Abstract
Interactions between the ureteric bud (UB) and metanephric mesenchyme are crucial for tubulogenesis during kidney development. Two immortalized cell lines derived from the day 11.5 embryonic kidney, UB cells, which appear to be epithelial (cytokeratin-positive, E-cadherin-positive, and ZO-1-positive by immunostaining) and BSN cells, which are largely mesenchymal (vimentin-positive, but negative for cytokeratin, cell surface E-cadherin, and cell surface ZO-1), were used to establish an in vitro tubulogenesis system. BSN cells expressed hepatocyte growth factor (HGF) and transforming growth factor-β1 mRNAs, and its conditioned medium (BSN-CM) contained factors capable of activating the epidermal growth factor (EGF) receptor (EGFR). When UB cells were cultured in an extracellular matrix gel in the presence of the embryonic kidney or BSN-CM, the UB cells underwent morphogenetic changes characteristic of early in vitro branching tubulogenesis. These changes were largely inhibited by a combination of neutralizing anti-HGF antibodies and the EGFR inhibitor tyrphostin AG1478, suggesting that EGFR ligands, together with HGF, account for much of this early morphogenetic activity. Nevertheless, there was a significant fraction of tubulogenic activity that could not be inhibited, suggesting the existence of other soluble factors. Whereas HGF, EGF, transforming growth factor α, basic fibroblast growth factor (bFGF), and insulin-like growth factor 1 (IGF-1), or a mixture of these growth factors, induced epithelial processes for up to 3 days, only IGF-1, possibly bFGF, and the mixture were able to sustain morphogenesis for longer periods, though not nearly to the same degree as BSN-CM. Moreover, only BSN-CM induced branching tubular structures with clear lumens, consistent with the existence of other soluble factors crucial for the formation and/or maintenance of branching tubular structures with lumens in vitro.
Keywords: kidney development, ureteric bud, metanephric mesenchyme, extracellular matrix
In the murine embryo, metanephrogenesis is initiated when the ureteric bud (UB) interacts with undifferentiated metanephric mesenchyme around 11.5 days after conception. The UB undergoes successive dichotomous branching steps as it invades the metanephric mesenchyme, developing into the kidney collecting system and ureteric tree. The cells of the metanephric mesenchyme, which have been induced by the UB, epithelialize and ultimately develop into the more proximal nephron from the glomerular capsule to the distal tubule (1, 2). Thus, UB interactions with the metanephric mesenchyme are essential for normal kidney development. Genetic approaches have recently implicated several potentially diffusible molecules in kidney development, including wnt-4 (3) and bone morphogenetic protein 7 (4, 5). Furthermore, organ culture studies suggest that a number of growth factors, including hepatocyte growth factor (HGF) (6), epidermal growth factor (EGF) receptor (EGFR) ligands (7), insulin-like growth factor 1 (IGF-1) (8, 9), and others, play at least a facilitory role in kidney development. Nevertheless, the spatiotemporal complexity of cell interactions in the developing kidney makes detailed analysis of cellular processes at the level of the whole embryo or in organ culture difficult.
In vitro cell culture systems, while possessing their own particular limitations, can be used to complement genetic or organ culture approaches. To the extent that this kind of approach reflects events occurring in vivo during development, it can be used to gain mechanistic insights into complex morphogenetic processes. One of the best-studied models employs kidney epithelial cell lines, such as Madin–Darby canine kidney (MDCK) and murine inner medullary collecting duct (mIMCD3), seeded in three-dimensional type I collagen gels to analyze mechanisms of epithelial tubulogenesis and branching morphogenesis (10–17). In the mIMCD3 cell–collagen gel system, HGF and EGFR ligands induce branching tubulogenesis (10, 11), whereas transforming growth factor (TGF)-β selectively inhibits branching events (14). Since these cells are derived from the collecting duct (and thus ultimately the UB), the results from this system have been used to propose a model whereby gradients of growth factors which might exist within the mesenchyme or elsewhere in the developing kidney lead to vectorial branching tubulogenesis such as occurs during collecting system development (17–19). The fact that multiple growth factors are capable of inducing branching tubulogenesis has also been used to argue for “relative redundancy” and explain why knockouts of individual growth factors often fail to exhibit obvious abnormalities in kidney development (20–24). However, the mIMCD3 and MDCK cells are derived from differentiated kidney epithelial cells, and the extent to which results from these models are applicable to the embryonic kidney has not been established. A more authentic in vitro model would be to use epithelial cells directly from the embryonic kidney. We have therefore developed an in vitro tubulogenesis system using immortalized UB cells and conditioned medium from cells which appear to be derived from the embryonic kidney mesenchyme. The results obtained with this model system suggest that, in addition to HGF and EGFR ligands, which are important for branching tubulogenesis in mIMCD3 cells, other known and yet-to-be-identified soluble factors play an important role in tubulogenesis in vitro.
MATERIALS AND METHODS
Cell Lines and Three-Dimensional Extracellular Matrix (ECM) Gel Culture.
The UB cell line was obtained from microdissected UB of a day-11.5 mouse embryo transgenic for simian virus 40 (SV40) large T antigen (Immortomouse, Charles River) as described previously (25). The UB cells were maintained in MEM, supplemented with 10% fetal calf serum (FCS) at 32°C in a 5% CO2 incubator. The BSN cell line was obtained from a day-11.5 murine embryonic kidney transgenic for the early region of SV40 [TgN(SV)7Bri, kindly supplied by R. L. Brinster, Univ. of Pennsylvania] (26)]. After embryonic kidneys were microdissected, the UB was carefully removed. The remaining metanephric mesenchyme was placed in DME/F12 medium, with 10% FCS at 37°C in a 5% CO2 incubator. The cells outgrown from the metanephric mesenchyme were transferred to plastic culture dishes and subselected. These BSN cells were maintained in DME/F12 with 10% FCS at 37°C in a 5% CO2 incubator. Passages 4–14, during which the cells appeared to have a relatively stable character by marker analysis, were used for experiments. Experiments with later passage cells had greater variability. To obtain BSN cell conditioned medium (BSN-CM), a confluent BSN cell monolayer was washed twice with serum-free DME/F12 medium, followed by application of serum-free DME/F12 and incubation in a CO2 incubator for 2–4 days. The collected BSN-CM was centrifuged at low speed to remove cell debris. The culture conditions for mIMCD3 cells have been previously described (10, 27).
Three-dimensional culture of UB cells was performed as previously described (10, 11, 14). In some experiments UB cells were suspended in the mixture of growth factor-depleted Matrigel and type I collagen. For the embryonic kidney coculture experiment, day-13 embryonic kidneys were placed on top of ECM gels in serum-free DME/F12 medium. The morphology of the suspended UB cells was analyzed by a phase-contrast microscope with Hoffman modulator. The three-dimensional cultures were maintained in a 32° or 37°C CO2 incubator with daily medium change for 5 days and every other day thereafter. At least morphologically, the incubation temperature did not seem to affect UB cell morphogenesis. Twenty randomly selected cells or colonies were evaluated for process formation (an early stage of tubulogenesis) or multicellular cord/tubule formation under the phase contrast microscope. The percentage of cells/colonies with processes was used as a semiquantitative indicator of tubulogenesity under each condition.
Cytochemistry for Cell Characterization.
Confluent monolayers of UB and BSN cells grown on glass coverslips were prepared for immunofluorescence analysis after methanol fixation as previously described (28). Antibodies and their dilutions were as follows; mouse anti-pan cytokeratin (1:1,000, Sigma) mouse anti-vimentin (1:200, Sigma), rat anti-ZO-1 (1:1, generous gift from D. A. Goodenough, Harvard), and rat anti-E-cadherin (1:200, Sigma). In the case of staining with Dolichos biflorus lectin, confluent monolayers of cells grown on glass coverslips were fixed with 4% paraformaldehyde/PBS at room temperature for 15 min. After a vigorous wash with PBS (with Ca2+ and Mg2+) coverslips were incubated with Texas red-conjugated Dolichos biflorus lectin (50 μg/ml in Ca2+- and Mg2+-containing PBS; Sigma) at room temperature for 1 hr, followed by washing with PBS with Ca2+ and Mg2+. To determine the specificity of the lectin staining, the lectin was preincubated with N-acetylgalactosamine (1 mg/ml in PBS with Ca2+ and Mg2+). Coincubation with the lectin binding-sugar abolished cell surface staining. The coverslips were mounted and examined with a fluorescence microscope.
Growth Factors, Inhibitors, and Antibodies in Three-Dimensional Cell Culture.
Sources and working concentrations of growth factors were as follows: HGF (R & D Systems) 40 ng/ml, EGF (Collaborative Research) 40 ng/ml, TGF-α (Collaborative Research) 40 ng/ml, basic fibroblast growth factor (bFGF; Upstate Biotechnology) 50 ng/ml, IGF-1 (Upstate Biotechnology) 50 ng/ml, glial cell line-derived neurotrophic factor (GDNF; R & D Systems) 100 ng/ml, TGF-β1 (R & D Systems) 1 ng/ml, and platelet-derived growth factor (PDGF; Upstate Biotechnology) 20 ng/ml. Growth factors were dissolved in the DME/F12 containing 0.1% BSA or 1% FCS and applied on top of the ECM gels.
The specific inhibitor for EGFR tyrosine kinase, tyrphostin AG 1478 (Calbiochem), was dissolved in dimethyl sulfoxide and applied to ECM gels at 0.3 μM. Neutralizing anti-HGF antibodies (generous gift from T. Nakamura, Osaka University, Japan) have been extensively characterized previously (6, 10). The antibodies react cleanly with HGF on Western blots, detect subnanomolar concentrations of HGF by ELISA, and inhibit HGF-induced mitogenesis (10) and tubulogenesis (6). The antibodies were applied at 10 μg/ml.
Western Blot Analysis.
Subconfluent mIMCD3 cells were stimulated with BSN-CM or EGF (20 ng/ml) for 5 min in the presence or absence of tyrphostin AG 1478 (0.3 μM). Tyrphostin AG 1478 was added 10 min before applying growth factors. Subsequently cells were lysed and subjected to SDS/PAGE as described previously (14, 28, 29). The membrane was probed with anti-phosphotyrosine antibodies (4G10, Upstate Biotechnology), followed by immunodetection.
Northern Blot Analysis.
Total RNA was extracted from confluent monolayers of UB and BSN cells by the acid/guanidine/phenol/chloroform method (30), followed by 1% agarose/formaldehyde gel electrophoresis. After transfer to a nylon membrane, the blot was hybridized with a 32P-labeled cDNA probe. Probes were as follows: HGF (generous gift from M. Park, McGill University), c-met (generous gift from M. Park), and TGF-β1 (generous gift from K. Totsune, Brigham and Women’s Hospital).
RESULTS
Characterization of UB and BSN Cell Lines.
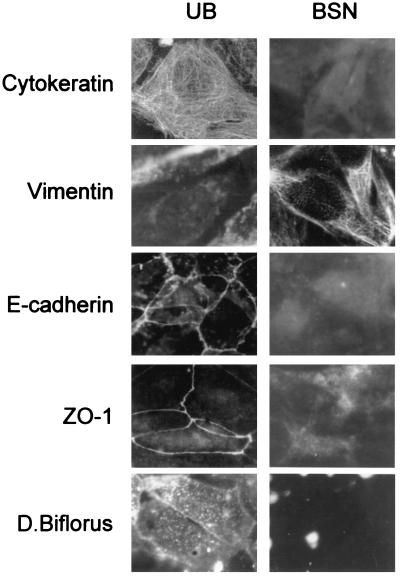
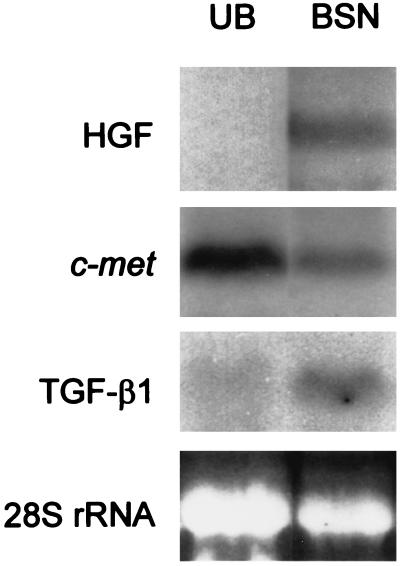
The BSN cell line was established as described above. To determine whether it was of epithelial or mesenchymal character, both it and the UB cell line were analyzed for the expression of E-cadherin, ZO-1, cytokeratin, and vimentin as well as lectin binding. As shown in Fig. 1, UB cells were cytokeratin-positive, E-cadherin-positive, ZO-1-positive, and partially (5–30%, depending on passage) vimentin-positive, consistent with an epithelial character as previously described (25). In contrast, BSN cells (passage 4–14) were negative for cytokeratin, though strongly positive for vimentin, consistent with a mesenchymal character. No cell surface staining for E-cadherin or ZO-1 was observed, though some faint cytoplasmic staining of ZO-1, which has been reported for a number of mesenchymal cell lines (31), was observed. The UB cells were also positive for cell surface staining by Dolichos biflorus lectin, known to bind a cell surface glycoprotein expressed in developing ureter but not mesenchyme, whereas BSN cells were negative for lectin staining (Fig. 1). The stain appeared to be specific, since preincubation with N-acetylgalactosamine eliminated the signal. We also examined HGF/c-met expression in these cells by Northern blotting. The UB cells were HGF-negative, c-met-positive, whereas the BSN cells were both HGF- and c-met- positive (Fig. 2). The BSN cells also expressed TGF-β1 mRNA. Together, these results confirm the origin and epithelial character of the UB cells derived from day-11.5 UB and the largely nonepithelial character of the BSN cells derived from embryonic kidney mesenchyme of approximately the same gestational day. In addition to expressing an intermediate filament and junctional molecule profile consistent with mesenchymal origin, the BSN cells also express mRNAs for growth factors believed to be made in the embryonic kidney mesenchyme (32, 33).
Figure 1.
Immunocytochemical characterization of UB and BSN cells. Confluent monolayers of UB and BSN cells were stained for cytokeratin, vimentin, E-cadherin, ZO-1, and binding of Dolichos biflorus lectin. (×450.)
Figure 2.
Northern blots of total RNA extracted from UB and BSN cell monolayers were probed for HGF, c-met, and TGF-β1. Ethidium bromide staining of 28S rRNA was used to estimate RNA loaded in each lane.
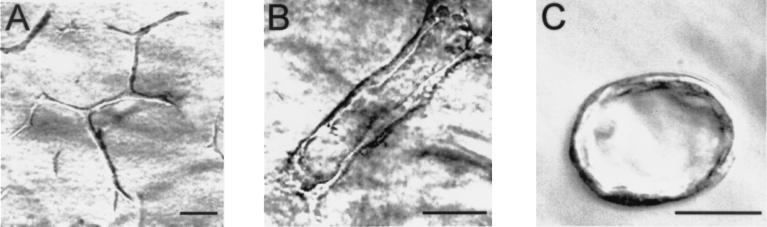
BSN-CM Induced UB Cell Tubulogenesis Comparable to That Induced by the Embryonic Kidney.
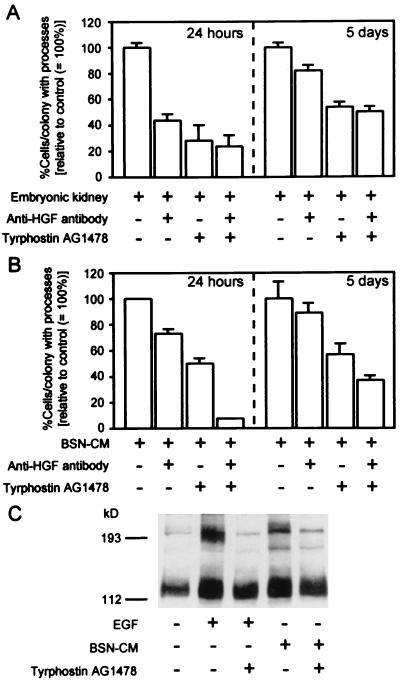
MDCK and mIMCD3 cells are known to undergo impressive branching morphogenesis when cocultured in ECM gels with the embryonic kidney (in the absence of apparent cell–cell contact between the embryonic kidney and the MDCK or mIMCD3 cells) due to the elaboration of soluble growth factors, including HGF and EGFR ligands (6, 10). When day-13 embryonic kidneys were placed on top of ECM gels containing UB cells, the embryonic kidneys were able to induce UB cell processes and multicellular cords, early steps in tubulogenesis in vitro. Thus, the embryonic kidney elaborates soluble factors capable of inducing UB cell morphogenesis; presumably these factors are in large part made in the mesenchyme. To determine if the BSN cells, which appear to be of mesenchymal origin, elaborated similar factors, BSN-CM was added to UB cells in three-dimensional ECM gel culture. A morphogenetic response comparable to that induced by the embryonic kidney was observed. The best response was obtained when the ECM gel consisted of 80% type I collagen mixed with 20% growth factor-reduced Matrigel (Fig. 3A and B). However, pure growth factor-reduced Matrigel suppressed tubule formation; instead, the matrix material promoted cyst formation even in the presence of BSN-CM (Fig. 3C). Similar modulation of tubulogenesis by ECM has been reported on MDCK cells in the presence of HGF (16). To determine whether the growth factors promoting UB cell morphogenesis that had been secreted by the embryonic kidney and/or BSN cells were the same as those which induce mIMCD3 cell tubulogenesis, we attempted to neutralize the activity of HGF and EGFR ligands separately and together with a well-characterized anti-HGF antibody that is known to inhibit the mitogenic and tubulogenic activity of HGF (6, 10) and a specific EGFR tyrosine kinase inhibitor, tyrphostin AG 1478, thus far not reported to inhibit the activity of any other tyrosine kinase (34, 35). Western blots with anti-phosphotyrosine antibodies confirmed the ability of tyrphostin AG 1478 to virtually completely inhibit EGFR autophosphorylation (Fig. 4C). At 24 hr, incubation with the neutralizing anti-HGF antibodies had an inhibitory effect on the ability of the embryonic kidney or the BSN-CM to induce process formation, the earliest step in in vitro tubulogenesis. Tyrphostin AG 1478 had a similar inhibitory effect. Both agents together were able to inhibit all but a small fraction of the soluble morphogenetic activity elaborated by the embryonic kidney and BSN cells, suggesting that HGF and EGFR ligands together account for the largest proportion of activity involved in early process formation in this system (Fig. 4 A and B left panels). To examine the effect of prolonged inhibition of HGF and EGFR, coculture was continued for up to 5 days. Anti-HGF antibodies were replaced every day. Although we administered tyrphostin AG 1478 only at the beginning of culture, its continuous presence prevented TGF-α-induced morphogenesis, suggesting long-term EGFR inactivation (data not shown). Continuous incubation with these inhibitory agents over 5 days, a period during which processes become more complex and develop into multicellular cords, was somewhat less effective in inhibiting the morphogenetic activity (Fig. 4 A and B right panels). While this suggests that the same set of factors (HGF and EGFR ligands) continues to play an important role in morphogenesis for up to 5 days, it also suggests that a significant fraction of the activity (≈50%) might not be accounted for by HGF and EGFR ligands.
Figure 3.
UB cells exhibit multicellular cords, tubules with lumens, and multicellular cysts in ECM gels in BSN-CM, depending on matrix composition. UB cells were suspended in 80% collagen type I/20% growth factor reduced Matrigel in the presence of BSN-CM containing 1% FCS. (A) After 7 days of culture, the cells grew to form multicellular cord-like structures. (B) After 15 days of culture, clear lumens could be seen in UB tubules. (C) When UB cells were suspended in 100% growth factor reduced Matrigel, even in the presence of BSN-CM and 1% FCS, they remained multicellular cysts even after 15 days of culture. (Bar = 50 μm.)
Figure 4.
BSN-CM shows tubulogenic activity comparable to that elaborated by the embryonic kidney upon UB cells grown in three-dimensional ECM gels. The percentages of UB cells/colonies exhibiting processes (an early step in tubulogenesis) were used for semiquantitative measures of tubulogenic activity under each condition. The percentages were standardized to untreated control (= 100%) and presented as means ± SE. The results were representative of four separate experiments. (A) Day-13 embryonic kidneys were placed on top of the three-dimensional collagen gel culture system. UB cell tubulogenesis was evaluated at 24 hr and 5 days of culture. At 24 hr, a large portion of tubulogenic activity was inhibited by a combination of neutralizing anti-HGF antibodies (10 μg/ml) and the EGFR inhibitor tyrphostin AG 1478 (0.3 μM). However, at 5 days about 50% of tubulogenic activity could not be inhibited. (B) BSN-CM with 1% FCS was applied on top of UB cells in three-dimensional collagen gel culture. As was the case for embryonic kidney coculture, there were differences between 24 hr and 5 days in terms of tubulogenic inhibition profile by anti-HGF antibodies and tyrphostin AG 1478. (C) BSN-CM phosphorylated tyrosine residues of EGFR. A subconfluent mIMCD3 cell monolayer was stimulated with EGF (20 ng/ml) or BSN-CM in the presence or absence of tyrphostin AG 1478 (0.3 μM). The whole cell lysates were subjected to Western blotting with anti-phosphotyrosine antibodies. In the second (EGF) and fourth (BSN-CM) lanes, ≈190-kDa protein was phosphorylated. The phosphorylation was completely inhibited by tyrphostin AG 1478 (third and fifth lanes).
A Number of Growth Factors Are Capable of Inducing in Vitro UB Cell Tubulogenesis.
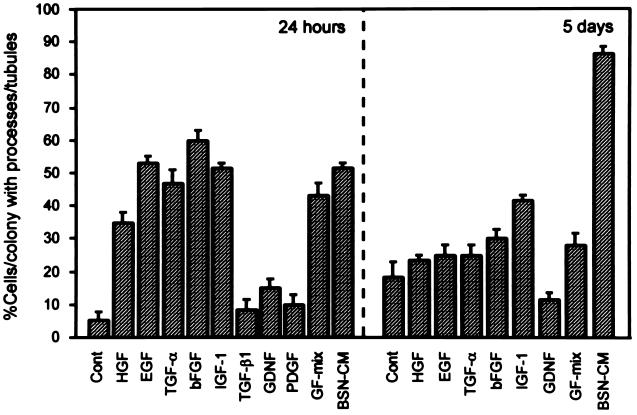
To more directly examine the role of HGF and EGFR ligands in UB cell tubulogenesis, and also to determine if other factors could be found that might account for the fraction of morphogenetic activity that could not be inhibited by the anti-HGF antibody and tyrphostin AG 1478, purified growth factors were added to the UB cells grown in type I collagen gels and compared with the effect of BSN-CM. [Collagen gels were employed because even growth factor-depleted Matrigel contains some remaining growth factor activity (16) that could complicate the interpretation.] During the initial 24–48 hr, several growth factors, including HGF, EGF, TGF-α, bFGF, and IGF-1, induced process formation of UB cells in three-dimensional culture (Fig. 5 Left; Table 1). GDNF, PDGF, and TGF-β1 did not induce initial process formation (Fig. 5, Left; Table 1). BSN-CM induced UB cell processes which became more complex over several days, eventually forming multicellular cords (Fig. 3A; Table 1). However, while HGF, EGF, TGF-α, bFGF, and IGF-1 continued to induce complex processes for 2–3 days, after this time only high concentrations of IGF-1 and bFGF were able to sustain multicellular cords, though not nearly to the same degree as BSN-CM (Fig. 5 Right; Table 1). Moreover, a mixture of HGF, EGF, bFGF, IGF-1, and GDNF, which initially appeared as potent as BSN-CM, did not sustain complex multicellular structures (either cysts or cords) after 5–6 days of culture (Fig. 5; Table 1), and the “quality” and complexity of the structures induced by BSN-CM was clearly superior. In fact, only BSN-CM consistently induced branching tubular structures with clearly distinguishable lumens (Table 1).
Figure 5.
BSN-CM maintains UB cell morphogenesis better than any other purified growth factor or their combination. Purified growth factors, a combination of growth factors (GF-mix: HGF, EGF, bFGF, IGF-1, and GDNF), or BSN-CM was applied on top of UB cells suspended in collagen gels. Tubulogenic activity was semiquantified by evaluating percentage of UB cells/colonies with processes/cords/tubules. At 24 hr of serum-free culture, HGF, EGF, TGF-α, bFGF, and IGF-1 were as capable of inducing UB cell processes as were GF-mix or BSN-CM medium. TGF-β1, PDGF, and GDNF were not effective. At 5 days of culture (with 1% serum), only high concentrations of bFGF and IGF-1 could sustain the growth of UB cell processes into cord-like structures. BSN-CM was much more potent than any other growth factors listed or their combination. Statistical significance was determined and is presented in Table 1, where a qualitative analysis of structures is also presented.
Table 1.
Qualitative effect of growth factors on UB cell tubulogenesis
| Growth factor | Process formation (24–48 hr) | Cord-like structure (5–6 days) | Tubules with lumen (>10 days) |
|---|---|---|---|
| HGF | + | ± | − |
| EGF | + | − | − |
| TGF-α | + | − | − |
| bFGF | + | ± | − |
| IGF-1 | + | + | − |
| PDGF | − | ND | ND |
| GDNF | − | − | − |
| TGF-β1 | − | ND | ND |
| BSN-CM | + | + | + |
| GF-mix | + | + | − |
Growth factors were applied on top of UB cell collagen gel suspension culture. Percentage of cells/colonies exhibiting tubules/processes was evaluated for each condition and compared to control condition (BSA or 1% FCS). If the specific growth factor treatment significantly increased tubulogenesis (i.e., P < 0.05 vs. control by unpaired Student’s t test), the growth factor was marked +. If not, the growth factor was marked −. ± indicates variable result among experiments. ND, not done. Process and formation data were derived from five separate experiments, cord-like structure data were derived from three separate experiments. Tubules with lumen data were derived from two separate experiments. Only BSN-CM induced tubules with lumens. GF-mix contains HGF, EGF, bFGF, IGF-1, and GDNF.
DISCUSSION
We have established a system in which soluble factors from an apparently mesenchymal cell line (BSN) derived from the early embryonic kidney are able to induce branching tubulogenesis in a UB cell line in a three-dimensional ECM gel system. Only minimal amounts of serum were used. Thus, this represents perhaps the simplest system for the study of mesenchymal–epithelial interactions relevant to early kidney development. Systems for branching tubulogenesis that have been employed previously utilize cell lines such as MDCK or mIMCD3 derived from the mature kidney (10–16, 36). In these cell lines, the major factors capable of inducing early cellular process formation leading to branching tubulogenesis appear to be HGF and EGFR ligands (e.g., EGF, TGF-α, heparin-binding EGF, amphiregulin, betacellulin) (10, 11). In contrast, the UB cells, apart from responding to HGF and EGFR ligands, also form processes in response to high concentrations of IGF-1 and bFGF (Fig. 5, Table 1). These growth factors can also induce the formation of multicellular cords in UB cells. Although a receptor for bFGF has been reported in the collecting duct (37), the fact that only high concentrations of bFGF induced UB cell tubulogenesis raises the possibility that this growth factor is not acting on classical bFGF receptors per se, but rather, on some other receptor that binds bFGF. However, neither alone nor in combination were these growth factors able to induce tubules with lumens. Only BSN-CM was capable of doing this (Table 1), suggesting that it contains additional growth factors capable of leading to the formation of branching tubules with lumens.
This raises an interesting question. Heretofore, the literature on in vitro tubulogenesis has been vague as to whether the phenomenon of tubule formation is simply the evolution of a single kind of process or whether multiple distinct steps are involved. Morphologically, there appear to be at least three clearly distinguishable morphogenetic events: the formation of cellular processes, the development into branching multicellular cords, and the establishment of tubules with lumens. Our data raise the possibility that these are distinct steps dependent on different sets of soluble factors. However, this issue probably cannot be clearly resolved until molecular markers specific for these steps are found, if indeed they are distinct, or relatively so.
Although perhaps somewhat less robust compared with the MDCK cell and mIMCD3 cell systems, the system we describe here may be viewed as a more authentic in vitro system for the study of branching tubulogenesis relevant to the developing kidney. Nevertheless, all these systems have potential limitations. The cell lines we have used express simian virus 40 antigen, and it is difficult to determine what effect this has on the behavior of the cells in the morphogenetic system. Nevertheless, the animals from which these cells were derived undergo apparently normal nephrogenesis with normal branching morphogenesis. It is also reassuring that coculturing the embryonic kidney with the UB cells and incubating the UB cells with the conditioned medium from the BSN cells gave similar results (Fig. 4). Thus, in both cases, EGFR ligands and HGF accounted for the bulk of branching tubulogenic activity, but there was a significant fraction (≈40–50%) of tubulogenic activity which could not be neutralized by the combination of neutralizing anti-HGF antibody and the specific EGFR antagonist. This was especially apparent in the long-term assays and is consistent with our finding that none of the growth factors we employed, individually or in combination, was capable of sustaining long-term morphogenesis leading to the formation of tubules with lumens. The nature of this additional factor remains to be determined. It is possible that this factor simply sustains tubular growth, thereby allowing other morphogens to act upon the developing multicellular structure, or that it functions as a true morphogen.
Although the UB cells express c-ret, at least by reverse transcription–polymerase chain reaction (25), we were unable to induce branching tubulogenesis with its ligand, GDNF (Fig. 5). This may be due to low levels of expression or the expression of a receptor that is not functionally active, at least in the context of the ECM gels we used. It is also conceivable that mesenchymal–epithelial cell contact is required for activation of the GDNF/c-ret axis.
Finally, while the BSN cells appear to be mesenchymal and exhibit clear-cut differences in marker profile from the UB cells (Fig. 1), it remains to be determined to what extent, if any, they themselves are capable of undergoing tubulogenesis and/or differentiating along the pathway leading to proximal tubule formation, as occurs in metanephric mesenchyme of the embryonic kidney. The fact that the BSN cells express c-met may suggest that, although largely mesenchymal in character, they have the potential for epithelialization or that they are at one of the earliest steps in this process. This constitutes an important area for future research.
Acknowledgments
This work was support by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases to S.K.N. This work was done during S.K.N.’s tenure as an Established Investigator of the American Heart Association. H.S. was supported by a Research Fellowship from the National Kidney Foundation. T.T. was supported in part by the Sandoz Foundation for Gerontological Research.
ABBREVIATIONS
- UB
ureteric bud
- HGF
hepatocyte growth factor
- EGF
epidermal growth factor
- EGFR
EGF receptor
- IGF-1
insulin-like growth factor 1
- bFGF
basic fibroblast growth factor
- ECM
extracellular matrix
- MDCK
Madin–Darby canine kidney
- mIMCD
murine inner medullary collecting duct
- TGF
transforming growth factor
- GDNF
glial cell line-derived neurotrophic factor
- PDGF
platelet-derived growth factor
- BSN-CM
BSN cell conditioned medium
References
- 1.Saxen L. Organogenesis of the Kidney. Cambridge, U.K.: Cambridge Univ. Press; 1987. [Google Scholar]
- 2.Nigam S K, Aperia A, Brenner B M. In: The Kidney. Brenner B M, editor. Philadelphia: Saunders; 1996. pp. 72–98. [Google Scholar]
- 3.Stark K, Vainio S, Vassileva G, McMahon A P. Nature (London) 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 4.Luo G, Hofmann C, Bronckers A L, Sohocki M, Bradley A, Karsenty G. Genes Dev. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- 5.Dudley A T, Lyons K M, Robertson E J. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 6.Santos O F, Barros E J, Yang X M, Matsumoto K, Nakamura T, Park M, Nigam S K. Dev Biol. 1994;163:525–529. doi: 10.1006/dbio.1994.1169. [DOI] [PubMed] [Google Scholar]
- 7.Rogers S A, Ryan G, Hammerman M R. Am J Physiol. 1992;262:F533–F539. doi: 10.1152/ajprenal.1992.262.4.F533. [DOI] [PubMed] [Google Scholar]
- 8.Rogers S A, Ryan G, Hammerman M R. J Cell Biol. 1991;113:1447–1453. doi: 10.1083/jcb.113.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z Z, Kumar A, Wallner E I, Wada J, Carone F A, Kanwar Y S. Eur J Cell Biol. 1994;65:378–391. [PubMed] [Google Scholar]
- 10.Barros E J, Santos O F, Matsumoto K, Nakamura T, Nigam S K. Proc Natl Acad Sci USA. 1995;92:4412–4416. doi: 10.1073/pnas.92.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantley L G, Barros E J, Gandhi M, Rauchman M, Nigam S K. Am J Physiol. 1994;267:F271–F280. doi: 10.1152/ajprenal.1994.267.2.F271. [DOI] [PubMed] [Google Scholar]
- 12.Derman M P, Cunha M J, Barros E J, Nigam S K, Cantley L G. Am J Physiol. 1995;268:F1211–F1217. doi: 10.1152/ajprenal.1995.268.6.F1211. [DOI] [PubMed] [Google Scholar]
- 13.Montesano R, Schaller G, Orci L. Cell. 1991;66:697–711. doi: 10.1016/0092-8674(91)90115-f. [DOI] [PubMed] [Google Scholar]
- 14.Sakurai H, Nigam S K. Am J Physiol. 1997;272:F139–F146. doi: 10.1152/ajprenal.1997.272.1.F139. [DOI] [PubMed] [Google Scholar]
- 15.Santos O F, Moura L A, Rosen E M, Nigam S K. Dev Biol. 1993;159:535–548. doi: 10.1006/dbio.1993.1262. [DOI] [PubMed] [Google Scholar]
- 16.Santos O F, Nigam S K. Dev Biol. 1993;160:293–302. doi: 10.1006/dbio.1993.1308. [DOI] [PubMed] [Google Scholar]
- 17.Rosen E M, Nigam S K, Goldberg I D. J Cell Biol. 1994;127:1783–1787. doi: 10.1083/jcb.127.6.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuart R O, Barros E J, Ribeiro E, Nigam S K. J Am Soc Nephrol. 1995;6:1151–1159. doi: 10.1681/ASN.V641151. [DOI] [PubMed] [Google Scholar]
- 19.Nigam S K. Curr Opin Nephrol Hypertension. 1995;4:209–214. doi: 10.1097/00041552-199505000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Nature (London) 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Nature (London) 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 22.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Nature (London) 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 23.Luetteke N C, Qui T H, Peiffer R L, Oliver P, Smithies O, Lee D C. Cell. 1993;73:263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- 24.Mann G B, Fowler K J, Gabriel A, Nice E C, Williams R L, Dunn A R. Cell. 1993;73:249–261. doi: 10.1016/0092-8674(93)90227-h. [DOI] [PubMed] [Google Scholar]
- 25.Barasch J, Pressler L, Connor J, Malik A. Am J Physiol. 1996;271:F50–F61. doi: 10.1152/ajprenal.1996.271.1.F50. [DOI] [PubMed] [Google Scholar]
- 26.Palmiter R D, Chen H Y, Messing A, Brinster R L. Nature (London) 1985;316:457–460. doi: 10.1038/316457a0. [DOI] [PubMed] [Google Scholar]
- 27.Rauchman M I, Nigam S K, Delpire E, Gullans S R. Am J Physiol. 1993;265:F416–F424. doi: 10.1152/ajprenal.1993.265.3.F416. [DOI] [PubMed] [Google Scholar]
- 28.Bush K T, Stuart R O, Li S-H, Moura L A, Sharp A H, Ross C A, Nigam S K. J Biol Chem. 1994;269:23694–23699. [PubMed] [Google Scholar]
- 29.Kuznetsov G, Chen L B, Nigam S K. J Biol Chem. 1997;272:3057–3063. doi: 10.1074/jbc.272.5.3057. [DOI] [PubMed] [Google Scholar]
- 30.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 31.Howarth A G, Hughes M R, Stevenson B R. Am J Physiol. 1992;262:C461–C469. doi: 10.1152/ajpcell.1992.262.2.C461. [DOI] [PubMed] [Google Scholar]
- 32.Karp S L, Ortiz-Arduan A, Li S, Neilson E G. Proc Natl Acad Sci USA. 1994;91:5286–5290. doi: 10.1073/pnas.91.12.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonnenberg E, Meyer D, Weidner K M, Birchmeier C. J Cell Biol. 1993;123:223–235. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fry D W, Kraker A J, McMichael A, Ambroso L A, Nelson J M, Leopold W R, Connors R W, Bridges A J. Science. 1994;265:1093–1095. doi: 10.1126/science.8066447. [DOI] [PubMed] [Google Scholar]
- 35.Levitzki A, Gazit A. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 36.Montesano R, Matsumoto K, Nakamura T, Orci L. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- 37.Wanaka A, Milbrandt J, Johoson E M., Jr Development (Cambridge, UK) 1991;111:455–468. doi: 10.1242/dev.111.2.455. [DOI] [PubMed] [Google Scholar]