Abstract
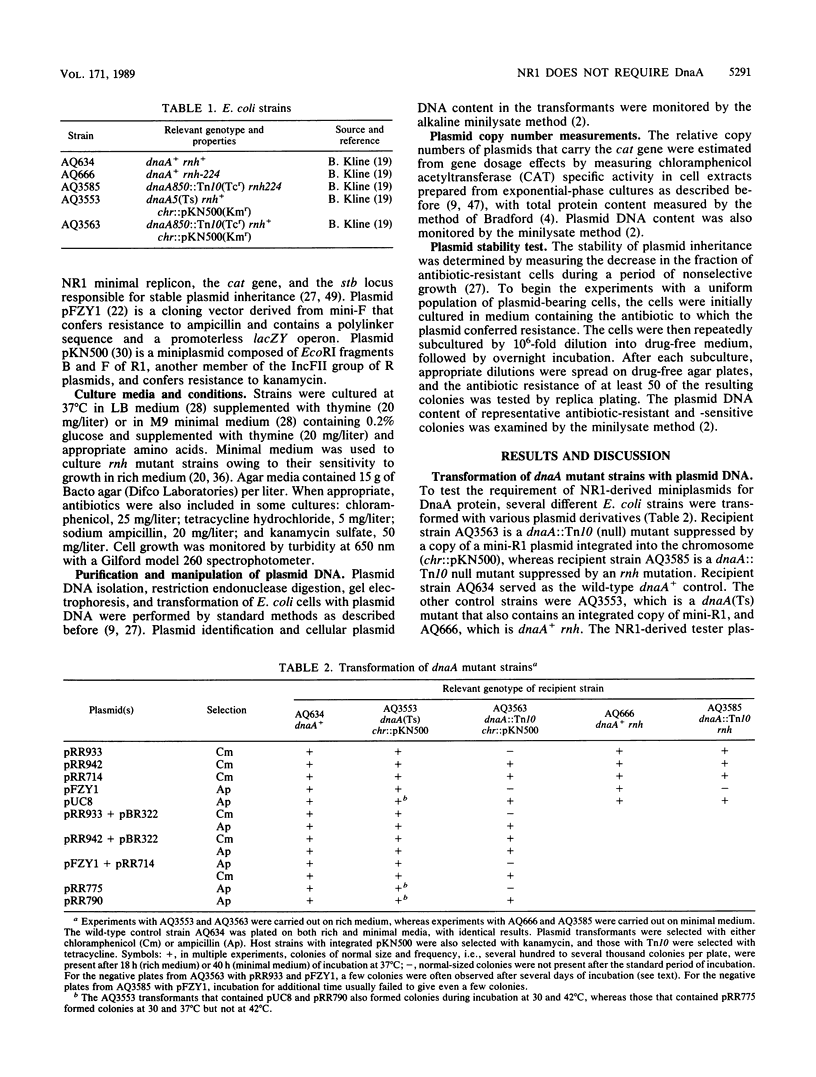
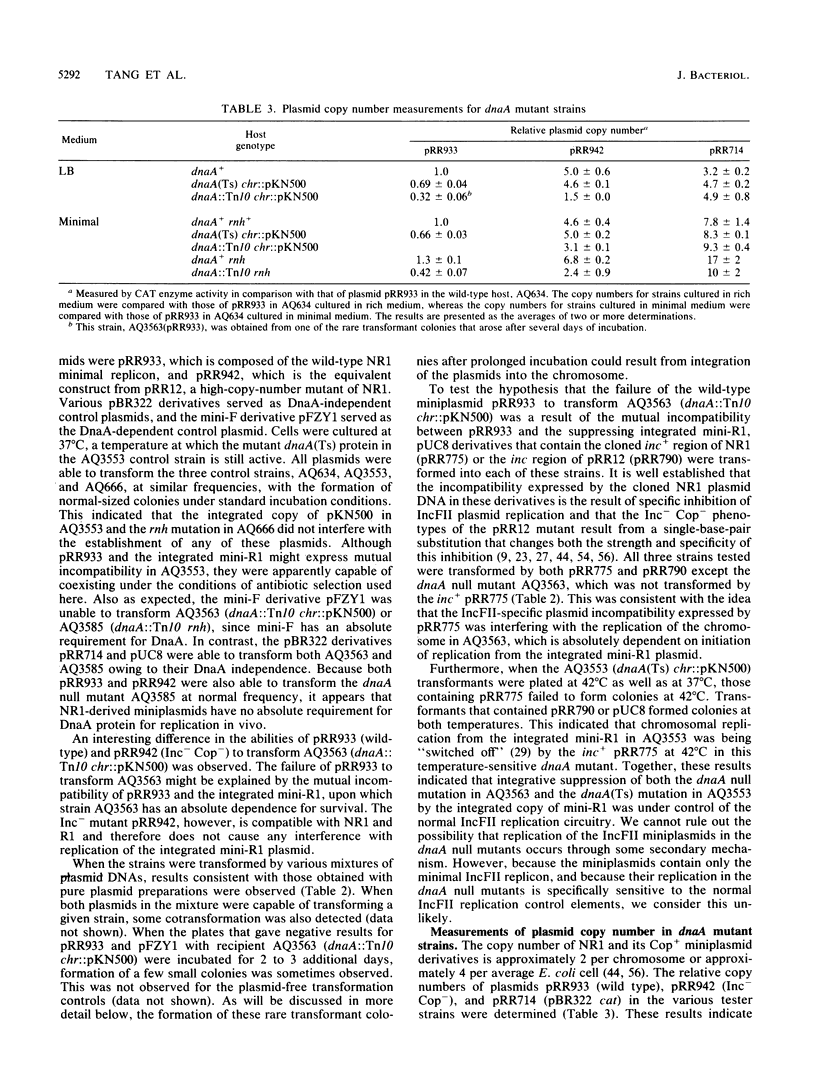
By transformation of dnaA null mutant host cells that are suppressed either by an rnh mutation or by chromosomal integration of a mini-R1 plasmid, it was shown that replication of miniplasmids composed of the NR1 minimal replicon had no absolute dependence upon DnaA protein. In addition, the suppression of the dnaA null mutation by the integrated mini-R1, which is an IncFII relative of NR1, was found to be sensitive to the expression of IncFII-specific plasmid incompatibility. This suggests that the integrative suppression by mini-R1 is under the control of the normal IncFII plasmid replication circuitry. Although NR1 replication had no absolute requirement for DnaA, the copy numbers of NR1-derived miniplasmids were lower in dnaA null mutants, and the plasmids exhibited a much reduced stability of inheritance during subculture in the absence of selection. This suggests that DnaA protein may participate in IncFII plasmid replication in some auxiliary way, such as by increasing the efficiency of formation of an open initiation complex at the plasmid replication origin. Such an auxiliary role for DnaA in IncFII replication would be different from that for replication of most other plasmids examined, for which DnaA has been found to be either essential or unimportant.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles A. L., Snyder K. M., Chattoraj D. K. P1 plasmid replication: replicon structure. J Mol Biol. 1984 Mar 5;173(3):307–324. doi: 10.1016/0022-2836(84)90123-2. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell. 1988 Mar 11;52(5):743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Chandler M., Silver L., Caro L. Suppression of an Escherichia coli dnaA mutation by the integrated R factor R100.1: origin of chromosome replication during exponential growth. J Bacteriol. 1977 Aug;131(2):421–430. doi: 10.1128/jb.131.2.421-430.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney R. H., Scott J. R., Vapnek D. Integration of the plasmid prophages P1 and P7 into the chromosome of Escherichia coli. J Mol Biol. 1979 May 15;130(2):161–173. doi: 10.1016/0022-2836(79)90424-8. [DOI] [PubMed] [Google Scholar]
- Easton A. M., Rownd R. H. The incompatibility product of IncFII R plasmid NR1 controls gene expression in the plasmid replication region. J Bacteriol. 1982 Nov;152(2):829–839. doi: 10.1128/jb.152.2.829-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton J., Wright A. Plasmid pSC101 replication in integratively suppressed cells requires dnaA function. Mol Gen Genet. 1979 Sep;175(2):231–233. doi: 10.1007/BF00425541. [DOI] [PubMed] [Google Scholar]
- Frey J., Chandler M., Caro L. The effects of an Escherichia coli dnaAts mutation on the replication of the plasmids colE1 pSC101, R100.1 and RTF-TC. Mol Gen Genet. 1979 Jul 13;174(2):117–126. doi: 10.1007/BF00268349. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Kornberg A. Purified dnaA protein in initiation of replication at the Escherichia coli chromosomal origin of replication. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5817–5821. doi: 10.1073/pnas.80.19.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W. The influence of DNA A and DNA C mutations on the initiation of plasmid DNA replication. Biochem Biophys Res Commun. 1973 Apr 16;51(4):1000–1007. doi: 10.1016/0006-291x(73)90026-0. [DOI] [PubMed] [Google Scholar]
- Hansen E. B., Yarmolinsky M. B. Host participation in plasmid maintenance: dependence upon dnaA of replicons derived from P1 and F. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4423–4427. doi: 10.1073/pnas.83.12.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasunuma K., Sekiguchi M. Replication of plasmid pSC101 in Escherichia coli K12: requirement for dnaA function. Mol Gen Genet. 1977 Sep 9;154(3):225–230. doi: 10.1007/BF00571277. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Kline B. C., Kogoma T., Tam J. E., Shields M. S. Requirement of the Escherichia coli dnaA gene product for plasmid F maintenance. J Bacteriol. 1986 Oct;168(1):440–443. doi: 10.1128/jb.168.1.440-443.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoma T., Kline B. C. Integrative suppression of dnaA(Ts) mutations mediated by plasmid F in Escherichia coli is a DnaA-dependent process. Mol Gen Genet. 1987 Dec;210(2):262–269. doi: 10.1007/BF00325692. [DOI] [PubMed] [Google Scholar]
- Kogoma T. RNase H-defective mutants of Escherichia coli. J Bacteriol. 1986 May;166(2):361–363. doi: 10.1128/jb.166.2.361-363.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop A. H., Hartley M. E., Bourgeois S. A low-copy-number vector utilizing beta-galactosidase for the analysis of gene control elements. Gene. 1987;52(2-3):245–256. doi: 10.1016/0378-1119(87)90051-5. [DOI] [PubMed] [Google Scholar]
- Light J., Molin S. Post-transcriptional control of expression of the repA gene of plasmid R1 mediated by a small RNA molecule. EMBO J. 1983;2(1):93–98. doi: 10.1002/j.1460-2075.1983.tb01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai H., Arai K. RepA and DnaA proteins are required for initiation of R1 plasmid replication in vitro and interact with the oriR sequence. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4781–4785. doi: 10.1073/pnas.84.14.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M., Oka A., Takanami M., Yasuda S., Hirota Y. Sites of dnaA protein-binding in the replication origin of the Escherichia coli K-12 chromosome. J Mol Biol. 1985 Aug 5;184(3):529–533. doi: 10.1016/0022-2836(85)90299-2. [DOI] [PubMed] [Google Scholar]
- Miki T., Easton A. M., Rownd R. H. Cloning of replication, incompatibility, and stability functions of R plasmid NR1. J Bacteriol. 1980 Jan;141(1):87–99. doi: 10.1128/jb.141.1.87-99.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin S., Nordström K. Control of plasmid R1 replication: functions involved in replication, copy number control, incompatibility, and switch-off of replication. J Bacteriol. 1980 Jan;141(1):111–120. doi: 10.1128/jb.141.1.111-120.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin S., Stougaard P., Uhlin B. E., Gustafsson P., Nordström K. Clustering of genes involved in replication, copy number control, incompatibility, and stable maintenance of the resistance plasmid R1drd-19. J Bacteriol. 1979 Apr;138(1):70–79. doi: 10.1128/jb.138.1.70-79.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. F., Hashimoto H., Mickel S., Rownd R. Round of replication mutant of a drug resistance factor. J Bacteriol. 1974 Jun;118(3):855–866. doi: 10.1128/jb.118.3.855-866.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Ohmori H., Yura T., Nagata T. Requirement of the Escherichia coli dnaA gene function for ori-2-dependent mini-F plasmid replication. J Bacteriol. 1987 Apr;169(4):1724–1730. doi: 10.1128/jb.169.4.1724-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murotsu T., Tsutsui H., Matsubara K. Identification of the minimal essential region for the replication origin of miniF plasmid. Mol Gen Genet. 1984;196(2):373–378. doi: 10.1007/BF00328075. [DOI] [PubMed] [Google Scholar]
- NAKAYA R., NAKAMURA A., MURATA Y. Resistance transfer agents in Shigella. Biochem Biophys Res Commun. 1960 Dec;3:654–659. doi: 10.1016/0006-291x(60)90081-4. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Caro L., Berg C. M., Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J Mol Biol. 1971 Feb 14;55(3):441–456. doi: 10.1016/0022-2836(71)90328-7. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Pickett G. G., Kogoma T., Kornberg A. RNase H confers specificity in the dnaA-dependent initiation of replication at the unique origin of the Escherichia coli chromosome in vivo and in vitro. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1040–1044. doi: 10.1073/pnas.81.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo H., Ryder T. B., Maeda Y., Armstrong K., Ohtsubo E. DNA replication of the resistance plasmid R100 and its control. Adv Biophys. 1986;21:115–133. doi: 10.1016/0065-227x(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Ortega S., Lanka E., Diaz R. The involvement of host replication proteins and of specific origin sequences in the in vitro replication of miniplasmid R1 DNA. Nucleic Acids Res. 1986 Jun 25;14(12):4865–4879. doi: 10.1093/nar/14.12.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B. C., Rownd R. H. Recombination sites in plasmid drug resistance gene amplification. J Bacteriol. 1985 Dec;164(3):1359–1361. doi: 10.1128/jb.164.3.1359-1361.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J., Ryder T., Inokuchi H., Ohtsubo H., Ohtsubo E. Genes and sites involved in replication and incompatibility of an R100 plasmid derivative based on nucleotide sequence analysis. Mol Gen Genet. 1980;179(3):527–537. doi: 10.1007/BF00271742. [DOI] [PubMed] [Google Scholar]
- Rosen J., Ryder T., Ohtsubo H., Ohtsubo E. Role of RNA transcripts in replication incompatibility and copy number control in antibiotic resistance plasmid derivatives. Nature. 1981 Apr 30;290(5809):794–797. doi: 10.1038/290794a0. [DOI] [PubMed] [Google Scholar]
- Rownd R. H., Womble D. D., Dong X. N., Luckow V. A., Wu R. P. Incompatibility and IncFII plasmid replication control. Basic Life Sci. 1985;30:335–354. doi: 10.1007/978-1-4613-2447-8_26. [DOI] [PubMed] [Google Scholar]
- Rownd R., Nakaya R., Nakamura A. Molecular nature of the drug-resistance factors of the Enterobacteriaceae. J Mol Biol. 1966 Jun;17(2):376–393. doi: 10.1016/s0022-2836(66)80149-3. [DOI] [PubMed] [Google Scholar]
- Scott J. R. Regulation of plasmid replication. Microbiol Rev. 1984 Mar;48(1):1–23. doi: 10.1016/b978-0-12-048850-6.50006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Tabuchi A., Min Y. N., Kim C. K., Fan Y. L., Womble D. D., Rownd R. H. Genetic organization and nucleotide sequence of the stability locus of IncFII plasmid NR1. J Mol Biol. 1988 Aug 5;202(3):511–525. doi: 10.1016/0022-2836(88)90282-3. [DOI] [PubMed] [Google Scholar]
- Tolun A., Helinski D. R. Separation of the minimal replication region of the F plasmid into a replication origin segment and a trans-acting segment. Mol Gen Genet. 1982;186(3):372–377. doi: 10.1007/BF00729456. [DOI] [PubMed] [Google Scholar]
- Tresguerres E. F., Nandadasa H. G., Pritchard R. H. Suppression of initiation-negative strains of Escherichia coli by integration of the sex factor F. J Bacteriol. 1975 Feb;121(2):554–561. doi: 10.1128/jb.121.2.554-561.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Womble D. D., Dong X., Luckow V. A., Wu R. P., Rownd R. H. Analysis of the individual regulatory components of the IncFII plasmid replication control system. J Bacteriol. 1985 Feb;161(2):534–543. doi: 10.1128/jb.161.2.534-543.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble D. D., Dong X., Wu R. P., Luckow V. A., Martinez A. F., Rownd R. H. IncFII plasmid incompatibility product and its target are both RNA transcripts. J Bacteriol. 1984 Oct;160(1):28–35. doi: 10.1128/jb.160.1.28-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble D. D., Rownd R. H. Genetic and physical map of plasmid NR1: comparison with other IncFII antibiotic resistance plasmids. Microbiol Rev. 1988 Dec;52(4):433–451. doi: 10.1128/mr.52.4.433-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble D. D., Rownd R. H. Regulation of IncFII plasmid DNA replication. A quantitative model for control of plasmid NR1 replication in the bacterial cell division cycle. J Mol Biol. 1986 Dec 5;192(3):529–547. doi: 10.1016/0022-2836(86)90274-3. [DOI] [PubMed] [Google Scholar]
- Womble D. D., Rownd R. H. Replication of plasmids in mutants of Escherichia coli temperature-sensitive for initiation of deoxyribonucleic acid synthesis. Plasmid. 1979 Jan;2(1):95–108. doi: 10.1016/0147-619x(79)90009-x. [DOI] [PubMed] [Google Scholar]
- Womble D. D., Sampathkumar P., Easton A. M., Luckow V. A., Rownd R. H. Transcription of the replication control region of the IncFII R-plasmid NR1 in vitro and in vivo. J Mol Biol. 1985 Feb 5;181(3):395–410. doi: 10.1016/0022-2836(85)90228-1. [DOI] [PubMed] [Google Scholar]
- Womble D. D., Taylor D. P., Rownd R. H. Method for obtaining more-accurate covalently closed circular plasmid-to-chromosome ratios from bacterial lysates by dye-buoyant density centrifugation. J Bacteriol. 1977 Apr;130(1):148–153. doi: 10.1128/jb.130.1.148-153.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]




