Abstract
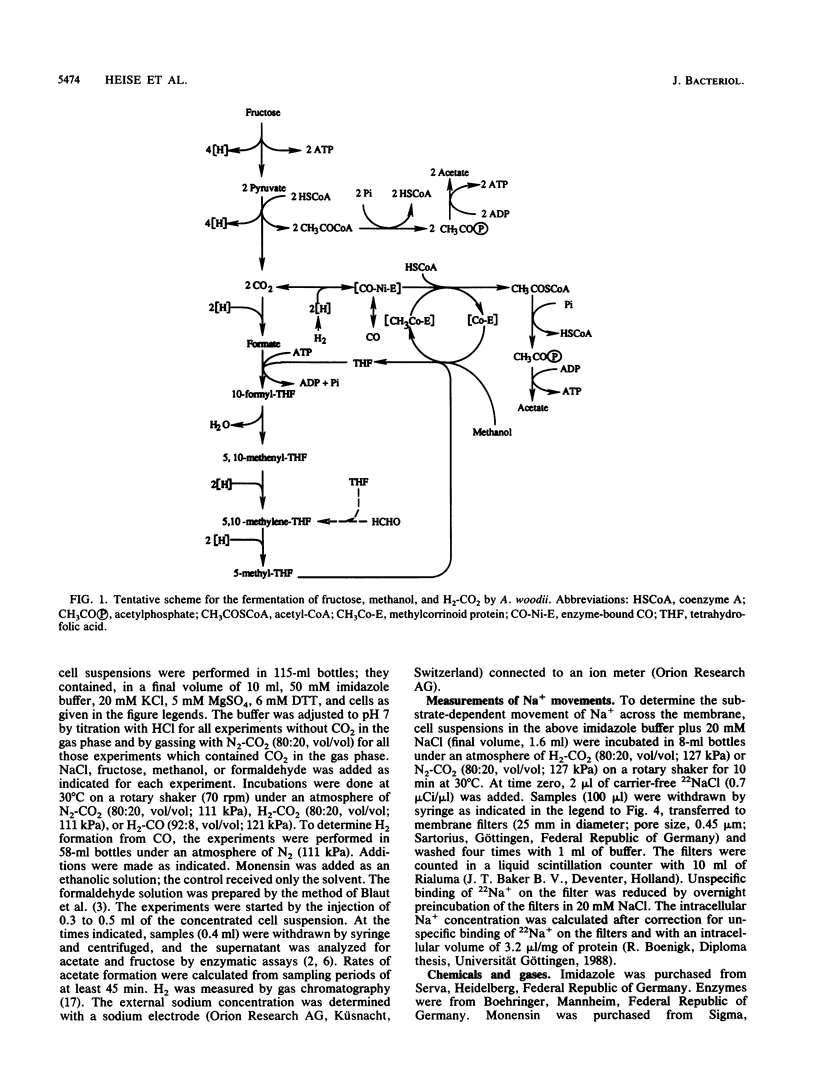
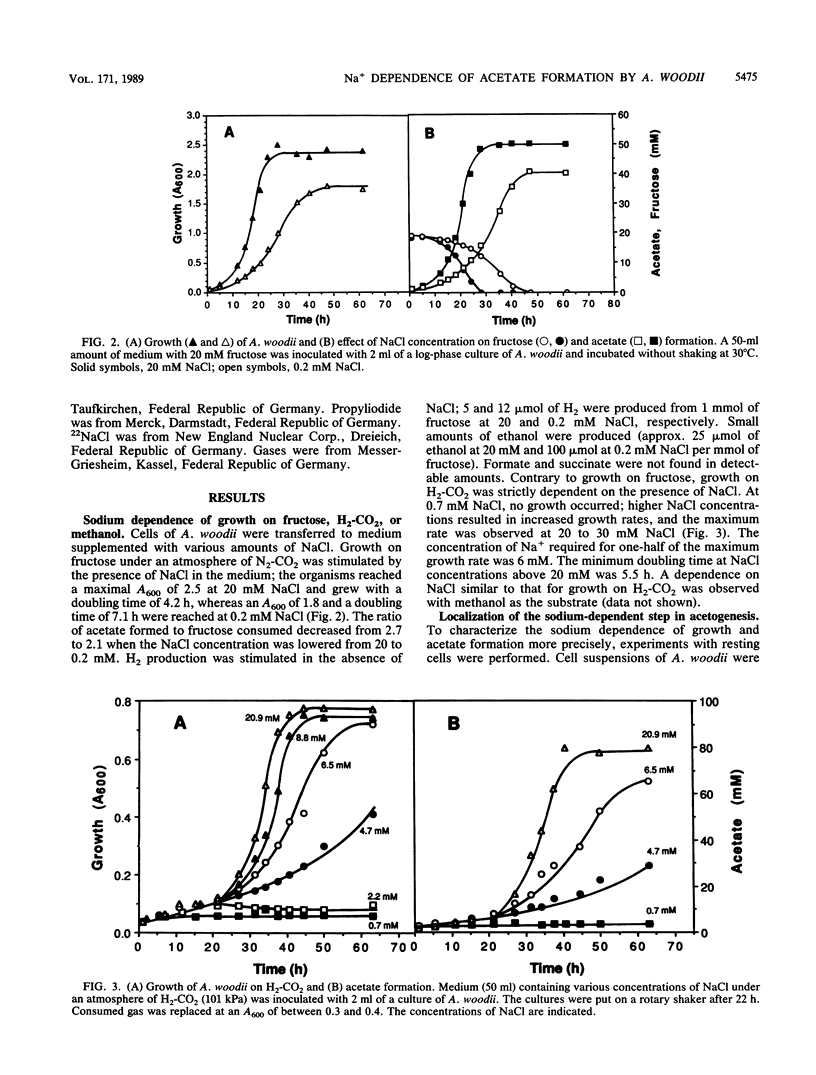
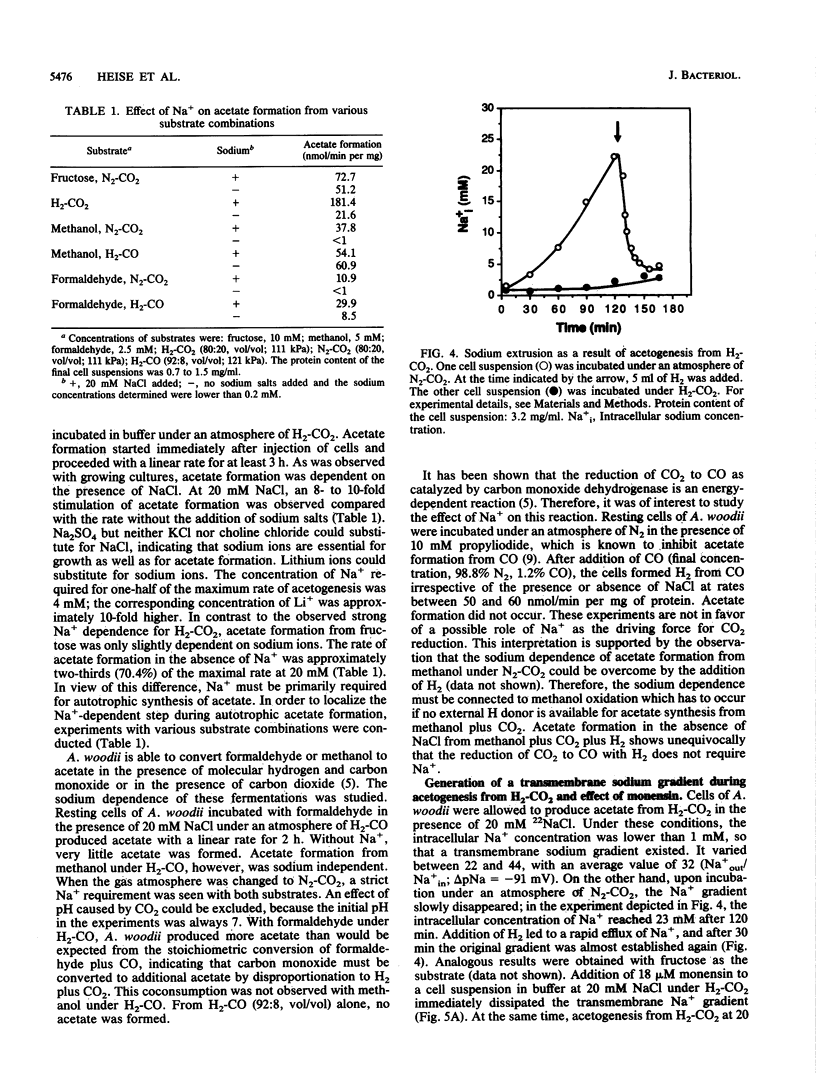
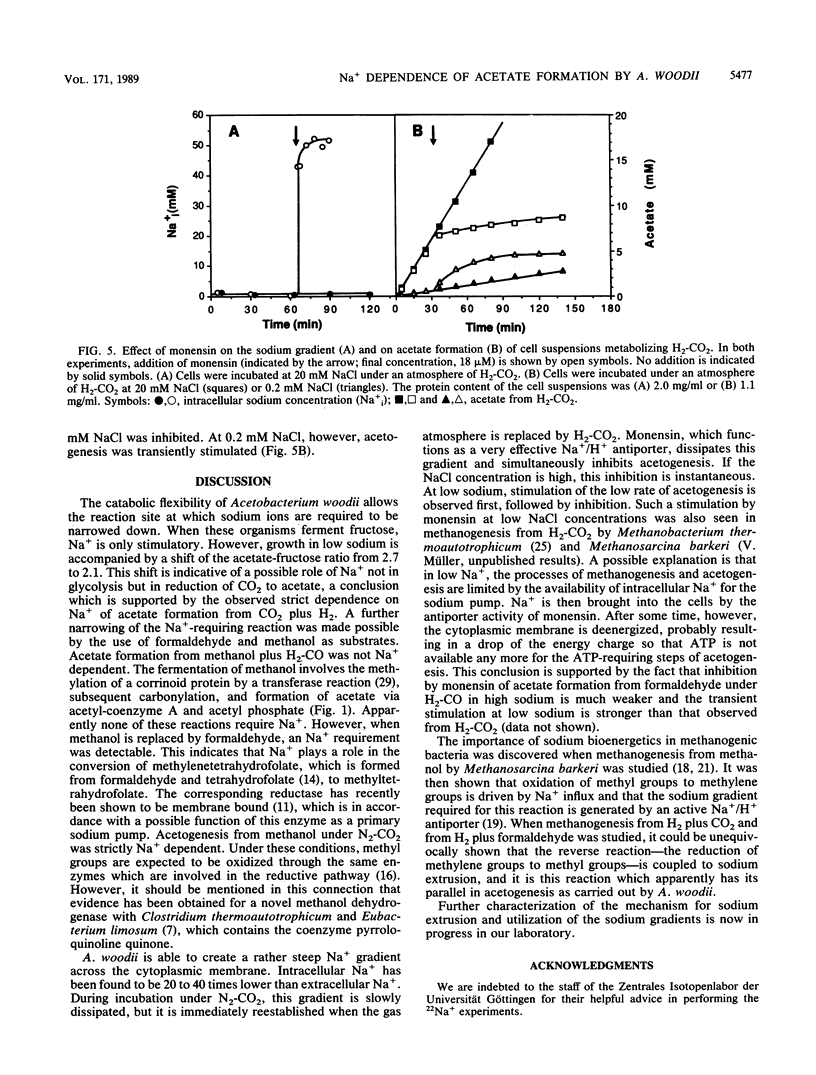
Growth of Acetobacterium woodii on fructose was stimulated by Na+; this stimulation was paralleled by a shift of the acetate-fructose ratio from 2.1 to 2.7. Growth on H2-CO2 or on methanol plus CO2 was strictly dependent on the presence of sodium ions in the medium. Acetate formation from formaldehyde plus H2-CO by resting cells required Na+, but from methanol plus H2-CO did not. This is analogous to H2-CO2 reduction to methane by Methanosarcina barkeri, which involves a sodium pump (V. Müller, C. Winner, and G. Gottschalk, Eur. J. Biochem. 178:519-525, 1988). This suggests that the reduction of methylenetetrahydrofolate to methyltetrahydrofolate is the Na+-requiring reaction. A sodium gradient (Na+ out/Na+ in = 32, delta pNa = -91 mV) was built up when resting cells of A. woodii were incubated under H2-CO2. Acetogenesis was inhibited when the delta pNa was dissipated by monensin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaut M., Müller V., Fiebig K., Gottschalk G. Sodium ions and an energized membrane required by Methanosarcina barkeri for the oxidation of methanol to the level of formaldehyde. J Bacteriol. 1985 Oct;164(1):95–101. doi: 10.1128/jb.164.1.95-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Dorn M., Andreesen J. R., Gottschalk G. Fermentation of fumarate and L-malate by Clostridium formicoaceticum. J Bacteriol. 1978 Jan;133(1):26–32. doi: 10.1128/jb.133.1.26-32.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghambeer R. K., Wood H. G., Schulman M., Ljungdahl L. Total synthesis of acetate from CO2. 3. Inhibition by alkylhalides of the synthesis from CO2, methyltetrahydrofolate, and methyl-B12 by Clostridium thermoaceticum. Arch Biochem Biophys. 1971 Apr;143(2):471–484. doi: 10.1016/0003-9861(71)90232-3. [DOI] [PubMed] [Google Scholar]
- Hugenholtz J., Ivey D. M., Ljungdahl L. G. Carbon monoxide-driven electron transport in Clostridium thermoautotrophicum membranes. J Bacteriol. 1987 Dec;169(12):5845–5847. doi: 10.1128/jb.169.12.5845-5847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen R. G., Jencks W. P. The mechanism of the condensation of formaldehyde with tetrahydrofolic acid. J Biol Chem. 1966 Dec 25;241(24):5851–5863. [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Müller V., Blaut M., Gottschalk G. Generation of a transmembrane gradient of Na+ in Methanosarcina barkeri. Eur J Biochem. 1987 Jan 15;162(2):461–466. doi: 10.1111/j.1432-1033.1987.tb10624.x. [DOI] [PubMed] [Google Scholar]
- Müller V., Blaut M., Gottschalk G. The transmembrane electrochemical gradient of Na+ as driving force for methanol oxidation in Methanosarcina barkeri. Eur J Biochem. 1988 Mar 15;172(3):601–606. doi: 10.1111/j.1432-1033.1988.tb13931.x. [DOI] [PubMed] [Google Scholar]
- Müller V., Blaut M., Gottschalk G. Utilization of Methanol plus Hydrogen by Methanosarcina barkeri for Methanogenesis and Growth. Appl Environ Microbiol. 1986 Aug;52(2):269–274. doi: 10.1128/aem.52.2.269-274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller V., Winner C., Gottschalk G. Electron-transport-driven sodium extrusion during methanogenesis from formaldehyde and molecular hydrogen by Methanosarcina barkeri. Eur J Biochem. 1988 Dec 15;178(2):519–525. doi: 10.1111/j.1432-1033.1988.tb14478.x. [DOI] [PubMed] [Google Scholar]
- SCHMIDT K., LIAAENJENSEN S., SCHLEGEL H. G. DIE CAROTINOIDE DER THIORHODACEAE. I. OKENON ALS HAUPTEAROTINOID VON CHROMATIUM OKENII PERTY. Arch Mikrobiol. 1963 Aug 1;46:117–126. [PubMed] [Google Scholar]
- Terracciano J. S., Schreurs W. J., Kashket E. R. Membrane H Conductance of Clostridium thermoaceticum and Clostridium acetobutylicum: Evidence for Electrogenic Na/H Antiport in Clostridium thermoaceticum. Appl Environ Microbiol. 1987 Apr;53(4):782–786. doi: 10.1128/aem.53.4.782-786.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



