Abstract
The purines ATP and adenosine play an important role in the communication between the photoreceptors and the retinal pigment epithelium (RPE). While the RPE is known to release ATP into subretinal space, the source of extracellular adenosine is unclear. In other tissues, ecto-nucleotidases mediate the consecutive dephosphorylation of ATP to AMP, and AMP is converted to adenosine by ecto-5′ nucleotidase (CD73). This study identifies ecto-5′ nucleotidase on RPE cells and investigates modulation of enzyme activity. The RPE was the most active site of 5′AMP dephosphorylation in the posterior rat eye. The ecto-5′ nucleotidase inhibitor αβmADP prevented the production adenosine by the apical membrane of the bovine RPE. Cultured human ARPE-19 cells expressed mRNA and protein for ecto-5′ nucleotidase. The production of phosphate from 5′AMP by ARPE-19 cells was inhibited by αβmADP, but the ecto-alkaline phosphatase inhibitor levamisole had no effect. Degradation of 5′AMP was blocked by norepinephrine, epinephrine and phenylephrine, with inhibition by antagonists prazosin and corynanthine implicating the α1 adrenergic receptor. The block of enzyme activity by norepinephrine was rapid, occurring within 1 min, and was similar at both 4 and 37°C, consistent with cleavage of the enzyme from its GPI anchor. HPLC measurements indicated norepinephrine reduced levels of adenosine in the bath. In the apical face of the bovine-RPE eyecup, norepinephrine reduced the production of phosphate from 5′AMP, suggesting that both receptor and enzyme face sub-retinal space. In conclusion, RPE cells express ecto-5′ nucleotidase, with activity on the apical membrane, and stimulation of α-1 adrenergic receptors downregulates activity. As epinephrine is released at light onset, and adenosine can inhibit phagocytosis, the corresponding decrease in subretinal adenosine levels may contribute to the enhanced the phagocytosis of rod outer segments that occurs at this time.
Key words: adenosine, ATP, CD73, ecto-5′ nucleotidase, epinephrine, eye, regulation of dephosphorylation, retinal pigment epithelium, subretinal space
Introduction
The retinal pigment epithelium (RPE) is in close physical alignment with the outer segments of the retinal photoreceptors and performs a variety of roles to maintain the optimal photoreceptor function. The complex interaction between the two cell types requires a tightly-regulated neurochemical communication, to which the purines ATP and adenosine contribute. The apical membrane of the RPE, which faces sub-retinal space, contains a P2Y2 receptor for ATP [1]. Stimulation of this receptor enhances movement of fluid across the tissue and into the choroidal circulation [2], and agonists for this receptor can reduce retinal detachments by increasing the rate of fluid reabsorption from the subretinal space [3]. Stimulation of an A2 receptor for adenosine can decrease phagocytosis of rod outer segments by the RPE [4]. This phagocytosis is an essential component of photoreceptor outer segment turnover which ensures the optimal photoreceptor function. The endogenous source and regulation of subretinal purines could consequently impact both fluid movement and phagocytosis.
ATP is known to be released across the apical membrane of the RPE into subretinal space [5, 6] and recent evidence indicates the cystic fibrosis transmembrane conductance regulator (CFTR) is involved in this release under at least some circumstances [7]. However, the source of extracellular adenosine is currently unclear. In some tissues, extracellular adenosine arises from the actions of an adenosine transporter [8], but in many epithelial cells the majority of adenosine is produced by the consecutive dephosphorylation of ATP to ADP, AMP and adenosine by a series of extracellular enzymes [9, 10]. ATP can be converted into AMP and pyrophosphate by members of the ectonucleotide pyrophosphatase/phosphodiesterase (eNPP) family, while ecto-nucleoside triphosphate diphosphohydrolase (NTPDases) can mediate either the single dephosphorylation of ATP into ADP (NTPDase 2) or the double dephosphorylation of ATP into AMP (NTPDases 1 and 3) [11, 12]. Multiple members of both the NTPDase and eNPP families have been identified in RPE cells [13].
The dephosphorylation of AMP into adenosine by the RPE is less well understood. A previous electron microscopy study demonstrated that enzyme activity on the apical membrane of rat RPE could produce phosphate from exogenous 5′AMP [14]. However the molecular identification of the enzyme, its physiologic impact on subretinal adenosine levels and its regulation remain unknown. The present study examines ecto-5′ nucleotidase activity on the apical membrane of RPE cells and how the activity of this enzyme can be modified. In the first part, ecto-5-nucleotidase is identified on fresh and cultured RPE cells using pharmacological, molecular and histochemical approaches. The second part indicates enzyme activity can be modified by catecholamines acting at a1 adrenergic receptors on the apical membrane. As epinephrine may be released by the retina following illumination [15], the production of adenosine by ecto-5′ nucleotidase may provide a functional link between light and RPE function.
Materials and methods
All reagents were obtained from Sigma Chemical Corp. (St. Louis, MO) unless otherwise indicated.
Cell culture
The human ARPE-19 cell line [16] was obtained from the American Type Culture Collection (Manassas, VA) and grown in a 1:1 mixture of Dulbecco’s modified Eagles medium (DMEM) and Ham’s F12 medium with 3 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 2.5 mg/ml Fungizone (all Invitrogen Corp, Carlsbad, CA) and 10% fetal bovine serum (FBS, HyClone Laboratories, Inc., Logan, UT). Cells were incubated at 37°C in 5% CO2 and subcultured with 0.25% trypsin and 0.02% EDTA.
Bovine RPE eyecup preparation
Bovine eyes were bisected at the ora serrata and the retina removed as described previously [7, 13]. This bovine RPE eyecup preparation has the apical membrane facing the cup interior, with samples from the cup consequently reflecting activity on the apical membrane. After rinsing the eyecup, 5′AMP or ATP was added in a total volume of 1 ml. For adenosine measurements, isotonic solution composed of (in mM) 105 NaCl; 5 KCl; 6 HEPES Acid; 4 NaHEPES; 5 NaHCO3; 60 Mannitol; 5 Glucose; 0.5 MgCl2; 1.3 CaCl2, at pH 7.4. For phosphate measurements, a glucose-free 0.1 mM TRIS HCl at pH 7.5. Norepinephrine was included in some preparations. After 20 min, a 600 µl sample was removed and frozen at −20°C. Samples were assayed for adenosine or phosphate as indicated.
Adenosine measurements
High Performance Liquid Chromatography (HPLC) analysis was performed as previously published [13]. To control for biological and experimental variability between samples obtained on different days, the amount of adenosine was normalized to control levels for each trial.
Lead precipitate assay
Activity of 5′ nucleotidase was assayed based upon method of Schoen and Kreutzberg [17] in which inorganic phosphate released in the conversion of 5′AMP to adenosine reacts with lead nitrate to produce a lead phosphate precipitate. Adult albino Sprague-Dawley rats were anesthetized with intraperitonial injection of 100 mg/kg ketamine and 10 mg/kg xylazine followed by a larger lethal dose, then perfused with 4% paraformaldehyde. All treatment of animals conformed to the ARVO Statement for Use of Animals in Ophthalmic and Vision Research. Enucleated eyes were usually postfixed with 4% paraformaldehyde for 3 h, followed by immersion in 30% sucrose before embedding in OCT and rapid freezing. Cryostat sections were cut at 14 µM and briefly washed with 4% paraformaldehyde before beginning the 5′-nucleotidase assay. Sections were incubated for 30 min at 37°C in 80 mM Tris malate buffer, 0.12% lead nitrate, 10 mM MgSO4 and either 50 mg/ml 5′AMP or 50 mg/ml 2′AMP (137 mM). After washing, the reaction was developed by incubating the tissue sections in 20% ammonium sulfide for 5 min at room temperature. Slides were washed and visualized with an upright microscope (Nikon Eclipse 600, Nikon) and digital images obtained with a 3-CCD digital camera (Toshiba America, Irvine, CA) and Image Pro Plus software (Media Cybernetics, Silver Spring, MD). While similar amounts of lead precipitate were formed on fixed and unfixed sections, the retinal structure was better maintained with the fixed tissue.
Immunohistochemistry
Retinal sections from adult albino rats were processed as described above. After washing in PBS, tissue was incubated with mouse anti-CD73 antibody diluted 1:1,000 (Clone Ty-73, BD Pharmingen, San Jose, CA) in PBS with 10% Superblock (Pierce Biotechnology Inc. Rockford, IL.) in 0.1% Triton X-100 and 0.1% Tween-20 for 4 h at 25°C. After wash, tissues were incubated with biotinylated donkey anti-mouse (Jackson ImmunoResearch, West Grove, PA, 1:500 in PBS) for 2 h at 25°C, followed by Streptavadin-conjugated Cy3 fluorophore for 1 h at 25°C. A parallel incubation with IgG (1:500, Jackson ImmunoResearch) acted as a negative control. After washing, cells were mounted with a fluorescent mounting medium containing DAPI to visualize the nuclei (H-1200, Vector Labs, Burlingame, CA). Images were taken on a Nikon Eclipse 600 upright microscope equipped for epifluorescence with a 3-CCD digital camera and analyzed on line using Image Pro Plus software: DAPI was imaged with 360 nM excitation and >515 nM emission, while Cy3 was excited at 540 nm with emission >590 nm.
ARPE-19 cells were grown on Lab-Tek-II Chamber slides (Nalgene Nunc Int. Corp., Naperville IL) overnight using culture medium described above. Cells were fixed using warm 4% paraformaldehyde added to the medium in a 1:1 ratio for 2 min, then straight warm 4% paraformaldehyde for 30 min. Cells were washed 3 × 5 min in PBS, after which cells were incubated for 18 h at 4°C with a goat polyclonal antibody to ecto-5′ nucleotidase CD73 (# SC-14684, Santa Cruz Biotechnology, Santa Cruz, CA; 1:250 in PBS). Cells were incubated with biotinylated anti-goat antibody (Jackson ImmunoResearch, 1:1,000 in PBS) for 2 h at 25°C, washed and incubated in streptavodin-linked Cy3 (Jackson ImmunoResearch, 1:1,000 in PBS) for 1 h at 25°C. To ensure specificity of the antibody binding, two controls were performed. Omission of the primary antibody eliminated all staining, while pre-incubating with CD73 peptide (Santa Cruz Biotechnology), eliminated >80% of the staining (data not shown).
RT-PCR
RNA was extracted from ARPE-19 cells using the Trizol reagent (Invitrogen, Carlsbad, CA) RNA was treated with RQ1 RNAse-free DNAse I (Promega, Madison, WI.) Reverse transcription was performed from 1 µg of total RNA using the SuperScript First Strand Synthesis system (Invitrogen, Carlsbad, CA). Primers were obtained from a previously published paper [18]: Sense: 5′-CACCAAGGTTCAGCAGATCCGC- 3′, Antisense: 5′-GTTCATCAATGGGCGACCGG-3′, 1,006 basepair product. PCR was performed with 2 ml SuperScript product, 2mM MgCl2 and 0.4 nM of each primer, using 0.5 µl AmpliTaq Gold Polymerase system (Applied Biosystems, Foster City, CA.) for 1 min 95°C, 1 min 55°C, 2 min 72°C, 35 cycles, with a final extension step of 20 min 72°C. SuperScript was omitted from the negative control. The band was visualized on a 2% agarose gel, photographed, and purifed from a 2% LMP agarose gel using the QIAEX II Gel Extraction kit (QIAGEN, Valencia, CA.) Cloning was performed using a TOPO TA cloning kit with Top 10 F′ cells (Invitrogen, Carlsbad, CA,) and purified using the Wizard Plus Minipreps kit (Promega, Madison, WI.) The DNA was sequenced at the University of Pennsylvania Cell Center Sequencing Facility. The resulting sequence was identified using BLAST nucleotide database (http://www.ncbi.nlm.nih.gov).
Measurement of phosphate production
Phosphate production was determined using the PiPer Phosphate Assay Kit in basic accordance with the manufacturer’s instructions (Molecular Probes/Invitrogen, Eugene, OR). A standard curve constructed with 0 to 400 µM Pi from 20 µM increments indicated that absorbance at 565 nm provided an accurate measure of Pi present in the bath. ARPE-19 cells were grown to confluence in 24 well dishes, washed twice with 0.1 mM TRIS HCl pH 7.5, and incubated with working solution (control), or solution including AMP with or without drugs. All experiments were performed for 30 min at 37°C according to the manufacturer’s protocol except where indicated in Figure 5. α1-adrenergic antagonists were present 30 min before and during the reaction. To test the effect of temperature, all solutions and plates were precooled to 4°C for 30 min before the experiment began, with reaction itself also occurring at the appropriate temperature. After all incubations, 0.3 ml was drawn from each well for analysis. Absorbance of each sample was read at 565 nm using a spectrophotometer (Milton Roy, Ivyland, PA). In experiments involving levamisole, the phosphate signal was assessed on cells grown in 96 well plates with a Fluroskan Ascent fluorimeter (ThermoElectron, Milford, MA). All drugs were tested on the assay in the absence of cells and found to have no effect.
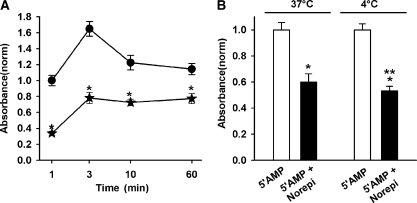
Figure 5.
Time and temperature dependence of inhibition. (A) Norepinephrine reduced phosphate production by >60% within the first minute of the reaction, with the reduction remaining throughout 60 min. Circles are 5′AMP alone and stars include 10 µM norepinephrine. *p < 0.05 vs 5′AMP for a given time point, n = 10–12. (B) The decrease in activity associated with 10 µM norepinephrine detected at the usual reaction temperature of 37°C was not affected by performing the reaction at 4°C. *p < 0.05 vs. 5′AMP alone, **not significantly different from Nor at 37°C, n = 14–16.
Data analysis and materials
Data are expressed as means ± SEM. Significance was evaluated using a one-way ANOVA with Tukey post test where more than two variables were present or an unpaired Student’s t test when only two variables were present. Significance was defined as p< 0.05.
Phosphate production was quantified by normalizing data to the mean value for control records (50AMP alone). The percent (%) block of enzyme activity was defined as:
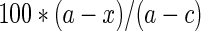 |
where a is the mean absorbance at 565 nm in the presence of 5′AMP, c is the mean absorbance in the absence of 5′AMP and x is each absorbance in the presence of 5′AMP + blocking compound after correction for background levels without added 50AMP. Phenylephrine concentration-response curve was fit with an exponential decay function
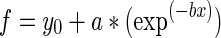 |
using the least squares method of Sigmaplot software (SPSS Inc, Chicago, Il) after subtracting control values before normalization to the mean level of 5′AMP alone of each day.
Results
Identification of ecto-5′ nucleotidase on fresh and cultured RPE cells
Initial experiments were performed to determine if RPE cells dephosphorylated AMP to adenosine and to identify the enzyme responsible for this conversion. First, a lead precipitate assay was performed on sections from the albino rat retina. Excess 5′AMP was used as a substrate to determine regions of ecto-5′ nucleotidase activity. Addition of 5′AMP to the reaction resulted in intense precipitate banding at the level of the RPE/outer segments (Figure 1A). Activity was considerably higher in the RPE layer than in any other tissue of the retina, although lighter precipitate was also detected at the level of the inner segments. The use of 2′AMP as a substrate, instead of 5′AMP, eliminated any reaction product.
Figure 1.
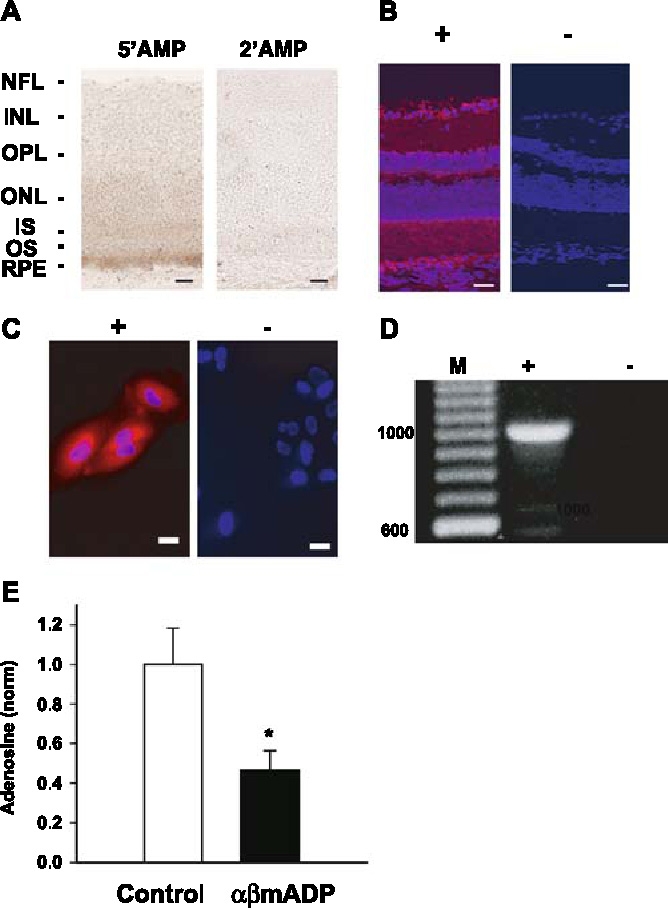
Identification of ecto-5′ nucleotidase on the RPE. (A) Sections of albino rat retina incubated with 5′AMP show an intense lead phosphate precipitate at the level of the RPE, indicating 5′ nucleotidase activity is concentrated there (left panel ). No reaction product was detected when 2′AMP was used as a substrate (right panel). NFL nerve fiber layer, INL inner nuclear layer, OPL outer plexiform layer, ONL outer nuclear layer, IS inner segments, OS outer segments, RPE retinal pigment epithelium. Scale bars = 25 µM. (B) Immunohistochemical staining localizes the ecto-5′ nucleotidase antigen in RPE layer in panel ‘+’; In panel ‘−’, IgG was used instead of the specific antibody as a control. Blue indicates nuclei stained with DAPI and confirms the identification of retinal layers. Scale bars = 50 µM. (C) Ecto-5′ nucleotidase antigen is also present on cultured human ARPE-19 cells. Panel ‘+’ indicates positive control while panel ‘−’ indicates absence of primary antibody. Scale bars = 25 µM. (D) RT-PCR reaction with ecto-5′ nucleotidase (CD73) primers produces band of predicted 1,006 bp size on RNA obtained from ARPE- 19 cells in lane ‘+’. No band was seen when reverse transcriptase was omitted from the reaction (lane ‘−’). Lane ‘M’ shows size markers. (E) The production of adenosine by the apical membrane of the fresh bovine RPE eyecup from ATP was inhibited by 100 µM ecto-5′ nucleotidase inhibitor αβmADP. Levels were normalized to the mean control values of area under the HPLC curve for each experimental set. *p = 0.018, n = 8–9.
The presence of ecto-5′ nucleotidase in the RPE was confirmed immunohistochemically. Bright staining was detected in the RPE layer, with more diffuse localization throughout the retina including the nerve fiber layer (Figure 1B). Counter staining of cell nuclei with DAPI allowed identification of retinal layers and emphasized that these layers were normal, displaying no sign of damage that can affect albino animals. Cultured human ARPE-19 cells also stained positively for the enzyme (Figure 1C), while RT-PCR on RNA from ARPE-19 cells confirmed the identification of ecto-5′ nucleotidase (CD73) on a molecular level. Primers for CD73 produced an intense band of predicted 1,006 base pairs (Figure 1D). The sequenced product showed >99% similarity with published CD73 sequences (accession # NM_002526, corresponding to ecto-5′-nucleotidase).
The polarity of enzyme activity was further examined using the bovine RPE-eyecup. In this preparation, the apical membrane of the eyecup is in contact with the solution. As the tight junctions of the RPE monolayers were intact, this likely reflects activity on the apical membrane [7]. The ability of extracellular enzymes to convert ATP to adenosine was measured directly by adding 100 nM ATP to the inside of the bovine RPE eyecup and measuring the adenosine levels with HPLC. The contribution of ecto-5′ nucleotidase to this conversion was determined with the compound αβmADP, an effective inhibitor of 5′ nucleotidase at physiologic pH [19]. Extracellular adenosine levels were reduced >50% by incubation with 100 µM αβmADP (Figure 1E). This is consistent with conversion of the 5′AMP to adenosine by ecto-5′ nucleotidase on the apical membrane of bovine RPE cells.
Production of phosphate
Phosphate production was measured with the PiPer phosphate assay in the presence of excess 5′AMP. Baseline phosphate levels were 30.8 ± 1.4 µM and rose to 99.1 ± 6.6 µM (n = 7) after a standard 30 min incubation with 100 µM 5′AMP, although the relative rise did vary somewhat in different trials. As phosphate can be produced by extracellular enzymes in addition to ecto-5′ nucleotidase, several controls were performed to ensure specificity of the reaction. Phosphate levels remained near baseline when cells were incubated with 2′AMP, suggesting this nucleotide was a negligible source of phosphate (Figure 2A). The production of phosphate from 5′AMP was also eliminated by 100 µM αβmADP (Figure 2B). However, the alkaline phosphatase inhibitor levamisole (10 µM) had no effect on phosphate production (Figure 2C). These results are consistent with activity of ecto-5′ nucleotidase, and support the predominant role of ecto-5′ nucleotidase in the production of phosphate from 5′AMP in these cells.
Figure 2.
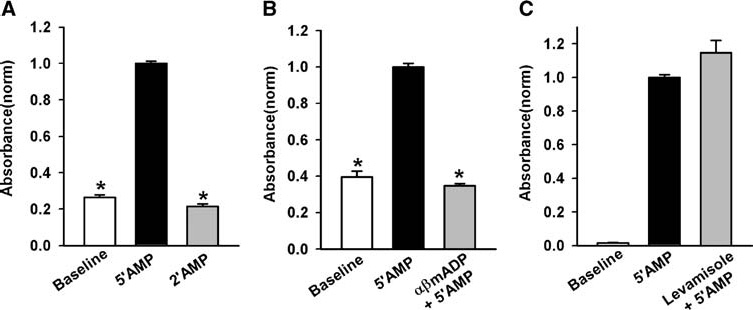
Verification of phosphate assay measured ecto-5′ nucleotidase activity. (A) Intact ARPE-19 cells produce considerably more Pi, as detected by absorbance of resorufin, when bathed with 5′AMP than 2′AMP when both nucleotides were used at 100 µM. Here and throughout the study, data are normalized to the mean absorbance value for 50AMP for each trail. Baseline represents background levels without added substrate. All compounds had no effect on the assay itself. *p < 0.05 compared to 5′AMP, 2′AMP vs. control not significant (NS), n = 4. (B) The production of Pi by intact ARPE-19 cells is completely blocked by the ecto-5′ nucleotidase inhibitor αβmADP (100 µM). Although cells from both 5′AMP and αβmADP populations were bathed in 100 µM 5′AMP, the inhibitor prevented the production of Pi. *p < 0.05 vs. 5′AMP, αβmADP vs. control NS, n = 3. (C) The alkaline phosphatase inhibitor levamisole (10 mM) had no effect on phosphate production in the presence of 100 µM 5′AMP. *p < 0.05 vs. 5′AMP, n = 12–20.
Regulation of ARPE-19 cells ecto-5′ nucleotidase activity by norepinephrine
Indirect evidence from studies on manganese-dependent pyrimidine 5′ nucleotidase suggests enzyme activity in the RPE may be decreased upon onset of light [20]. Numerous changes accompany the transition from dark to light, including the release of epinephrine from the retina at light onset [15]. We thus asked whether catecholamines could reduce the activity of the ecto-50 nucleotidase present on the RPE.
Addition of 10 µM norepinephrine to the assay mixture bathing ARPE-19 cells reduced the extracellular production of phosphate by 51%, corresponding to a decrease of 80% when baseline Pi levels in the absence of added 5′AMP were subtracted (n = 10–13, Figure 3A). Additional experiments were performed to verify the ability of norepinephrine to decrease ecto-5′ nucleotidase activity. ATP was added to the solution bathing the ARPE-19 cells and levels of adenosine and AMP present in the bath 20 min later were determined by HPLC. Inclusion of 10 µM norepinephrine was found to decrease adenosine levels by 50% (Figure 3B). Levels of AMP increased by 41%; although this increase was not significant, the parallel decrease in adenosine and increase in 5′AMP levels is consistent with a decline in catalyzing the dephosphorylation of AMP into adenosine by ecto-5′ nucleotidase.
Figure 3.
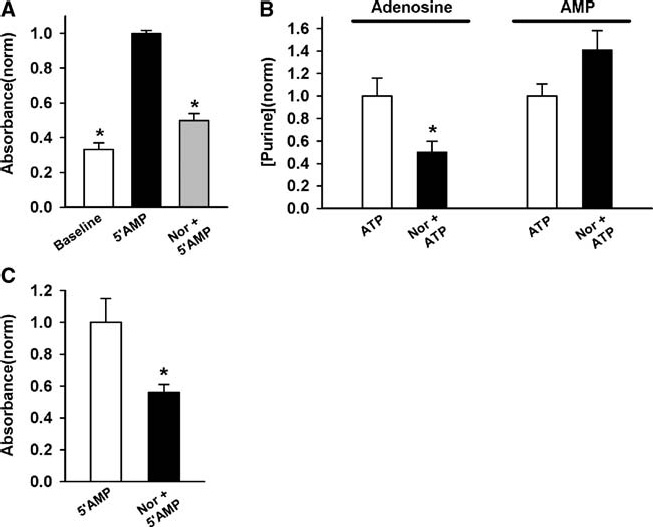
Norepinephrine inhibits ecto-5′ nucleotidase activity of RPE cells. (A) Norepinephrine (Nor + 5′AMP, 10 and 100 µM, respectively) reduced conversion of 5′AMP to Pi. Baseline values were obtained without added 5′AMP. *p < 0.0001 vs. 5′AMP alone, n = 12–13. (B) When 100 µM ATP was added to the bath surrounding ARPE-19 cells, 10 µM norepinephrine reduced the amount of adenosine present 20 min later as determined with HPLC analysis (*p = 0.039). The levels of AMP in the bath showed a small but insignificant rise, consistent with an inhibition of ecto-5′ nucleotidase (p = 0.095); n = 4. (C) Levels of Pi produced from 100 µM 5′AMP by the apical membrane of the fresh bovine RPE eyecup were also reduced by 10 µM norepinephrine (Nor + 5′AMP) *p = 0.017, n = 8–9.
Regulation of activity on fresh bovine RPE by norepinephrine
While the experiments above provide strong evidence that catecholamines down-regulate ecto-5′ nucleotidase activity on ARPE-19 cells, the regulation of the enzyme on fresh RPE cells was investigated. As mentioned, the fresh bovine RPE eyecup preparation allows activity to be measured specifically from the apical membrane of RPE cells, providing potential information about adenosine production in sub-retinal space. Experiments were repeated in the fresh bovine RPE eyecup. The production of phosphate was reduced 44% by norepinephrine (Figure 3C). This suggests that stimulation of adrenergic receptors on the apical membrane of fresh RPE can decrease activity of ecto-5′ nucleotidase on the same membrane.
Decrease in activity mediated by a1-adrenergic receptor
The receptor responsible for the effects of norepinephrine on ecto-5′ nucleotidase activity was determined pharmacologically. Epinephrine reduced phosphate production from 5′AMP, reducing levels by 87 ± 3% over baseline (n = 6, Figure 4A). The a1-adrenergic agonist phenylephrine also reduced the production of phosphate; the inhibition was dose-dependent over the range tested (Figure 4B). To confirm a role for the a1 receptor the effect of two different antagonists were examined. At 10 µM, prazosin completely prevented the effects of norepinephrine (Figure 4C). The antagonist corynanthine also produced a significant inhibition, although 10 µM of this antagonist led to a partial inhibition (Figure 4D). To rule out contributions from b-adrenergic receptors linked to Gs, the effect of raising intracellular cAMP with a cocktail was examined. The cocktail consisted of 10 µM forskolin, 100 µM 3-isobutyl-1-methylxanthine (IBMX) and 500 µM 8-(4-chlorophenylthio (cpt)) cAMP, and has previously used to produce changes in RPE cells indicative of an elevation in intracellular cAMP [7]. However, the cocktail had no effect on phosphate production (Figure 4E).
Figure 4.
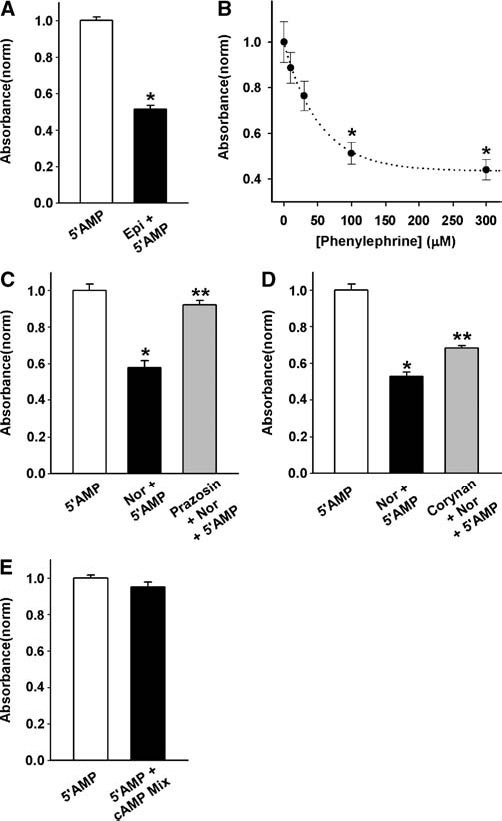
Inhibition of ecto-5′ nucleotidase activity mediated by aladrenergic receptor. (A) Epinephrine reduced the 5′AMP-derived production of Pi from ARPE-19 cells. In the presence of 100 µM 5′AMP (5′AMP), Pi production was inhibited by co-incubation with of 3 µM epinephrine (5′AMP + Epi) p < 0.0001 vs. 5′AMP alone, n = 6. (B) The α1 agonist phenylephrine inhibited production of Pi from 5′AMP by ARPE- 19 cells in a dose dependent manner. The dotted line is an exponential fit to the data, with r2 = 0.99, Y0 = 0.435, a = 0.560 and b = 0.019 (see Materials and methods). *p < 0.05 vs. 5′AMP alone, n = 6–11. (C) The inhibition produced by 10 µM norepinephrine was prevented by 10 µM of the α1 adrenergic antagonist prazosin. Levels with prazosin were not significantly different from 5′AMP alone. *p < 0.001 vs. 5′AMP, **p < 0.001 vs. 5′AMP + norepinephrine, n = 6. (D) The α1 adrenergic antagonist corynanthine (corynan, 10 µM) also blocked the effect of norepinephrine. *p < 0.001 vs. 5′AMP, **p < 0.05 vs. 5′AMP + norepinephrine, n = 7–8. (E) A mixture of 100 µM IBMX, 10 µM forskolin and 500 µM cpt cAMP had no significant effect on phosphate production in the presence of 5′AMP, n = 9–10.
Mechanism of catecholamine block
Ecto-5′ nucleotidase is linked to the membrane by a glycosylphosphatidylinositol (GPI) anchor, and activity is frequently regulated by changing the amount of enzyme present on the membrane surface. As an initial investigation into the mechanisms underlying the drop in enzyme activity with catecholamines, the effects of temperature and time on the reduction of phosphate production were examined.
Norepinephrine led to a very rapid block of ecto-5′ nucleotidase activity. Only 1 min after addition of substrates, phosphate production was reduced by >60% when compared to the production under baseline conditions (Figure 5A). This reduction was sustained for the 60 min that measurements were made, although the effective block became proportionally less with time. The rapid nature of the block argues against a role for transcription or translation in the decrease. The reduction in phosphate production was not affected by performing the reaction at 4°C as opposed to the normal 37°C (Figure 5B). This implies that changes in the rate of enzyme internalization or trafficking are not responsible for the decreased activity.
Discussion
The present study has functionally identified the enzyme ecto-5′ nucleotidase on RPE cells. Enzyme activity was localized to the apical membrane of bovine RPE cells, the antigen was detected on fresh rat RPE cells and ARPE-19 cultured human RPE cells, while mRNA message and protein for the enzyme was found in ARPE-19 cells. The production of phosphate by ARPE-19 cells was greatly enhanced in the presence of 5′AMP, and this production was prevented by αβmADP. Phosphate production by ARPE-19 cells was also inhibited by epinephrine, norepinephrine and phenylephrine. Norepinephrine decreased the amount of adenosine bathing ARPE-19 cells, and reduced the production of phosphate from 5′AMP by the apical membrane of bovine RPE cells. The effect of norepinephrine on phosphate production from 5′AMPwas evident within 1 min and was prevented by a1 adrenergic antagonists, but reducing the temperature did not alter the block. Together these findings suggest ecto-5′ nucleotidase on the apical membrane of RPE cells produces adenosine, and this activity is reduced by stimulating α1 adrenergic receptors.
Enzyme identification
Several lines of evidence support our conclusion that ecto-50′ nucleotidase is the enzyme responsible for the production of phosphate and adenosine on the apical membrane of RPE cells. RT-PCR experiments indicate mRNA message for ecto-5′ nucleotidase is present in these cells. The ecto-5′ nucleotidase inhibitor αβmADP blocked >50% of adenosine production, and 98% of the phosphate production in the presence of 5′AMP. As αβmADP has no effect on cytoplasmic form of the enzyme [21], this block is consistent with ecto-5′ nucleotidase activity. The lack of phosphate production after incubation with 2′AMP relative to 5′AMP agrees with the substrate preference reported for ecto-5′ nucleotidase [22]. A study on airway epithelial cells indicated that both ecto-5′ nucleotidase and non-specific alkaline phosphatase were responsible for the degradation of 5′AMP, with alkaline phosphatase contributing 3 fold more at a substrate concentration of 100 µM [10]. However the alkaline phosphatase inhibitor levamisole did not alter phosphate production from 5′AMP in RPE cells. This is consistent with large block of phosphate production by αβmADP, and with recent data indicating levamisole did not alter the rate of ATP degradation in these cells [13]. This suggests the contribution of alkaline phosphatases to nucleotide degradation in ARPE-19 cells is minimal.
These findings support previous studies demonstrating that the outer retina contains a considerable amount of 5′ nucleotidase activity. Close inspection of the lead precipitate found by Kreuzberg and Hussain on albino mice shows the darkest staining at the proximal end of the outer segments [23]. While this staining was interpreted as evidence for activity on Müller cells, the pattern is consistent with activity on the apical membrane of the RPE. An immunohistochemical study of adult albino rat retina also displayed considerable fluorescence at the RPE/outer segment region, although staining at the Müller cells microvilli was more intense, particularly at the tips [24]. This difference may represent a distinction between maximal enzyme levels and maximal activity levels in the retina. However, all reports do stress the importance of the enzyme in the outer retinal region, and emphasize the potential for both P2 and P1 receptors in signaling throughout the outer retina.
Mechanism for ecto-5′ nucleotidase regulation
Ecto-5′ nucleotidase is linked to the plasma membrane by a GPI anchor and activity is typically regulated by modifying enzyme expression on the membrane. Intracellular pools of enzyme are cycled to the cell surface and either endocytosed to reenter the cycle or cleaved off the anchor [25]. Modulation of enzyme activity has been linked to changes in surface expression, with lovastatin reducing the rate of endocytosis by interfering with Rho-GTPases, and hypoxia reducing endocytosis by increasing the proportion of saturated fatty acids in the membrane [26, 27]. However, endocytosis is temperature dependent; as lowering the temperature from 37 to 4°C had no effect on ability of norepinephrine to decrease activity in the present study, this decrease is unlikely to result from a change in enzyme trafficking. The speed of the change in RPE cell activity shown above also makes transcriptional or translational modulation unlikely to play a role. Adenosine increases activity of ecto-5′ nucleotidase through enhanced transcription, but these changes take place over many hours [28]. Likewise, elevation of cAMP increased the synthesis of ecto-5′ nucleotidase, but at least 12 h was necessary to detect any increase [29], consistent with our inability to detect an effect of the cAMP cocktail over 30 min.
In the heart, stimulation of the a1-adrenergic receptor enhances activity of ecto-5′ nucleotidase by activating PKC [30, 31]. While this is the opposite of what we have found in the present study, this likely reflects a real difference as we observed this decline with three different agonists, blocked it with two antagonists, corroborated it with changes in adenosine levels and found this decrease in both cultured human and fresh bovine RPE cells. This discrepancy may be explained by a difference in mechanism. The enhancement of activity in cardiac cells took at least 15–30 min after presentation of norepinephrine, and is hypothesized to involve phosphorylation of ecto-5′ nucleotidase from inside the membrane. The rapid time scale of our changes suggest the enzyme is instead sheared off the membrane of RPE cells. Stimulation of the a1 adrenergic receptor can activate phospholipase C (PLC) and activation of phospholipase C can cleave ecto-5′ nucleotidase from the membrane [32]. While the activity of the cleaved enzyme generally increases, this increase is dependent upon the lipid constituents of the membrane [32], and a decrease in ecto-5′ nucleotidase activity was found after cleavage from endothelial cells by TNF-α [33]. As the space between the apical membrane of the RPE and the photoreceptor outer segments is filled with a complex protein scaffolding [34], ecto-5′ nucleotidase cleaved off the RPE cells could subsequently bind to a site on this interphotoreceptor matrix and modulate signaling closer to the outer segments. While our investigations do not enable us to precisely define the mechanism responsible for the decrease on RPE cells, they do emphasize that a particular receptor may have multiple effects on ecto-5′ nucleotidase activity, reflecting the many modes for enzyme regulation that are now apparent.
Physiologic implications
The inhibition of ecto-5′ nucleotidase activity following stimulation of the a1 adrenergic receptor links observations by several investigators. Epinephrine is released from the neural retina during the light [15]. Our findings here indicate that epinephrine can reduce ecto-5′ nucleotidase activity, potentially leading to a reduction in adenosine production at light onset. Rod outer segments are shed by the photoreceptors and phagocytozed by the RPE, with peak activity levels at the onset of light [35]. The process of disc shedding and phagocytosis is complex, and the predominant temporal regulator is the circadian shedding of tips. However the rate of phagocytosis itself can be altered, and stimulation of A2 adenosine receptors in the RPE membrane can decrease this rate [4]. The light-dependent release of epinephrine would lead to less ecto-5′ nucleotidase activity and thus less adenosine available to stimulate these receptors, enabling an increase in phagocytosis shortly after light onset. This is supported by studies on pyrimidine 5′ nucleotidase where enzyme activity declined after light onset [20]. Although the enzyme preferentially used pyrimidines as a substrate under acid conditions, 5′AMP was used as a substrate at physiologic pH [36], suggesting the regulated enzyme activity was ecto-5′ nucleotidase. Our findings are consistent with epinephrine causing this decrease in enzyme activity when lights go on and imply that the activity of ecto-5′ nucleotidase may modulate the neurochemical communication between the retinal and RPE.
In addition to altering the light-dependent interactions of the RPE and photoreceptors, the extracellular production of adenosine may have implications for the ability of ATP to trigger signaling in RPE cells. Recently it was suggested that ATP and adenosine act synergistically to raise intracellular Ca2+ levels in RPE cells, enabling low levels of ATP to trigger cellular responses [37]. As the elevation of Ca2+ by ATP in these cells can lead to enhanced fluid movement [2], this synergism may be key component to RPE physiology. It remains to be seen whether epinephrine can reduce Ca2+ signaling by reducing the availability of adenosine available for this synergism.
Acknowledgement
The authors would like to thank Tatyana Shekherman, Tim Baradet and Constantin Friedman for technical assistance, and Alice McGlinn for advice with the molecular biology. This work was supported by grants from the NIH EY013434 (CHM), EY10009 (ALM), EY07354 and EY001583 (RAS), A Core Vision Grant to the University of Pennsylvania from the NIH (EY001583), Research to Prevent Blindness (RAS), the Paul and Evanina Bell Mackall Foundation Trust (RAS), and grant 304718503, from the Chinese National Scientific Research Fund. Portions of this work have been presented previously in abstract form [38, 39].
References
- 1.Sullivan DM, Erb L, Anglade E et al. Identification and characterization of P2Y2 nucleotide receptors in human retinal pigment epithelial cells. J Neurosci Res 1997; 49(1): 43–2. [DOI] [PubMed]
- 2.Peterson WM, Meggyesy C, Yu K, Miller SS. Extracellular ATP activates calcium signaling, ion, and fluid transport in retinal pigment epithelium. J Neurosci 1997; 17(7): 2324–7. [DOI] [PMC free article] [PubMed]
- 3.Maminishkis A, Jalickee S, Blaug SA et al. The P2Y(2) receptor agonist INS37217 stimulates RPE fluid transport in vitro and retinal reattachment in rat. Investig Ophthalmol Vis Sci 2002; 43(11): 3555–6. [PubMed]
- 4.Gregory CY, Abrams TA, Hall MO. Stimulation of A2 adenosine receptors inhibits the ingestion of photoreceptor outer segments by retinal pigment epithelium. Investig Ophthalmol Vis Sci 1994; 35(3): 819–5. [PubMed]
- 5.Mitchell CH. Release of ATP by a human retinal pigment epithelial cell line: Potential for autocrine stimulation through subretinal space. J Physiol 2001; 534(1): 193–02. [DOI] [PMC free article] [PubMed]
- 6.Eldred JA, Sanderson J, Wormstone M et al. Stress-induced ATP release from and growth modulation of human lens and retinal pigment epithelial cells. Biochem Soc Trans 2003; 31(6): 1213–5. [DOI] [PubMed]
- 7.Reigada D, Mitchell CH. Release of ATP from RPE cells involves both CFTR and vesicular transport. Am J Physiol, Cell Physiol 2005; 288(1): C132–0. [DOI] [PubMed]
- 8.Thorn JA, Jarvis SM. Adenosine transporters. Gen Pharmacol 1996; 27(4): 613–0. [DOI] [PubMed]
- 9.Lynge J, Juel C, Hellsten Y. Extracellular formation and uptake of adenosine during skeletal muscle contraction in the rat: Role of adenosine transporters. J Physiol 2001; 537(2): 597–05. [DOI] [PMC free article] [PubMed]
- 10.Picher M, Burch LH, Hirsh AJ et al. Ecto 5′-nucleotidase and nonspecific alkaline phosphatase. Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J Biol Chem 2003; 278(15): 13468–9. [DOI] [PubMed]
- 11.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg’s Arch Pharmacol 2000; 362(4–): 299–09. [DOI] [PubMed]
- 12.Zimmermann H. Ecto-nucleotidases. In Williams M, Abbracchio MP (eds): Purinergic and Pyraminergic Signalling: I. Molecular, Nervous and Urogenitary System Function. Berlin Heidelberg New York: Springer 2001; 209–0.
- 13.Reigada D, Lu W, Zhang X et al. Degradation of extracellular ATP by the retinal pigment epithelium. Am J Physiol, Cell Physiol 2005; 289(1): C617–4. [DOI] [PubMed]
- 14.Takizawa T. 5′-nucleotidase in rat photoreceptor cells and pigment epithelial cells processed by rapid-freezing enzyme cytochemistry. J Histochem Cytochem 1998; 46(9): 1091–. [DOI] [PubMed]
- 15.Hadjiconstantinou M, Cohen J, Neff NH. Epinephrine: A potential neurotransmitter in retina. J Neurochem 1983; 41(5): 1440–. [DOI] [PubMed]
- 16.Dunn KC, Marmorstein AD, Bonilha VL et al. Use of the ARPE-19 cell line as a model of RPE polarity: Basolateral secretion of FGF5. Investig Ophthalmol Vis Sci 1998; 39(13): 2744–. [PubMed]
- 17.Schoen SW, Kreutzberg GW. 5′-nucleotidase enzyme cytochemistry as a tool for revealing activated glial cells and malleable synapses in CNS development and regeneration. Brain Res Brain Res Prot 1997; 1(1): 33–3. [DOI] [PubMed]
- 18.Berchtold S, Ogilvie AL, Bogdan C et al. Human monocyte derived dendritic cells express functional P2X and P2Y receptors as well as ecto-nucleotidases. FEBS Letts 1999; 458(3): 424–. [DOI] [PubMed]
- 19.Naito Y, Lowenstein JM. 5′-nucleotidase from rat heart membranes. Inhibition by adenine nucleotides and related compounds. Biochem J 1985; 226(3): 645–1. [DOI] [PMC free article] [PubMed]
- 20.Irons MJ. Redistribution of Mn++-dependent pyrimidine 5′-nucleotidase (MDPNase) activity during shedding and phagocytosis. Investig Ophthalmol Vis Sci 1987; 28(1): 83–1. [PubMed]
- 21.Le Hir M, Kaissling B. Distribution and regulation of renal ecto-5′-nucleotidase: Implications for physiological functions of adenosine. Am J Physiol 1993; 264: F377–7. [DOI] [PubMed]
- 22.Zimmermann H. 5′-Nucleotidase: Molecular structure and functional aspects. Biochem J 1992; 285(2): 345–5. [DOI] [PMC free article] [PubMed]
- 23.Kreutzberg GW, Hussain ST. Cytochemical heterogeneity of the glial plasma membrane: 5′-nucleotidase in retinal Muller cells. J Neurocytol 1982; 11(1): 53–4. [DOI] [PubMed]
- 24.Braun N, Brendel P, Zimmermann H. Distribution of 5′-nucleotidase in the developing mouse retina. Brain Res Dev Brain Res 1995; 88(1): 79–6. [DOI] [PubMed]
- 25.Widnell CC, Schneider YJ, Pierre B et al. Evidence for a continual exchange of 5′-nucleotidase between the cell surface and cytoplasmic membranes in cultured rat fibroblasts. Cell 1982; 28(1): 61–0. [DOI] [PubMed]
- 26.Ledoux S, Laouari D, Essig M et al. Lovastatin enhances ecto-5′-nucleotidase activity and cell surface expression in endothelial cells:Implication of rho-family GTPases. Circ Res 2002; 90(4): 420–. [DOI] [PubMed]
- 27.Ledoux S, Runembert I, Koumanov K et al. Hypoxia enhances ecto-5′-nucleotidase activity and cell surface expression in endothelial cells: Role of membrane lipids. Circ Res 2003; 92(8): 848–5. [DOI] [PubMed]
- 28.Narravula S, Lennon PF, Mueller BU, Colgan SP. Regulation of endothelial CD73 by adenosine: Paracrine pathway for enhanced endothelial barrier function. J Immunol 2000; 165(9): 5262–. [DOI] [PubMed]
- 29.Savic V, Blanchard A, Vlahovic P et al. Cyclic adenosine monophosphate-stimulating agents induce ecto-5′-nucleotidase activity and inhibit DNA synthesis in rat cultured mesangial cells. Arch Biochem Biophys 1991; 290(1): 202–. [DOI] [PubMed]
- 30.Sato T, Obata T, Yamanaka Y, Arita M. Stimulation of alpha 1-adrenoceptors and protein kinase C-mediated activation of ecto-5′-nucleotidase in rat hearts in vivo. J Physiol 1997; 503(1): 119–7. [DOI] [PMC free article] [PubMed]
- 31.Obata T, Kubota S, Yamanaka Y. Histamine increases interstitial adenosine concentration via activation of ecto-5′-nucleotidase in rat hearts in vivo. J Pharmacol Exp Ther 2001; 298(1): 71–. [PubMed]
- 32.Lehto MT, Sharom FJ. Release of the glycosylphosphatidylinositol-anchored enzyme ecto-5′-nucleotidase by phospholipase C: Catalytic activation and modulation by the lipid bilayer. Biochem J 1998; 332(1): 101–. [DOI] [PMC free article] [PubMed]
- 33.Kalsi K, Lawson C, Dominguez M et al. Regulation of ecto-5′-nucleotidase by TNF-alpha in human endothelial cells. Mol Cell Biochem 2002; 232(1–): 113–. [DOI] [PubMed]
- 34.Hageman GS, Kuehn MH. Biology of the interphotoreceptor matrix–retinal pigment epithelium–retina interface. In Marmor M, Wolfensberger TJ (eds): The Retinal Pigment Epithelium. New York: Oxford University Press 1998; 361–1.
- 35.LaVail MM. Rod outer segment disc shedding in relation to cyclic lighting. Exp Eye Res 1976; 23(2): 277–0. [DOI] [PubMed]
- 36.Irons MJ, O’Brien PJ. Biochemical evidence for Mn2+-dependent 5′-nucleotidase activity in isolated rod outer segments. Exp Eye Res 1987; 45(6): 813–1. [DOI] [PubMed]
- 37.Collison DJ, Tovell VE, Coombes LJ et al. Potentiation of ATP-induced Ca2+ mobilisation in human retinal pigment epithelial cells. Exp Eye Res 2005; 80(4): 465–5. [DOI] [PubMed]
- 38.Friedman C, Zhang X, Laties AM, Mitchell CH. Ecto-nucleotidases on RPE cells: A potential source of extracellular adenosine. Investig Ophthalmol Vis Sci 2002; 43: U197 (abs).
- 39.Mitchell CH, Zhang X, Pendrak K, Stone RA. Ecto ATDPase and ecto-5′ nucleotidase activity on the apical membrane of the RPE. Investig Ophthalmol Vis Sci 2003; 44: U383 (abs).