Abstract
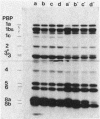
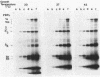
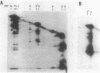
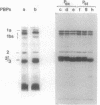
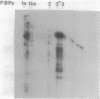
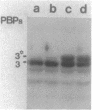
In this paper we describe a new beta-lactam-binding protein from the cell envelope of Escherichia coli. It can be detected in cells grown at either 37 or 42 degrees C in medium containing glucose but not in cells grown at 30 degrees C. This novel component has an apparent molecular size that is 2.0 kilodaltons larger than that of penicillin-binding protein 3 and is derived from the latter through a divalent-cation-mediated process probably catalyzed by components located in the periplasmic space. The significance of this protein with regard to regulation of the amount of functional penicillin-binding protein 3 in the cell is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayala J. A., Plá J., Desviat L. R., de Pedro M. A. A lacZ-pbpB gene fusion coding for an inducible hybrid protein that recognizes localized sites in the inner membrane of Escherichia coli. J Bacteriol. 1988 Aug;170(8):3333–3341. doi: 10.1128/jb.170.8.3333-3341.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas J. A., Díaz J., Rodríguez-Tébar A., Vázquez D. Specific location of penicillin-binding proteins within the cell envelope of Escherichia coli. J Bacteriol. 1986 Jan;165(1):269–275. doi: 10.1128/jb.165.1.269-275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome-Smith J. K., Edelman A., Yousif S., Spratt B. G. The nucleotide sequences of the ponA and ponB genes encoding penicillin-binding protein 1A and 1B of Escherichia coli K12. Eur J Biochem. 1985 Mar 1;147(2):437–446. doi: 10.1111/j.1432-1033.1985.tb08768.x. [DOI] [PubMed] [Google Scholar]
- Broome-Smith J. K., Hedge P. J., Spratt B. G. Production of thiol-penicillin-binding protein 3 of Escherichia coli using a two primer method of site-directed mutagenesis. EMBO J. 1985 Jan;4(1):231–235. doi: 10.1002/j.1460-2075.1985.tb02340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Goldberg A. L., Swamy K. H., Chung C. H., Larimore F. S. Proteases in Escherichia coli. Methods Enzymol. 1981;80(Pt 100):680–702. doi: 10.1016/s0076-6879(81)80052-3. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Hara H., Suzuki H., Hirota Y. Lipid modification of Escherichia coli penicillin-binding protein 3. J Bacteriol. 1988 Nov;170(11):5392–5395. doi: 10.1128/jb.170.11.5392-5395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. H., Young K. D. Unequal distribution of penicillin-binding proteins among inner membrane vesicles of Escherichia coli. J Bacteriol. 1988 Aug;170(8):3660–3667. doi: 10.1128/jb.170.8.3660-3667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Maruyama I. N., Soma M., Kato J., Suzuki H., Horota Y. On the process of cellular division in Escherichia coli: nucleotide sequence of the gene for penicillin-binding protein 3. Mol Gen Genet. 1983;191(1):1–9. doi: 10.1007/BF00330881. [DOI] [PubMed] [Google Scholar]
- Pisabarro A. G., Prats R., Váquez D., Rodríguez-Tébar A. Activity of penicillin-binding protein 3 from Escherichia coli. J Bacteriol. 1986 Oct;168(1):199–206. doi: 10.1128/jb.168.1.199-206.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Tébar A., Arán V., Vázquez D. Labelling and cross-linking of Escherichia coli penicillin-binding proteins with bis-beta-lactam antibiotics. Eur J Biochem. 1984 Mar 1;139(2):287–293. doi: 10.1111/j.1432-1033.1984.tb08006.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Tébar A., Barbas J. A., Vázquez D. Location of some proteins involved in peptidoglycan synthesis and cell division in the inner and outer membranes of Escherichia coli. J Bacteriol. 1985 Jan;161(1):243–248. doi: 10.1128/jb.161.1.243-248.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo F., Ayala J. A., De Pedro M. A., Vázquez D. Analysis of the different molecular forms of penicillin-binding protein 1B in Escherichia coli ponB mutants lysogenized with specialized transducing lambda (ponB+) bacteriophages. Eur J Biochem. 1984 Nov 2;144(3):571–576. doi: 10.1111/j.1432-1033.1984.tb08503.x. [DOI] [PubMed] [Google Scholar]
- Rojo F., Ayala J. A., de la Rosa E. J., de Pedro M. A., Arán V., Berenguer J., Vázquez D. Binding of 125I-labeled beta-lactam antibiotics to the penicillin binding proteins of Escherichia coli. J Antibiot (Tokyo) 1984 Apr;37(4):389–393. doi: 10.7164/antibiotics.37.389. [DOI] [PubMed] [Google Scholar]
- Rojo F., Berenguer J., Ayala J. A., de Pedro M. A. Variability in the posttranslational processing of penicillin-binding protein 1b among different strains of Escherichia coli. Biochem Cell Biol. 1987 Jan;65(1):62–67. doi: 10.1139/o87-009. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kato J., Sakagami Y., Mori M., Suzuki A., Hirota Y. Conversion of the alpha component of penicillin-binding protein 1b to the beta component in Escherichia coli. J Bacteriol. 1987 Feb;169(2):891–893. doi: 10.1128/jb.169.2.891-893.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Ayala J. A., de Pedro M. A., Aldea M., Vicente M. Interaction of FtsA and PBP3 proteins in the Escherichia coli septum. J Bacteriol. 1986 Jun;166(3):985–992. doi: 10.1128/jb.166.3.985-992.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Vicente M. The ftsA gene product participates in formation of the Escherichia coli septum structure. J Bacteriol. 1984 Mar;157(3):779–784. doi: 10.1128/jb.157.3.779-784.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]