Abstract
dnaQ (mutD) encodes the editing exonuclease subunit (epsilon) of DNA polymerase III. Previously described mutations in dnaQ include dominant and recessive mutator alleles as well as leaky temperature-sensitive alleles. We describe the properties of strains bearing null mutations (deletion-substitution alleles) of this gene. Null mutants exhibited a growth defect as well as elevated spontaneous mutation. As a consequence of the poor growth of dnaQ mutants and their high mutation rate, these strains were replaced within single colonies by derivatives carrying an extragenic suppressor mutation that compensated the growth defect but apparently not the mutator effect. Sixteen independently derived suppressors mapped in the vicinity of dnaE, the gene for the polymerization subunit (alpha) of DNA polymerase III, and one suppressor that was sequenced encoded an altered alpha polypeptide. Partially purified DNA polymerase III containing this altered alpha subunit was active in polymerization assays. In addition to their dependence on a suppressor mutation affecting alpha, dnaQ mutants strictly required DNA polymerase I for viability. We argue from these data that in the absence of epsilon, DNA replication falters unless secondary mechanisms, including genetically coded alteration in the intrinsic replication capacity of alpha and increased use of DNA polymerase I, come into play. Thus, epsilon plays a role in DNA replication distinct from its known role in controlling spontaneous mutation frequency.
Full text
PDF


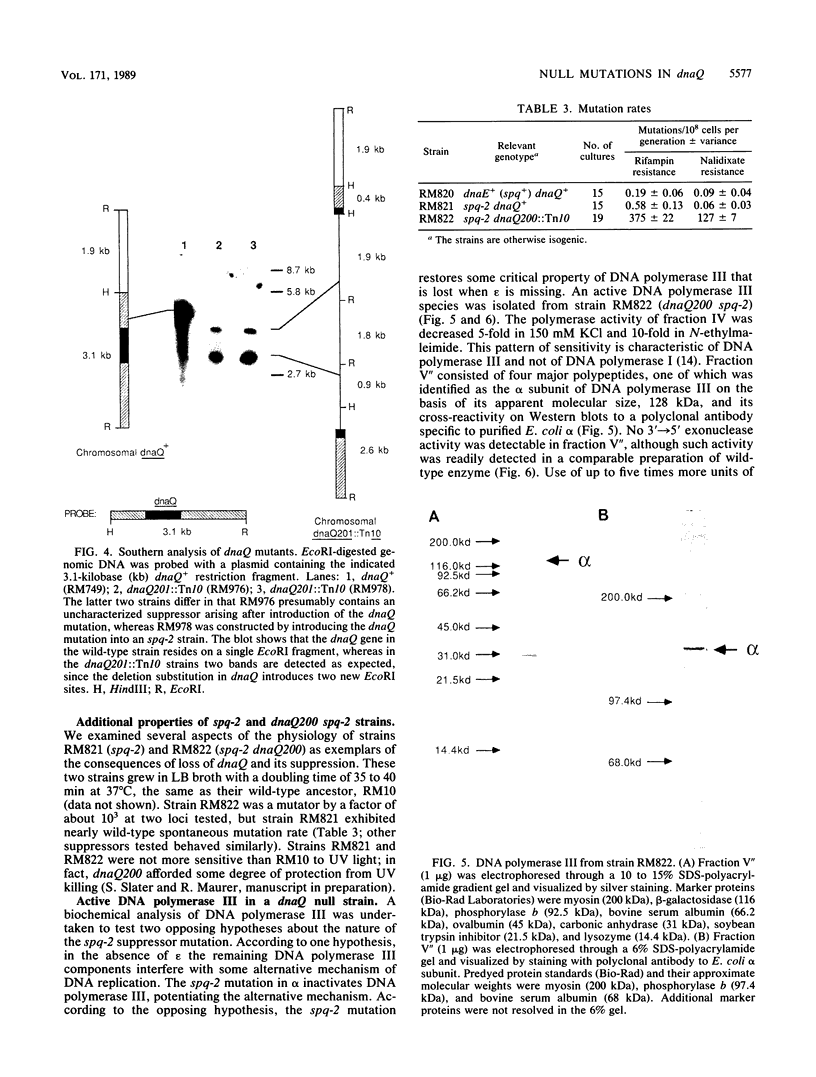
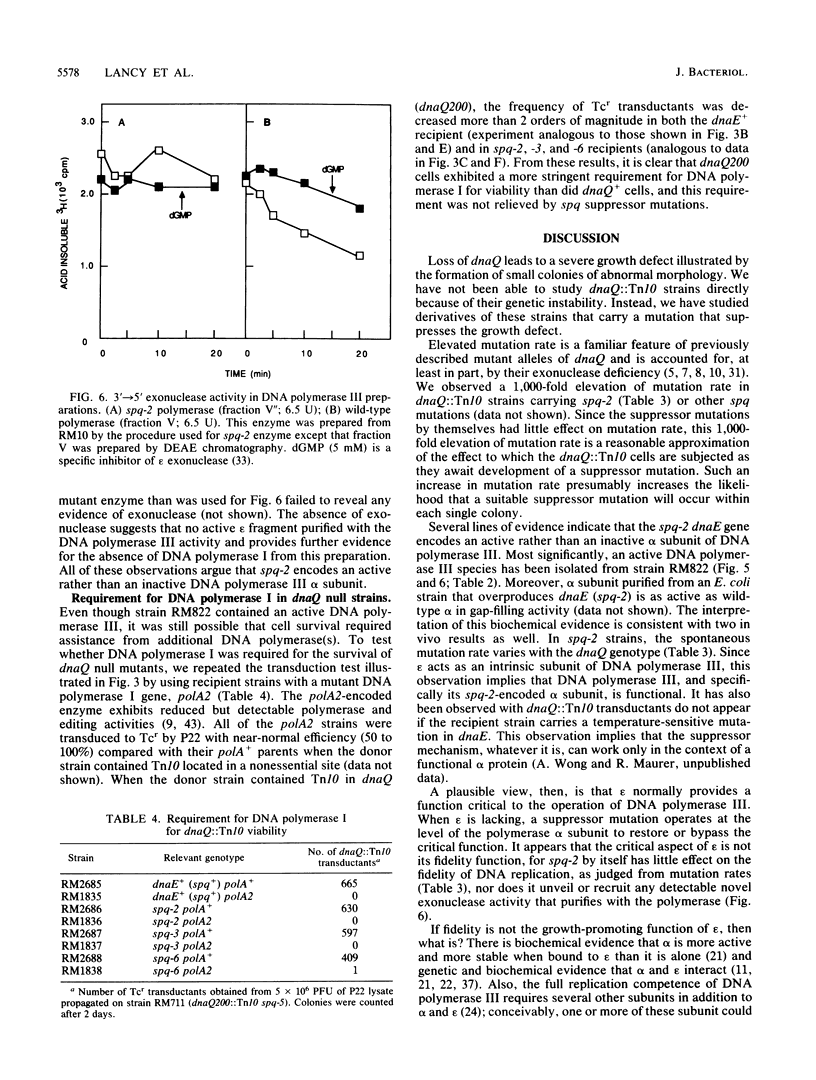


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3' leads to 5' exonuclease activity in deoxyribonucleic acid polymerases. J Biol Chem. 1972 Jan 10;247(1):241–248. [PubMed] [Google Scholar]
- Bryan S. K., Moses R. E. Map location of the pcbA mutation and physiology of the mutant. J Bacteriol. 1984 Apr;158(1):216–221. doi: 10.1128/jb.158.1.216-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E. C., Horner D. L. Dominant mutators in Escherichia coli. Genetics. 1982 Jan;100(1):7–18. doi: 10.1093/genetics/100.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnen G. E., Cox E. C. Conditional mutator gene in Escherichia coli: isolation, mapping, and effector studies. J Bacteriol. 1974 Feb;117(2):477–487. doi: 10.1128/jb.117.2.477-487.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H., Lu C., Burgers P. M. Mutator strains of Escherichia coli, mutD and dnaQ, with defective exonucleolytic editing by DNA polymerase III holoenzyme. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2189–2192. doi: 10.1073/pnas.80.8.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler M. J., Bessman M. J. Characterization of a mutator DNA polymerase I from Salmonella typhimurium. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):929–935. doi: 10.1101/sqb.1979.043.01.102. [DOI] [PubMed] [Google Scholar]
- Horiuchi T., Maki H., Sekiguchi M. A new conditional lethal mutator (dnaQ49) in Escherichia coli K12. Mol Gen Genet. 1978 Jul 25;163(3):277–283. doi: 10.1007/BF00271956. [DOI] [PubMed] [Google Scholar]
- Horiuchi T., Maki H., Sekiguchi M. Conditional lethality of Escherichia coli strains carrying dnaE and dnaQ mutations. Mol Gen Genet. 1981;181(1):24–28. doi: 10.1007/BF00339000. [DOI] [PubMed] [Google Scholar]
- Jonczyk P., Fijalkowska I., Ciesla Z. Overproduction of the epsilon subunit of DNA polymerase III counteracts the SOS mutagenic response of Escherichia coli. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9124–9127. doi: 10.1073/pnas.85.23.9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukral A. M., Strauch K. L., Maurer R. A., Miller C. G. Genetic analysis in Salmonella typhimurium with a small collection of randomly spaced insertions of transposon Tn10 delta 16 delta 17. J Bacteriol. 1987 May;169(5):1787–1793. doi: 10.1128/jb.169.5.1787-1793.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lahue R. S., Modrich P. Methyl-directed DNA mismatch repair in Escherichia coli. Mutat Res. 1988 Mar;198(1):37–43. doi: 10.1016/0027-5107(88)90037-1. [DOI] [PubMed] [Google Scholar]
- Lancy E. D., Lifsics M. R., Munson P., Maurer R. Nucleotide sequences of dnaE, the gene for the polymerase subunit of DNA polymerase III in Salmonella typhimurium, and a variant that facilitates growth in the absence of another polymerase subunit. J Bacteriol. 1989 Oct;171(10):5581–5586. doi: 10.1128/jb.171.10.5581-5586.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki H., Kornberg A. Proofreading by DNA polymerase III of Escherichia coli depends on cooperative interaction of the polymerase and exonuclease subunits. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4389–4392. doi: 10.1073/pnas.84.13.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki H., Kornberg A. The polymerase subunit of DNA polymerase III of Escherichia coli. II. Purification of the alpha subunit, devoid of nuclease activities. J Biol Chem. 1985 Oct 25;260(24):12987–12992. [PubMed] [Google Scholar]
- Maurer R., Osmond B. C., Botstein D. Genetic analysis of DNA replication in bacteria: dnaB mutations that suppress dnaC mutations and dnaQ mutations that suppress dnaE mutations in Salmonella typhimurium. Genetics. 1984 Sep;108(1):25–38. doi: 10.1093/genetics/108.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R., Osmond B. C., Shekhtman E., Wong A., Botstein D. Functional interchangeability of DNA replication genes in Salmonella typhimurium and Escherichia coli demonstrated by a general complementation procedure. Genetics. 1984 Sep;108(1):1–23. doi: 10.1093/genetics/108.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry C. S., Crow W. DNA polymerase III of Escherichia coli. Purification and identification of subunits. J Biol Chem. 1979 Mar 10;254(5):1748–1753. [PubMed] [Google Scholar]
- McHenry C. S. DNA polymerase III holoenzyme of Escherichia coli. Annu Rev Biochem. 1988;57:519–550. doi: 10.1146/annurev.bi.57.070188.002511. [DOI] [PubMed] [Google Scholar]
- Niwa O., Bryan S. K., Moses R. E. Alternate pathways of DNA replication: DNA polymerase I-dependent replication. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7024–7027. doi: 10.1073/pnas.78.11.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T., Aiba H., Ishihama A. Transcriptional organization of the convergent overlapping dnaQ-rnh genes of Escherichia coli. J Biol Chem. 1985 Jun 10;260(11):7122–7125. [PubMed] [Google Scholar]
- Orrego C., Eisenstadt E. An inducible pathway is required for mutagenesis in Salmonella typhimurium LT2. J Bacteriol. 1987 Jun;169(6):2885–2888. doi: 10.1128/jb.169.6.2885-2888.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Liljeström P., Harayama S. Cosmid cloning and transposon mutagenesis in Salmonella typhimurium using phage lambda vehicles. Mol Gen Genet. 1981;181(2):153–157. doi: 10.1007/BF00268420. [DOI] [PubMed] [Google Scholar]
- Quiñones A., Kaasch J., Kaasch M., Messer W. Induction of dnaN and dnaQ gene expression in Escherichia coli by alkylation damage to DNA. EMBO J. 1989 Feb;8(2):587–593. doi: 10.1002/j.1460-2075.1989.tb03413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M. Escherichia coli mutator mutD5 is defective in the mutHLS pathway of DNA mismatch repair. Genetics. 1989 Feb;121(2):205–212. doi: 10.1093/genetics/121.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermann R. H., Echols H. A separate editing exonuclease for DNA replication: the epsilon subunit of Escherichia coli DNA polymerase III holoenzyme. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7747–7751. doi: 10.1073/pnas.81.24.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermann R., Tam S., Burgers P. M., Lu C., Echols H. Identification of the epsilon-subunit of Escherichia coli DNA polymerase III holoenzyme as the dnaQ gene product: a fidelity subunit for DNA replication. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7085–7089. doi: 10.1073/pnas.80.23.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Schollmeier K., Hillen W. Transposon Tn10 contains two structural genes with opposite polarity between tetA and IS10R. J Bacteriol. 1984 Nov;160(2):499–503. doi: 10.1128/jb.160.2.499-503.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shwartz H., Shavitt O., Livneh Z. The role of exonucleolytic processing and polymerase-DNA association in bypass of lesions during replication in vitro. Significance for SOS-targeted mutagenesis. J Biol Chem. 1988 Dec 5;263(34):18277–18285. [PubMed] [Google Scholar]
- Takano K., Nakabeppu Y., Maki H., Horiuchi T., Sekiguchi M. Structure and function of dnaQ and mutD mutators of Escherichia coli. Mol Gen Genet. 1986 Oct;205(1):9–13. doi: 10.1007/BF02428026. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Villani G., Boiteux S., Radman M. Mechanism of ultraviolet-induced mutagenesis: extent and fidelity of in vitro DNA synthesis on irradiated templates. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3037–3041. doi: 10.1073/pnas.75.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch M. M., McHenry C. S. Cloning and identification of the product of the dnaE gene of Escherichia coli. J Bacteriol. 1982 Oct;152(1):351–356. doi: 10.1128/jb.152.1.351-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield H. J., Levine G. Isolation and characterization of a mutant of Salmonella typhimurium deficient in a major deoxyribonucleic acid polymerase activity. J Bacteriol. 1973 Oct;116(1):54–58. doi: 10.1128/jb.116.1.54-58.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgate R., Bridges B. A., Herrera G., Blanco M. Mutagenic DNA repair in Escherichia coli. XIII. Proofreading exonuclease of DNA polymerase III holoenzyme is not operational during UV mutagenesis. Mutat Res. 1987 Jan;183(1):31–37. doi: 10.1016/0167-8817(87)90042-3. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]