Abstract
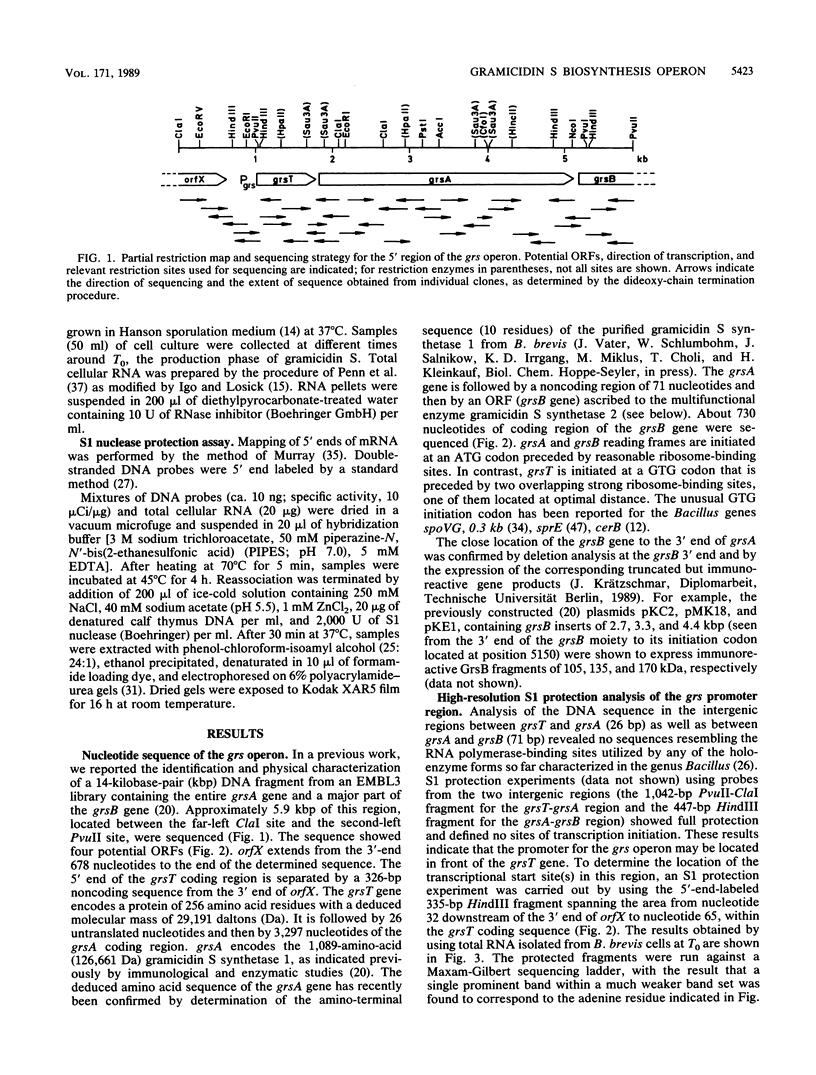
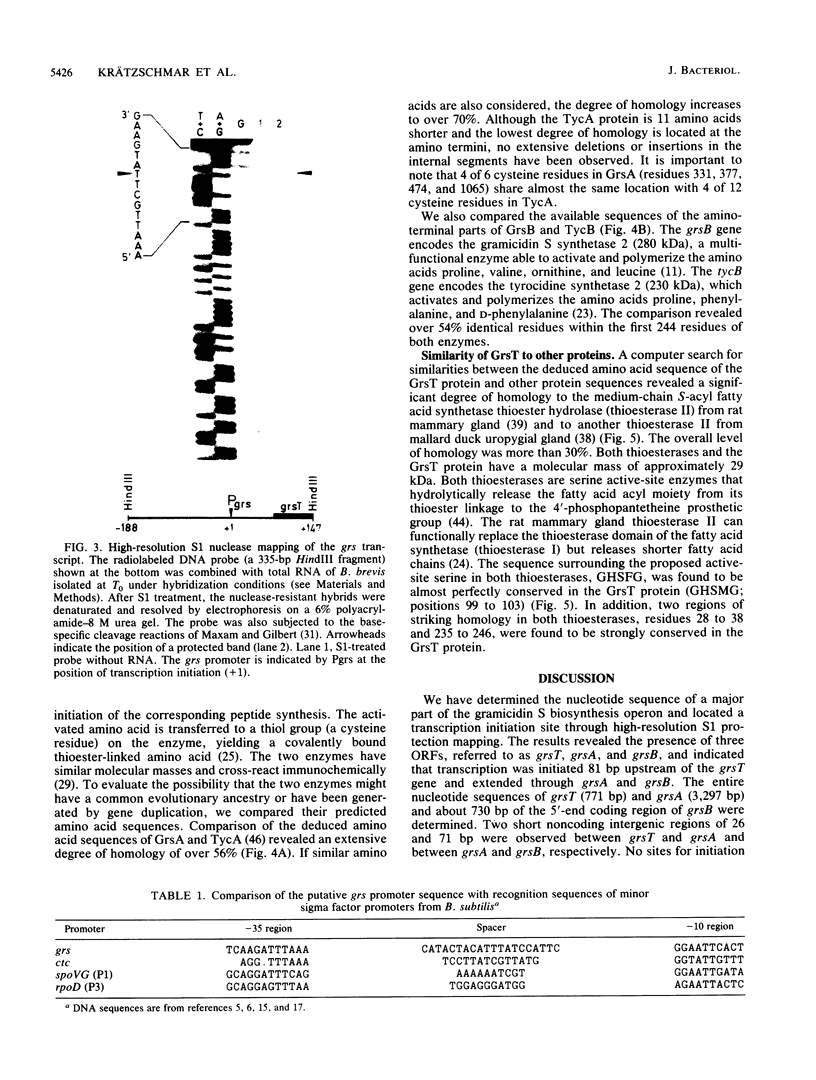
The DNA sequence of about 5.9 kilobase pairs (kbp) of the gramicidin S biosynthesis operon (grs) was determined. Three open reading frames were identified; the corresponding genes, called grsT, grsA, and grsB, were found to be organized in one transcriptional unit, not two as previously reported (M. Krause and M. A. Marahiel, J. Bacteriol. 170:4669-4674, 1988). The entire nucleotide sequence of grsA, coding for the 126.663-kilodalton gramicidin S synthetase 1, grsT, encoding a 29.191-kilodalton protein of unknown function, and 732 bp of the 5' end of grsB, encoding the gramicidin S synthetase 2, were determined. A single initiation site of transcription 81 bp upstream of the grsT initiation condon GTG was identified by high-resolution S1 mapping studies. The sequence of the grsA gene product showed a high degree of homology to the tyrocidine synthetase 1 (TycA protein), and that of grsT exhibited a significant degree of homology to vertebrate fatty acid thioesterases.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banner C. D., Moran C. P., Jr, Losick R. Deletion analysis of a complex promoter for a developmentally regulated gene from Bacillus subtilis. J Mol Biol. 1983 Aug 5;168(2):351–365. doi: 10.1016/s0022-2836(83)80023-0. [DOI] [PubMed] [Google Scholar]
- Binnie C., Lampe M., Losick R. Gene encoding the sigma 37 species of RNA polymerase sigma factor from Bacillus subtilis. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5943–5947. doi: 10.1073/pnas.83.16.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm S. P., Staal S. P., Hoch J. A. Phenotypes of pleiotropic-negative sporulation mutants of Bacillus subtilis. J Bacteriol. 1973 Sep;115(3):1063–1070. doi: 10.1128/jb.115.3.1063-1070.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter H. L., 3rd, Moran C. P., Jr New RNA polymerase sigma factor under spo0 control in Bacillus subtilis. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9438–9442. doi: 10.1073/pnas.83.24.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter H. L., 3rd, Wang L. F., Doi R. H., Moran C. P., Jr rpoD operon promoter used by sigma H-RNA polymerase in Bacillus subtilis. J Bacteriol. 1988 Apr;170(4):1617–1621. doi: 10.1128/jb.170.4.1617-1621.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse G. F., Frischauf A., Lehrach H. An integrated and simplified approach to cloning into plasmids and single-stranded phages. Methods Enzymol. 1983;101:78–89. doi: 10.1016/0076-6879(83)01006-x. [DOI] [PubMed] [Google Scholar]
- Engeser H., Hübner K., Straub J., Lynen F. Identity of malonyl and palmitoyl transferase of fatty acid synthetase from yeast. 2. A comparison of active-site peptides. Eur J Biochem. 1979 Nov;101(2):413–422. doi: 10.1111/j.1432-1033.1979.tb19734.x. [DOI] [PubMed] [Google Scholar]
- Ferrari E., Henner D. J., Perego M., Hoch J. A. Transcription of Bacillus subtilis subtilisin and expression of subtilisin in sporulation mutants. J Bacteriol. 1988 Jan;170(1):289–295. doi: 10.1128/jb.170.1.289-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers W., Kleinkauf H., Lipmann F. The activation of amino acids for biosynthesis of gramicidin S. Proc Natl Acad Sci U S A. 1968 May;60(1):269–276. doi: 10.1073/pnas.60.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore M. S., Cruz-Rodz A. L., Leimeister-Wächter M., Kreft J., Goebel W. A Bacillus cereus cytolytic determinant, cereolysin AB, which comprises the phospholipase C and sphingomyelinase genes: nucleotide sequence and genetic linkage. J Bacteriol. 1989 Feb;171(2):744–753. doi: 10.1128/jb.171.2.744-753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán P., Westpheling J., Youngman P. Characterization of the promoter region of the Bacillus subtilis spoIIE operon. J Bacteriol. 1988 Apr;170(4):1598–1609. doi: 10.1128/jb.170.4.1598-1609.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo M. M., Losick R. Regulation of a promoter that is utilized by minor forms of RNA polymerase holoenzyme in Bacillus subtilis. J Mol Biol. 1986 Oct 20;191(4):615–624. doi: 10.1016/0022-2836(86)90449-3. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Hara N., Iwabuchi T. Molecular cloning and expression in Escherichia coli of the Bacillus licheniformis bacitracin synthetase 2 gene. J Bacteriol. 1989 Mar;171(3):1705–1711. doi: 10.1128/jb.171.3.1705-1711.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. C., Moran C. P., Jr, Losick R. Two RNA polymerase sigma factors from Bacillus subtilis discriminate between overlapping promoters for a developmentally regulated gene. Nature. 1983 Apr 28;302(5911):800–804. doi: 10.1038/302800a0. [DOI] [PubMed] [Google Scholar]
- Katz E., Demain A. L. The peptide antibiotics of Bacillus: chemistry, biogenesis, and possible functions. Bacteriol Rev. 1977 Jun;41(2):449–474. doi: 10.1128/br.41.2.449-474.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney T. J., Moran C. P., Jr Organization and regulation of an operon that encodes a sporulation-essential sigma factor in Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):3329–3339. doi: 10.1128/jb.169.7.3329-3339.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Marahiel M. A. Organization of the biosynthesis genes for the peptide antibiotic gramicidin S. J Bacteriol. 1988 Oct;170(10):4669–4674. doi: 10.1128/jb.170.10.4669-4674.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi K. Biosynthesis of small peptides. Annu Rev Biochem. 1974;43(0):445–459. doi: 10.1146/annurev.bi.43.070174.002305. [DOI] [PubMed] [Google Scholar]
- Laland S. G., Zimmer T. L. The protein thiotemplate mechanism of synthesis for the peptide antibiotics produced by Bacillus brevis. Essays Biochem. 1973;9:31–57. [PubMed] [Google Scholar]
- Lee S. G., Lipmann F. Tyrocidine synthetase system. Methods Enzymol. 1975;43:585–602. doi: 10.1016/0076-6879(75)43121-4. [DOI] [PubMed] [Google Scholar]
- Libertini L. J., Smith S. Purification and properties of a thioesterase from lactating rat mammary gland which modifies the product specificity of fatty acid synthetase. J Biol Chem. 1978 Mar 10;253(5):1393–1401. [PubMed] [Google Scholar]
- Lipmann F. Bacterial production of antibiotic polypeptides by thiol-linked synthesis on protein templates. Adv Microb Physiol. 1980;21:227–266. doi: 10.1016/s0065-2911(08)60357-4. [DOI] [PubMed] [Google Scholar]
- Losick R., Youngman P., Piggot P. J. Genetics of endospore formation in Bacillus subtilis. Annu Rev Genet. 1986;20:625–669. doi: 10.1146/annurev.ge.20.120186.003205. [DOI] [PubMed] [Google Scholar]
- Marahiel M. A., Danders W., Krause M., Kleinkauf H. Biological role of gramicidin S in spore functions. Studies on gramicidin-S-negative mutants of Bacillus brevis ATCC9999. Eur J Biochem. 1979 Aug 15;99(1):49–55. doi: 10.1111/j.1432-1033.1979.tb13229.x. [DOI] [PubMed] [Google Scholar]
- Marahiel M. A., Krause M., Skarpeid H. J. Cloning of the tyrocidine synthetase 1 gene from Bacillus brevis and its expression in Escherichia coli. Mol Gen Genet. 1985;201(2):231–236. doi: 10.1007/BF00425664. [DOI] [PubMed] [Google Scholar]
- Marahiel M. A., Zuber P., Czekay G., Losick R. Identification of the promoter for a peptide antibiotic biosynthesis gene from Bacillus brevis and its regulation in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2215–2222. doi: 10.1128/jb.169.5.2215-2222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mittenhuber G., Weckermann R., Marahiel M. A. Gene cluster containing the genes for tyrocidine synthetases 1 and 2 from Bacillus brevis: evidence for an operon. J Bacteriol. 1989 Sep;171(9):4881–4887. doi: 10.1128/jb.171.9.4881-4887.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Murray M. G. Use of sodium trichloroacetate and mung bean nuclease to increase sensitivity and precision during transcript mapping. Anal Biochem. 1986 Oct;158(1):165–170. doi: 10.1016/0003-2697(86)90605-6. [DOI] [PubMed] [Google Scholar]
- Naggert J., Witkowski A., Mikkelsen J., Smith S. Molecular cloning and sequencing of a cDNA encoding the thioesterase domain of the rat fatty acid synthetase. J Biol Chem. 1988 Jan 25;263(3):1146–1150. [PubMed] [Google Scholar]
- Penn M. D., Thireos G., Greer H. Temporal analysis of general control of amino acid biosynthesis in Saccharomyces cerevisiae: role of positive regulatory genes in initiation and maintenance of mRNA derepression. Mol Cell Biol. 1984 Mar;4(3):520–528. doi: 10.1128/mcb.4.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulose A. J., Rogers L., Cheesbrough T. M., Kolattukudy P. E. Cloning and sequencing of the cDNA for S-acyl fatty acid synthase thioesterase from the uropygial gland of mallard duck. J Biol Chem. 1985 Dec 15;260(29):15953–15958. [PubMed] [Google Scholar]
- Randhawa Z. I., Smith S. Complete amino acid sequence of the medium-chain S-acyl fatty acid synthetase thio ester hydrolase from rat mammary gland. Biochemistry. 1987 Mar 10;26(5):1365–1373. doi: 10.1021/bi00379a024. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Roberts L. M., Höltke H. J., Takabayashi K., Höllerer E., Hoffmann B., Müller G., Köttig H., Schweizer E. The pentafunctional FAS1 gene of yeast: its nucleotide sequence and order of the catalytic domains. Mol Gen Genet. 1986 Jun;203(3):479–486. doi: 10.1007/BF00422073. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Takabayashi K., Laux T., Beck K. F., Schreglmann R. Rat mammary gland fatty acid synthase: localization of the constituent domains and two functional polyadenylation/termination signals in the cDNA. Nucleic Acids Res. 1989 Jan 25;17(2):567–586. doi: 10.1093/nar/17.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. Medium-chain fatty acyl-s-4'-phosphopantetheine-fatty acid synthase thioester hydrolase from lactating mammary gland of rat. Methods Enzymol. 1981;71(Pt 100):188–200. doi: 10.1016/0076-6879(81)71027-9. [DOI] [PubMed] [Google Scholar]
- Vater J., Kleinkauf H. Gramicidin S-synthetase. A further characterization of phenylalanine racemase, the light enzyme of gramicidin s-synthetase. Biochim Biophys Acta. 1976 May 13;429(3):1062–1072. doi: 10.1016/0005-2744(76)90351-x. [DOI] [PubMed] [Google Scholar]
- Weckermann R., Fürbass R., Marahiel M. A. Complete nucleotide sequence of the tycA gene coding the tyrocidine synthetase 1 from Bacillus brevis. Nucleic Acids Res. 1988 Dec 23;16(24):11841–11841. doi: 10.1093/nar/16.24.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. L., Price C. W., Goldfarb D. S., Doi R. H. The subtilisin E gene of Bacillus subtilis is transcribed from a sigma 37 promoter in vivo. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1184–1188. doi: 10.1073/pnas.81.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]