Abstract
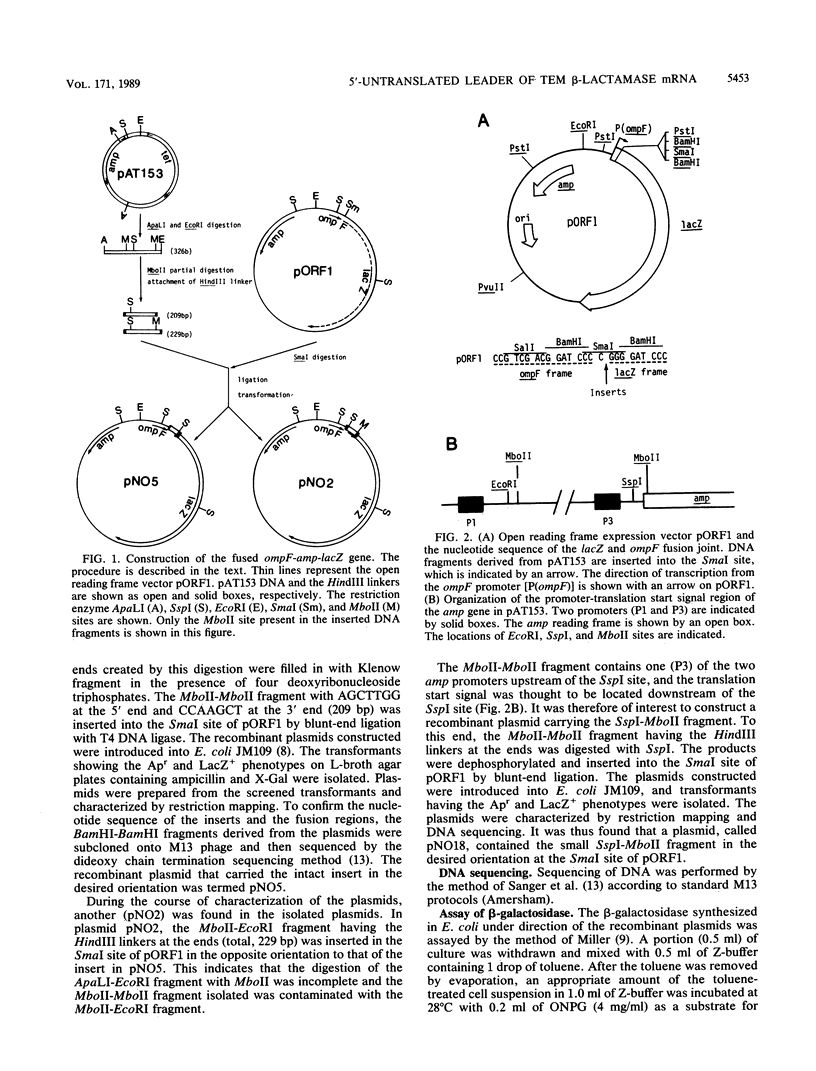
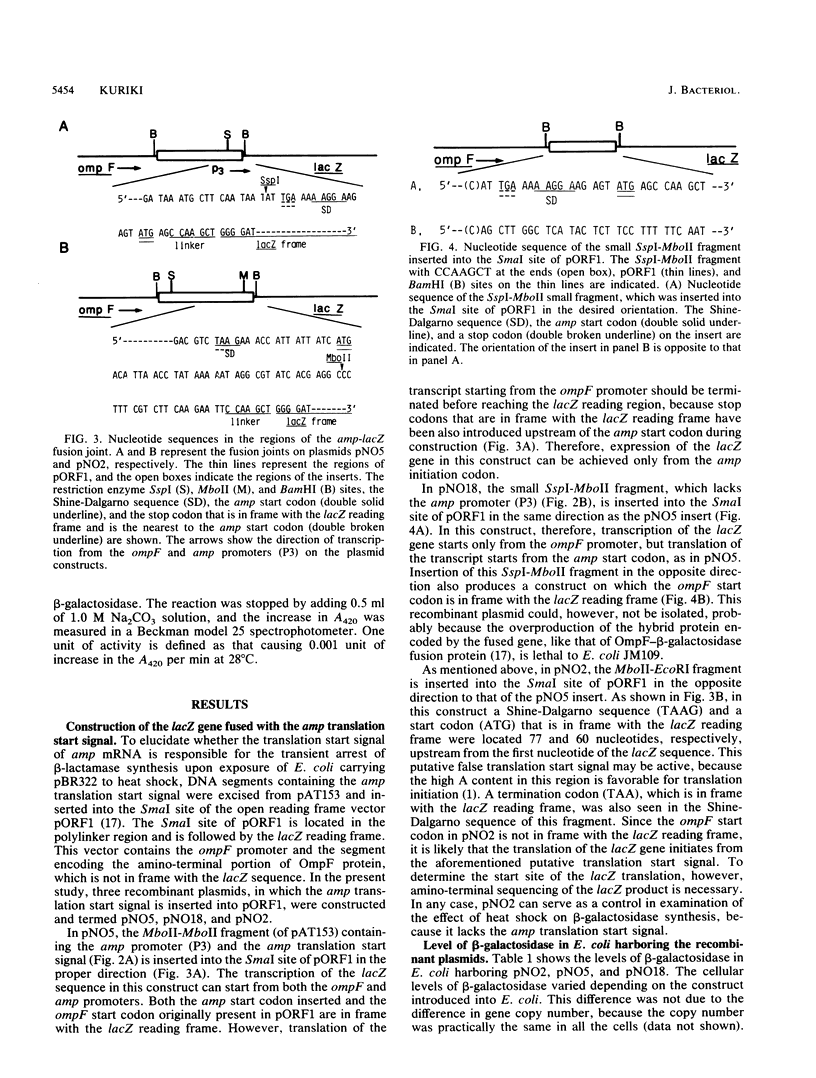
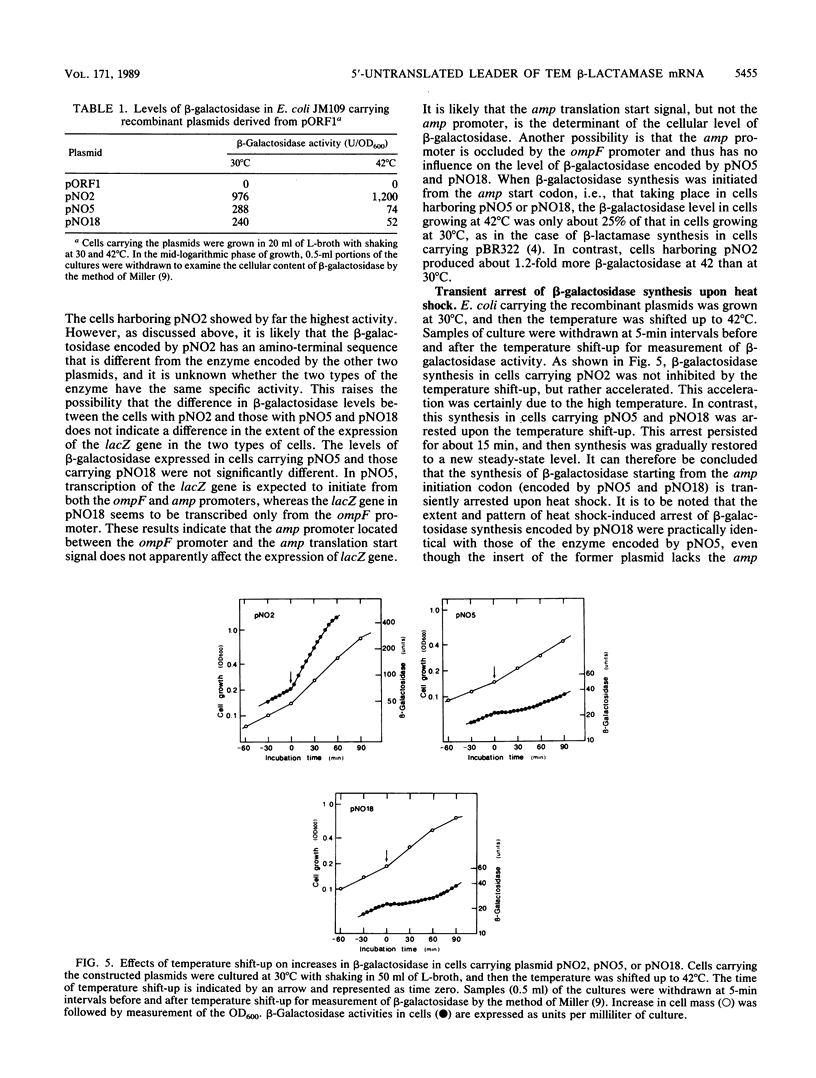
pBR322 contains the amp gene encoding beta-lactamase. When Escherichia coli carrying this plasmid is exposed to heat shock, beta-lactamase synthesis is repressed transiently at the translational level. To identify the DNA element responsible for this translational repression, DNA segments containing the translation start region of the amp gene were excised from pAT153 and fused in frame with the lacZ reading frame in the open reading frame vector pORF1. These constructs were introduced into E. coli, and the effect of heat shock of the cells on the synthesis of beta-galactosidase starting from the amp start codon was examined. As is the case for pBR322-encoded synthesis of beta-lactamase, the synthesis of beta-galactosidase encoded by the fused genes also ceased transiently upon heat shock. It is concluded that the heat shock-induced repression of the amp gene occurs at the initiation step of translation. As far as the present study is concerned, the minimum DNA segment responsible for the repression is AT TGA AAA AGG AAG AGT ATG AG, which includes the Shine-Dalgarno sequence (AAGGA) and the initiation codon (ATG).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dreyfus M. What constitutes the signal for the initiation of protein synthesis on Escherichia coli mRNAs? J Mol Biol. 1988 Nov 5;204(1):79–94. doi: 10.1016/0022-2836(88)90601-8. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriki Y. Heat shock inactivates a supernatant factor(s) specifically required for efficient expression of the amp gene in Escherichia coli. FEBS Lett. 1987 Oct 19;223(1):127–130. doi: 10.1016/0014-5793(87)80522-7. [DOI] [PubMed] [Google Scholar]
- Kuriki Y. Requirement of a heat-labile factor(s) for in vitro expression of the amp gene of pBR322. J Bacteriol. 1987 Dec;169(12):5856–5858. doi: 10.1128/jb.169.12.5856-5858.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriki Y. Response to temperature shifts of expression of the amp gene on pBR322 in Escherichia coli K-12. J Bacteriol. 1987 May;169(5):2294–2297. doi: 10.1128/jb.169.5.2294-2297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriki Y. Stimulation in vitro of expression of the amp gene of pBR322 by soluble protein fractions isolated from E. coli. Biochem Int. 1986 Apr;12(4):593–602. [PubMed] [Google Scholar]
- Lemaux P. G., Herendeen S. L., Bloch P. L., Neidhardt F. C. Transient rates of synthesis of individual polypeptides in E. coli following temperature shifts. Cell. 1978 Mar;13(3):427–434. doi: 10.1016/0092-8674(78)90317-3. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., ap Rhys C., Berman M. L., Hampar B., Jackson D., Silhavy T. J., Weisemann J., Zweig M. Open reading frame expression vectors: a general method for antigen production in Escherichia coli using protein fusions to beta-galactosidase. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4432–4436. doi: 10.1073/pnas.80.14.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]


