Feb;170(2):980–984. doi: 10.1128/jb.170.2.980-984.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Dilworth M. J. Ammonia assimilation by rhizobium cultures and bacteroids. J Gen Microbiol. 1975 Jan;86(1):39–48. doi: 10.1099/00221287-86-1-39. [DOI] [PubMed] [Google Scholar]
- Carlson T. A., Guerinot M. L., Chelm B. K. Characterization of the gene encoding glutamine synthetase I (glnA) from Bradyrhizobium japonicum. J Bacteriol. 1985 May;162(2):698–703. doi: 10.1128/jb.162.2.698-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson T. A., Martin G. B., Chelm B. K. Differential transcription of the two glutamine synthetase genes of Bradyrhizobium japonicum. J Bacteriol. 1987 Dec;169(12):5861–5866. doi: 10.1128/jb.169.12.5861-5866.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Darrow R. A., Knotts R. R. Two forms of glutamine synthetase in free-living root-nodule bacteria. Biochem Biophys Res Commun. 1977 Sep 23;78(2):554–559. doi: 10.1016/0006-291x(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Donald R. G., Ludwig R. A. Rhizobium sp. strain ORS571 ammonium assimilation and nitrogen fixation. J Bacteriol. 1984 Jun;158(3):1144–1151. doi: 10.1128/jb.158.3.1144-1151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmands J., Noridge N. A., Benson D. R. The actinorhizal root-nodule symbiont Frankia sp. strain CpI1 has two glutamine synthetases. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6126–6130. doi: 10.1073/pnas.84.17.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filser D. M., Moscatelli C., Lamberti A., Vincze E., Guida M., Salzano G., Iaccarino M. Characterization and cloning of two Rhizobium leguminosarum genes coding for glutamine synthetase activities. J Gen Microbiol. 1986 Sep;132(9):2561–2569. doi: 10.1099/00221287-132-9-2561. [DOI] [PubMed] [Google Scholar]
- Fuchs R. L., Keister D. L. Comparative properties of glutamine synthetases I and II in Rhizobium and Agrobacterium spp. J Bacteriol. 1980 Nov;144(2):641–648. doi: 10.1128/jb.144.2.641-648.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R. L., Keister D. L. Identification of two glutamine synthetases in Agrobacterium. J Bacteriol. 1980 Feb;141(2):996–998. doi: 10.1128/jb.141.2.996-998.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Kahn M. L. Genetic analysis of carbamoylphosphate synthesis in Rhizobium meliloti 104A14. J Gen Microbiol. 1988 Apr;134(4):921–929. doi: 10.1099/00221287-134-4-921. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Kahn M. L. Symbiotic phenotypes of auxotrophic mutants of Rhizobium meliloti 104A14. J Gen Microbiol. 1988 Apr;134(4):913–919. doi: 10.1099/00221287-134-4-913. [DOI] [PubMed] [Google Scholar]
- Ludwig R. A. Control of ammonium assimilation in Rhizobium 32H1. J Bacteriol. 1978 Jul;135(1):114–123. doi: 10.1128/jb.135.1.114-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R. A. Physiological roles of glutamine synthetases I and II in ammonium assimilation in Rhizobium sp. 32H1. J Bacteriol. 1980 Mar;141(3):1209–1216. doi: 10.1128/jb.141.3.1209-1216.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R. A. Regulation of Rhizobium nitrogen fixation by the unadenylylated glutamine synthetase I system. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5817–5821. doi: 10.1073/pnas.77.10.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil T., MacNeil D., Tyler B. Fine-structure deletion map and complementation analysis of the glnA-glnL-glnG region in Escherichia coli. J Bacteriol. 1982 Jun;150(3):1302–1313. doi: 10.1128/jb.150.3.1302-1313.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osburne M. S. Rhizobium meliloti mutants altered in ammonium utilization. J Bacteriol. 1982 Sep;151(3):1633–1636. doi: 10.1128/jb.151.3.1633-1636.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Albright L. M., Ausubel F. M. Rhizobium meliloti ntrA (rpoN) gene is required for diverse metabolic functions. J Bacteriol. 1987 Jun;169(6):2424–2431. doi: 10.1128/jb.169.6.2424-2431.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Suicide plasmid vehicles for insertion mutagenesis in Rhizobium meliloti and related bacteria. J Bacteriol. 1983 Dec;156(3):1292–1300. doi: 10.1128/jb.156.3.1292-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatters R. G., Somerville J. E., Kahn M. L. Regulation of glutamine synthetase II activity in Rhizobium meliloti 104A14. J Bacteriol. 1989 Sep;171(9):5087–5094. doi: 10.1128/jb.171.9.5087-5094.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville J. E., Kahn M. L. Cloning of the glutamine synthetase I gene from Rhizobium meliloti. J Bacteriol. 1983 Oct;156(1):168–176. doi: 10.1128/jb.156.1.168-176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Carbohydrate, organic Acid, and amino Acid composition of bacteroids and cytosol from soybean nodules. Plant Physiol. 1987 Nov;85(3):768–773. doi: 10.1104/pp.85.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn F. J., Rossbach S., Schneider M., Ratet P., Messmer S., Szeto W. W., Ausubel F. M., Schell J. Rhizobium meliloti 1021 has three differentially regulated loci involved in glutamine biosynthesis, none of which is essential for symbiotic nitrogen fixation. J Bacteriol. 1989 Mar;171(3):1673–1682. doi: 10.1128/jb.171.3.1673-1682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



