Abstract
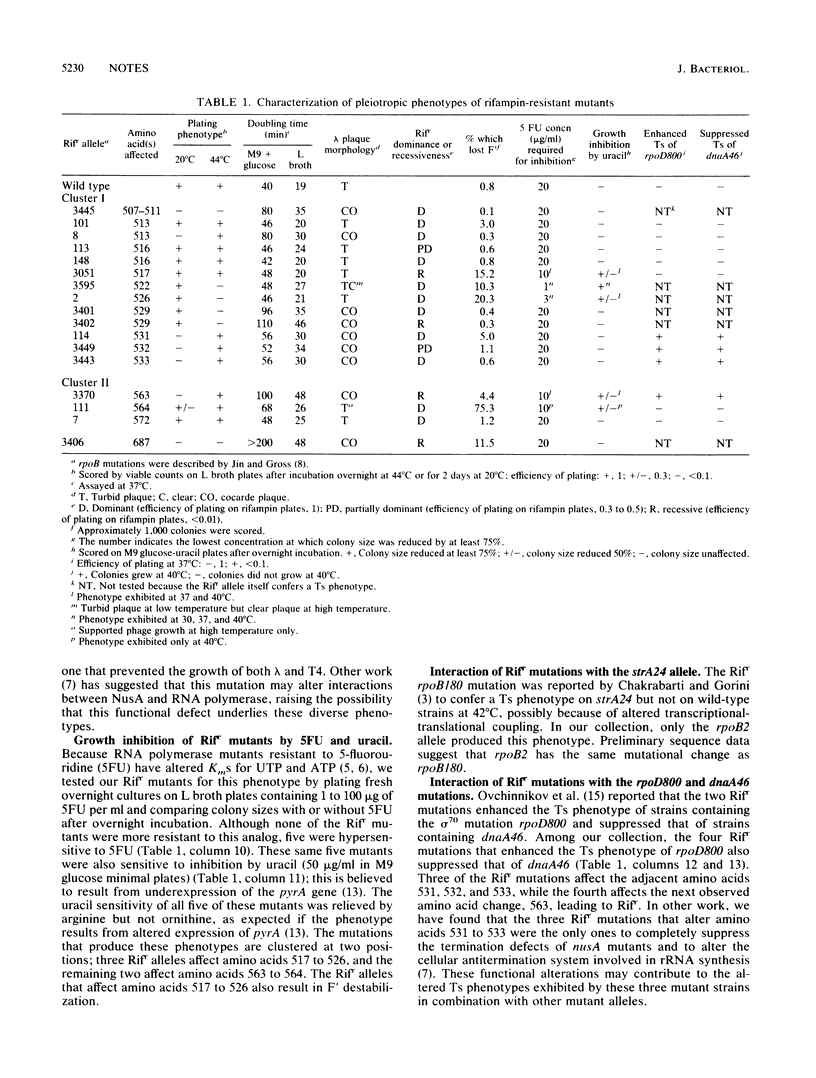
We used our collection of 17 sequenced rifampin resistance alleles in rpoB to perform a systematic analysis of the phenotypes historically reported with this class of mutants, including growth phenotype, ability to support the growth of different bacteriophages, ability to maintain the F' episome, interaction with mutant alleles at other loci, sensitivity to uracil, inhibition by 5-fluorouridine, and dominance. We found that mutational changes leading to the same phenotype were often located together and that certain phenotypes were associated with one another.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babinet C. Propriétés de dominance de quelques mutations conférant la résitance à la rifampicine chez E. coli K12. Biochimie. 1971;53(4):507–516. doi: 10.1016/s0300-9084(71)80168-2. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M. M., Izakowska M., Bagdasarian M. Suppression of the DnaA phenotype by mutations in the rpoB cistron of ribonucleic acid polymerase in Salmonella typhimurium and Escherichia coli. J Bacteriol. 1977 May;130(2):577–582. doi: 10.1128/jb.130.2.577-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S. L., Gorini L. Interaction between mutations of ribosomes and RNA polymerase: a pair of strA and rif mutants individually temperature-insensitive but temperature-sensitive in combination. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1157–1161. doi: 10.1073/pnas.74.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. W., Jr, Chao L. Altered stability and integration frequency of a F' factor in RNA polymerase mutants of Escherichia coli. Mol Gen Genet. 1981;182(1):12–18. doi: 10.1007/BF00422760. [DOI] [PubMed] [Google Scholar]
- Jensen K. F., Fast R., Karlström O., Larsen J. N. Association of RNA polymerase having increased Km for ATP and UTP with hyperexpression of the pyrB and pyrE genes of Salmonella typhimurium. J Bacteriol. 1986 Jun;166(3):857–865. doi: 10.1128/jb.166.3.857-865.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. F., Neuhard J., Schack L. RNA polymerase involvement in the regulation of expression of Salmonella typhimurium pyr genes. Isolation and characterization of a fluorouracil-resistant mutant with high, constitutive expression of the pyrB and pyrE genes due to a mutation in rpoBC. EMBO J. 1982;1(1):69–74. doi: 10.1002/j.1460-2075.1982.tb01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D. J., Cashel M., Friedman D. I., Nakamura Y., Walter W. A., Gross C. A. Effects of rifampicin resistant rpoB mutations on antitermination and interaction with nusA in Escherichia coli. J Mol Biol. 1988 Nov 20;204(2):247–261. doi: 10.1016/0022-2836(88)90573-6. [DOI] [PubMed] [Google Scholar]
- Jin D. J., Gross C. A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988 Jul 5;202(1):45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- Jin D. J., Walter W. A., Gross C. A. Characterization of the termination phenotypes of rifampicin-resistant mutants. J Mol Biol. 1988 Jul 20;202(2):245–253. doi: 10.1016/0022-2836(88)90455-x. [DOI] [PubMed] [Google Scholar]
- Kawai M., Ishihama A., Yura T. RNA polymerase mutants of Escherichia coli. III. A temperature-sensitive rifampicin-resistant mutant. Mol Gen Genet. 1976 Feb 2;143(3):233–241. doi: 10.1007/BF00269399. [DOI] [PubMed] [Google Scholar]
- Kirschbaum J. B., Konrad E. B. Isolation of a specialized lambda transducing bacteriophage carrying the beta subunit gene for Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1973 Nov;116(2):517–526. doi: 10.1128/jb.116.2.517-526.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecocq J., Dambly C. A bacterial RNA polymerase mutant that renders lambda growth independent of the N and cro functions at 42 degrees C. Mol Gen Genet. 1976 Apr 23;145(1):53–64. doi: 10.1007/BF00331557. [DOI] [PubMed] [Google Scholar]
- Neuhard J., Jensen K. F., Stauning E. Salmonella typhimurium mutants with altered expression of the pyrA gene due to changes in RNA polymerase. EMBO J. 1982;1(9):1141–1145. doi: 10.1002/j.1460-2075.1982.tb01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A., Hayward R. S. Cloning of DNA of the rpoBC operon from the chromosome of Escherichia coli K12. Mol Gen Genet. 1980 Feb;177(3):527–533. doi: 10.1007/BF00271493. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Monastyrskaya G. S., Guriev S. O., Kalinina N. F., Sverdlov E. D., Gragerov A. I., Bass I. A., Kiver I. F., Moiseyeva E. P., Igumnov V. N. RNA polymerase rifampicin resistance mutations in Escherichia coli: sequence changes and dominance. Mol Gen Genet. 1983;190(2):344–348. doi: 10.1007/BF00330662. [DOI] [PubMed] [Google Scholar]
- Reid P. Isolation of cold sensitive-rifampicin resistant RNA polymerase mutants of Escherichia coli. Biochem Biophys Res Commun. 1971 Aug 6;44(3):737–744. doi: 10.1016/s0006-291x(71)80145-6. [DOI] [PubMed] [Google Scholar]
- Schwarz T. F., Yeats S. M., Connolly P., McConnell D. J. Altered transcriptional termination in a rifampicin-resistant mutant of Escherichia coli which inhibits the growth of bacteriophage T7. Mol Gen Genet. 1981;183(1):181–186. doi: 10.1007/BF00270159. [DOI] [PubMed] [Google Scholar]
- Snyder L. R. An RNA polymerase mutant of Escherichia coli defective in the T4 viral transcription program. Virology. 1972 Nov;50(2):396–403. doi: 10.1016/0042-6822(72)90391-1. [DOI] [PubMed] [Google Scholar]


