Abstract
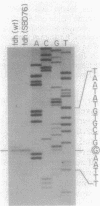
The tdh operon of Escherichia coli consists of two genes whose products catalyze sequential steps in the formation of glycine and acetyl coenzyme A from threonine. The operation of the tdh pathway can potentially confer at least two capabilities on the cell: the first is to provide a biosynthetic source of glycine, serine, or both that is an alternative to the conventional (triose phosphate) pathway; the second is to enable cells to utilize threonine as the sole carbon source. The latter capability is referred to as the Tuc+ phenotype. In wild-type E. coli, the tdh operon is expressed at levels that are too low to bestow the Tuc+ phenotype, even in leucine-supplemented media, where the operon is induced eightfold. In eight Tuc+ mutants, the expression of the tdh operon was elevated 100-fold relative to the uninduced wild-type operon. The physical state of the DNA at the tdh locus in these Tuc+ strains was analyzed by Southern blotting and by DNA sequencing. In eight independent isolates the mobile genetic element IS3 was found to lie within the tdh promoter region in identical orientations. In six cases that were examined by DNA sequencing, IS3 occupied identical sites between the -10 and -35 elements of the tdh promoter. The transcription start points for the wild-type tdh promoter and one IS3-activated tdh promoter were identical. In effect, the repeatedly observed transposition event juxtaposed an IS3-borne -35 region and the tdh-specific -10 region, generating a hybrid promoter whose utilization led to elevated, constitutive expression of the tdh operon. This is the first case of promoter activation by IS3 where the site of transcription initiation is unaltered.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A., Bidwell K., Musso R. Internal rearrangements of IS2 in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):141–151. doi: 10.1101/sqb.1981.045.01.023. [DOI] [PubMed] [Google Scholar]
- Aronson B. D., Ravnikar P. D., Somerville R. L. Nucleotide sequence of the 2-amino-3-ketobutyrate coenzyme A ligase (kbl) gene of E. coli. Nucleic Acids Res. 1988 Apr 25;16(8):3586–3586. doi: 10.1093/nar/16.8.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson B. D., Somerville R. L., Epperly B. R., Dekker E. E. The primary structure of Escherichia coli L-threonine dehydrogenase. J Biol Chem. 1989 Mar 25;264(9):5226–5232. [PubMed] [Google Scholar]
- Berman M. L., Jackson D. E. Selection of lac gene fusions in vivo: ompR-lacZ fusions that define a functional domain of the ompR gene product. J Bacteriol. 1984 Aug;159(2):750–756. doi: 10.1128/jb.159.2.750-756.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefoy V., Fons M., Ratouchniak J., Pascal M. C., Chippaux M. Aerobic expression of the nar operon of Escherichia coli in a fnr mutant. Mol Microbiol. 1988 May;2(3):419–425. doi: 10.1111/j.1365-2958.1988.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Boylan S. A., Dekker E. E. Growth, enzyme levels, and some metabolic properties of an Escherichia coli mutant grown on L-threonine as the sole carbon source. J Bacteriol. 1983 Oct;156(1):273–280. doi: 10.1128/jb.156.1.273-280.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan S. A., Dekker E. E. L-threonine dehydrogenase. Purification and properties of the homogeneous enzyme from Escherichia coli K-12. J Biol Chem. 1981 Feb 25;256(4):1809–1815. [PubMed] [Google Scholar]
- Chan T. T., Newman E. B. Threonine as a carbon source for Escherichia coli. J Bacteriol. 1981 Mar;145(3):1150–1153. doi: 10.1128/jb.145.3.1150-1153.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier D., Piette J., Glansdorff N. IS3 can function as a mobile promoter in E. coli. Nucleic Acids Res. 1982 Oct 11;10(19):5935–5948. doi: 10.1093/nar/10.19.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Miller R. H. A rapid and convenient method for the preparation and storage of competent bacterial cells. Nucleic Acids Res. 1988 Apr 25;16(8):3580–3580. doi: 10.1093/nar/16.8.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P. A., Dekker E. E. L-threonine dehydrogenase from Escherichia coli K-12: thiol-dependent activation by Mn2+. Biochemistry. 1986 Apr 22;25(8):1870–1876. doi: 10.1021/bi00356a005. [DOI] [PubMed] [Google Scholar]
- Curtis S. E. Genes encoding the beta and epsilon subunits of the proton-translocating ATPase from Anabaena sp. strain PCC 7120. J Bacteriol. 1987 Jan;169(1):80–86. doi: 10.1128/jb.169.1.80-86.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple B., Caspers P., Arber W. Nucleotide sequence of the prokaryotic mobile genetic element IS30. EMBO J. 1984 Sep;3(9):2145–2149. doi: 10.1002/j.1460-2075.1984.tb02104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P., Goss T. J., Omnaas J. R., Patil R. V. Covalent structure of biodegradative threonine dehydratase of Escherichia coli: homology with other dehydratases. Proc Natl Acad Sci U S A. 1987 Jan;84(2):393–397. doi: 10.1073/pnas.84.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deonier R. C., Hadley R. G., Hu M. Enumeration and identification of IS3 elements in Escherichia coli strains. J Bacteriol. 1979 Mar;137(3):1421–1424. doi: 10.1128/jb.137.3.1421-1424.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J., Newman E. B. Derivation of glycine from threonine in Escherichia coli K-12 mutants. J Bacteriol. 1975 Jun;122(3):810–817. doi: 10.1128/jb.122.3.810-817.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glansdorff N., Charlier D., Zafarullah M. Activation of gene expression by IS2 and IS3. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):153–156. doi: 10.1101/sqb.1981.045.01.024. [DOI] [PubMed] [Google Scholar]
- Hall B. G., Parker L. L., Betts P. W., DuBose R. F., Sawyer S. A., Hartl D. L. IS103, a new insertion element in Escherichia coli: characterization and distribution in natural populations. Genetics. 1989 Mar;121(3):423–431. doi: 10.1093/genetics/121.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton D. M., Musso R. E. Transcription initiation sites within an IS2 insertion in a Gal-constitutive mutant of Escherichia coli. Nucleic Acids Res. 1982 Aug 25;10(16):5015–5031. doi: 10.1093/nar/10.16.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Loeb L. A. DNA sequences of random origin as probes of Escherichia coli promoter architecture. J Biol Chem. 1988 Oct 15;263(29):14724–14731. [PubMed] [Google Scholar]
- Horwitz M. S., Loeb L. A. Promoters selected from random DNA sequences. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7405–7409. doi: 10.1073/pnas.83.19.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M., Deonier R. C. Comparison of IS1, IS2 and IS3 copy number in Escherichia coli strains K-12, B and C. Gene. 1981 Dec;16(1-3):161–170. doi: 10.1016/0378-1119(81)90072-x. [DOI] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaurin B., Normark S. Insertion of IS2 creates a novel ampC promoter in Escherichia coli. Cell. 1983 Mar;32(3):809–816. doi: 10.1016/0092-8674(83)90067-3. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Meyer B. J., Maurer R., Ptashne M. Gene regulation at the right operator (OR) of bacteriophage lambda. II. OR1, OR2, and OR3: their roles in mediating the effects of repressor and cro. J Mol Biol. 1980 May 15;139(2):163–194. doi: 10.1016/0022-2836(80)90303-4. [DOI] [PubMed] [Google Scholar]
- Mollet B., Iida S., Shepherd J., Arber W. Nucleotide sequence of IS26, a new prokaryotic mobile genetic element. Nucleic Acids Res. 1983 Sep 24;11(18):6319–6330. doi: 10.1093/nar/11.18.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. B., Kapoor V., Potter R. Role of L-threonine dehydrogenase in the catabolism of threonine and synthesis of glycine by Escherichia coli. J Bacteriol. 1976 Jun;126(3):1245–1249. doi: 10.1128/jb.126.3.1245-1249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter R., Kapoor V., Newman E. B. Role of threonine dehydrogenase in Escherichia coli threonine degradation. J Bacteriol. 1977 Nov;132(2):385–391. doi: 10.1128/jb.132.2.385-391.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Teter B., Chandler M., Galas D. J. Functional promoters created by the insertion of transposable element IS1. J Mol Biol. 1986 Oct 5;191(3):383–393. doi: 10.1016/0022-2836(86)90134-8. [DOI] [PubMed] [Google Scholar]
- Ravnikar P. D., Somerville R. L. Genetic characterization of a highly efficient alternate pathway of serine biosynthesis in Escherichia coli. J Bacteriol. 1987 Jun;169(6):2611–2617. doi: 10.1128/jb.169.6.2611-2617.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnikar P. D., Somerville R. L. Localization of the structural gene for threonine dehydrogenase in Escherichia coli. J Bacteriol. 1986 Oct;168(1):434–436. doi: 10.1128/jb.168.1.434-436.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnikar P. D., Somerville R. L. Structural and functional analysis of a cloned segment of Escherichia coli DNA that specifies proteins of a C4 pathway of serine biosynthesis. J Bacteriol. 1987 Oct;169(10):4716–4721. doi: 10.1128/jb.169.10.4716-4721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimmann C., Moore R., Little S., Savioz A., Willetts N. S., Haas D. Genetic structure, function and regulation of the transposable element IS21. Mol Gen Genet. 1989 Feb;215(3):416–424. doi: 10.1007/BF00427038. [DOI] [PubMed] [Google Scholar]
- Reynolds A. E., Mahadevan S., LeGrice S. F., Wright A. Enhancement of bacterial gene expression by insertion elements or by mutation in a CAP-cAMP binding site. J Mol Biol. 1986 Sep 5;191(1):85–95. doi: 10.1016/0022-2836(86)90424-9. [DOI] [PubMed] [Google Scholar]
- Salser W., Gesteland R. F., Bolle A. In vitro synthesis of bacteriophage lysozyme. Nature. 1967 Aug 5;215(5101):588–591. doi: 10.1038/215588a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E., Herberger C., Rak B. Second-element turn-on of gene expression in an IS1 insertion mutant. Mol Gen Genet. 1988 Feb;211(2):282–289. doi: 10.1007/BF00330605. [DOI] [PubMed] [Google Scholar]
- Schweizer H., Boos W. Transfer of the delta (argF-lac)U169 mutation between Escherichia coli strains by selection for a closely linked Tn10 insertion. Mol Gen Genet. 1983;192(1-2):293–294. doi: 10.1007/BF00327683. [DOI] [PubMed] [Google Scholar]
- Sommer H., Cullum J., Saedler H. Integration of IS3 into IS2 generates a short sequence duplication. Mol Gen Genet. 1979;177(1):85–89. doi: 10.1007/BF00267256. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Timmerman K. P., Tu C. P. Complete sequence of IS3. Nucleic Acids Res. 1985 Mar 25;13(6):2127–2139. doi: 10.1093/nar/13.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wang A., Roth J. R. Activation of silent genes by transposons Tn5 and Tn10. Genetics. 1988 Dec;120(4):875–885. doi: 10.1093/genetics/120.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertman K. F., Little J. W., Mount D. W. Rapid mutational analysis of regulatory loci in Escherichia coli K-12 using bacteriophage M13. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3801–3805. doi: 10.1073/pnas.81.12.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa B., Meyer F., Reiser J., Weissmann C. Unusual splice sites revealed by mutagenic inactivation of an authentic splice site of the rabbit beta-globin gene. Nature. 1983 Jan 6;301(5895):38–43. doi: 10.1038/301038a0. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y., Ohtsubo H., Ohtsubo E. Repressor gene finO in plasmids R100 and F: constitutive transfer of plasmid F is caused by insertion of IS3 into F finO. J Bacteriol. 1987 Feb;169(2):619–623. doi: 10.1128/jb.169.2.619-623.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. M., Reznikoff W. S. Deletion analysis of the CAP-cAMP binding site of the Escherichia coli lactose promoter. Nucleic Acids Res. 1984 Jul 11;12(13):5449–5464. doi: 10.1093/nar/12.13.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafarullah M., Charlier D., Glansdorff N. Insertion of IS3 can "turn-on" a silent gene in Escherichia coli. J Bacteriol. 1981 Apr;146(1):415–417. doi: 10.1128/jb.146.1.415-417.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]