Abstract
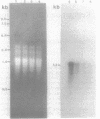
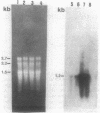
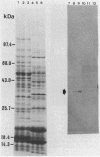
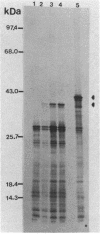
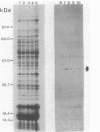
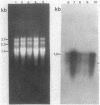
The expression of the cyanobacterial recA gene, isolated from Anabaena variabilis, has been examined at the levels of transcript and protein abundance. Exposure of the cyanobacterium to a variety of DNA-damaging agents, including mitomycin C, methyl methanesulfonate, and UV irradiation, results in a rapid increase in the abundance of the recA transcript above basal levels as determined by Northern (RNA) blot analysis. A concomitant increase in the abundance of a 37- to 38-kilodalton polypeptide was also detected by Western (immuno-) blot analysis of soluble cyanobacterial polypeptides using polyclonal antiserum directed against the Escherichia coli recA protein. The cyanobacterial polypeptide is of the same molecular mass as that synthesized by an in vitro, DNA-directed procaryotic transcription-translation system primed with an A. variabilis genomic fragment containing the recA gene. Nucleotide sequence analysis of the cyanobacterial gene revealed a protein of 358 amino acids with a molecular weight of 38,403 daltons. The A. variabilis and E. coli recA genes share similarity at 58% of the amino acid residues; however, an E. coli-like lexA repressor-binding site is not present in the A. variabilis promoter region. The similarities of A. variabilis and E. coli recA expression and gene sequence are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dzelzkalns V. A., Bogorad L. Stable transformation of the cyanobacterium Synechocystis sp. PCC 6803 induced by UV irradiation. J Bacteriol. 1986 Mar;165(3):964–971. doi: 10.1128/jb.165.3.964-971.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Geoghegan C. M., Houghton J. A. Molecular cloning and isolation of a cyanobacterial gene which increases the UV and methyl methanesulphonate survival of recA strains of Escherichia coli K12. J Gen Microbiol. 1987 Jan;133(1):119–126. doi: 10.1099/00221287-133-1-119. [DOI] [PubMed] [Google Scholar]
- Goldberg I., Mekalanos J. J. Cloning of the Vibrio cholerae recA gene and construction of a Vibrio cholerae recA mutant. J Bacteriol. 1986 Mar;165(3):715–722. doi: 10.1128/jb.165.3.715-722.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Horn J. M., Ohman D. E. Autogenous regulation and kinetics of induction of Pseudomonas aeruginosa recA transcription as analyzed with operon fusions. J Bacteriol. 1988 Oct;170(10):4699–4705. doi: 10.1128/jb.170.10.4699-4705.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn J. M., Ohman D. E. Transcriptional and translational analyses of recA mutant alleles in Pseudomonas aeruginosa. J Bacteriol. 1988 Apr;170(4):1637–1650. doi: 10.1128/jb.170.4.1637-1650.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keener S. L., McNamee K. P., McEntee K. Cloning and characterization of recA genes froM Proteus vulgaris, Erwinia carotovora, Shigella flexneri, and Escherichia coli B/r. J Bacteriol. 1984 Oct;160(1):153–160. doi: 10.1128/jb.160.1.153-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokjohn T. A., Miller R. V. Molecular cloning and characterization of the recA gene of Pseudomonas aeruginosa PAO. J Bacteriol. 1985 Aug;163(2):568–572. doi: 10.1128/jb.163.2.568-572.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Marrero R., Yasbin R. E. Cloning of the Bacillus subtilis recE+ gene and functional expression of recE+ in B. subtilis. J Bacteriol. 1988 Jan;170(1):335–344. doi: 10.1128/jb.170.1.335-344.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. C., Bryant D. A., Porter R. D., de Marsac N. T. Molecular cloning and characterization of the recA gene from the cyanobacterium Synechococcus sp. strain PCC 7002. J Bacteriol. 1987 Jun;169(6):2739–2747. doi: 10.1128/jb.169.6.2739-2747.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owttrim G. W., Coleman J. R. Molecular cloning of a recA-like gene from the cyanobacterium Anabaena variabilis. J Bacteriol. 1987 May;169(5):1824–1829. doi: 10.1128/jb.169.5.1824-1829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Cohen-Bazire G. Phototrophic prokaryotes: the cyanobacteria. Annu Rev Microbiol. 1977;31:225–274. doi: 10.1146/annurev.mi.31.100177.001301. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Szalay A. A. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene. 1983 Sep;24(1):37–51. doi: 10.1016/0378-1119(83)90129-4. [DOI] [PubMed] [Google Scholar]