Abstract
Six independent lines of evidence point to the existence of heme-containing compounds and/or hemoglobin breakdown products in extracts of trabecular tissues of the large theropod dinosaur Tyrannosaurus rex. These include signatures from nuclear magnetic resonance and electron spin resonance that indicate the presence of a paramagnetic compound consistent with heme. In addition, UV/visible spectroscopy and high performance liquid chromatography data are consistent with the Soret absorbance characteristic of this molecule. Resonance Raman profiles are also consistent with a modified heme structure. Finally, when dinosaurian tissues were extracted for protein fragments and were used to immunize rats, the resulting antisera reacted positively with purified avian and mammalian hemoglobins. The most parsimonious explanation of this evidence is the presence of blood-derived hemoglobin compounds preserved in the dinosaurian tissues.
Hemoglobin is a large (64-Kd), multiple, subunit globular protein necessary for gas exchange in living organisms. It has long been the focus of studies in molecular biology and was one of the first proteins for which structure and sequence were determined (1). A large body of data exists pertinent to its structure, function, and evolution (2). The amino acid sequence of hemoglobin has been determined for many taxa, making it an important tool in studies of evolution (2–4).
The heme prosthetic group at the core of hemoglobin consists of a porphyrin ring with one iron atom at its center. Although porphyrins are common constituents of many compounds (5), the incorporation of iron within the ring structure imparts unique characteristics to heme-containing molecules, and a variety of analytical techniques have been used to characterize both hemoglobin and the related protein, myoglobin. These include nuclear magnetic resonance (NMR) (6, 7), Raman and resonance Raman spectroscopy (RR) (8–11), and electron spin resonance (ESR) (12, 13).
The portions of hemoglobin that interact with the heme prosthetic group are highly constrained and conserved across vertebrate taxa (2, 14). Consequently, these regions of hemoglobin are appropriate for determining deep divergences. Other regions of the molecule allow for much more variation and are more appropriate for determining closer relationships, even to delineating species (15). Additionally, the association of the hemoglobin molecule and its organophosphate allosteric effectors may be a useful indicator of metabolic rates (15, 16).
The functional and chromophoric heme prosthetic group in hemoglobin contains iron as either a d5 Fe (III) form in met–heme proteins or as a d6 Fe (II) center in the reduced ferrous proteins. In either oxidation state, the iron–protoporphyrin–IX complex provides a spectroscopically rich ensemble that has been characterized thoroughly for a wide range of systems (17, 18). Moreover, the extended aromatic stabilization provided by the 18-electron π delocalization in porphyrins and closely related tetrapyrrole-based macrocycles and their complexes is thought to contribute to the stability of these compounds, many of which are identifiable across geological time (19).
Hemoglobin crystallizes fairly easily, and in crystalline form the stability of the protein may be enhanced. The stability of the core porphyrin ring also may contribute to the longevity of this biomolecule. Hemoglobin fragments containing antigenic components have been identified in fossil bone (20, 21) up to 4500 years old (22). Blood residues bearing antigenic properties also have been isolated from stone implements up to 100,000 years old (23–25). In some cases, erythrocyte cellular morphology has been reported to be preserved to the extent that pathological conditions can be recognized (26, 27).
Most erythrocytes undergo hemolysis when the organism dies. Hemoglobin is released with a resultant red discoloration of surrounding bony tissues (22). However, under the anoxic conditions that prevail deep in the endosteal tissues of massive animals (28), hemoglobin molecules may be protected from early stages of oxidative degradation. The proteins may then become complexed with apatite, the mineral phase of bone matrix. This association may well be a prerequisite for the survival of biomolecular compounds across geological time (29, 30). Protein and other organic compounds are protected from degradation when stabilized by interaction with mineral crystal aggregates (31). In addition, by adsorbing to a stabilizing mineral matrix, biomolecules are effectively isolated from water, thereby retarding hydrolytic damage (32).
Traditionally, there was little hope that biomolecules might be recovered from bone more than a few thousand years old. However, 20 years ago, partial amino acid sequences were identified from the shells of mollusks ≈80 million years old (33). Gurley et al. (34) followed with a report of amino acids in the bony tissues of the Late Jurassic (≈150 million years ago) sauropod dinosaur, Seismosaurus, and more recently the small and highly acidic bone protein, osteocalcin, has been recognized immunologically in extracts of dinosaurian bone (35). Stable isotope studies (36), including those done on the specimen used in the following study (37), indicate that at least some of these molecules are endogenous to the fossils, rather than arising from younger exogenous contaminants. These results suggest that significant protein remnants may exist in fossil bone. In light of the above studies, it was decided to examine nonpermineralized dinosaur bone for biomolecular degradation products, including hemoglobin.
MATERIALS AND METHODS
A near-complete specimen of the Late Cretaceous dinosaur Tyrannosaurus rex [Museum of Rockies (MOR) 555] was collected by MOR from the Hell Creek formation (67–65 million years ago) of eastern Montana in 1990. The completeness and articulation of the skeleton indicated that burial was rapid enough to forestall damage by scavenging and weathering but not rapid enough to prevent some minimal displacement. The specimen was surrounded by a consolidated white sandstone, buried under 1–1.5 m of stream channel sediments that contained abundant coalified plant material. The specimen was collected with 0.5–0.6 m of sediment in place between the unexposed hind limb used in this study and the pelvis, which had been exposed before collection. Preliminary examination of trabecular bone elements of the specimen revealed little or no evidence of internal permineralization or replacement. This relatively unaltered state may be due to minimal exposure to water. Dehydration would favor preservation of endogenous biomolecules, including hemoglobin, so an attempt was made to detect their presence in the tissues of MOR 555.
Trabecular tissues were harvested from MOR 555 immediately upon removal of the surrounding sediment from the bones. Gloves were worn at all times, and only sterilized instruments and containers were used. The tissues were wrapped in foil and stored in desiccation jars at −20°C. Tissues were extracted by grinding the bone to a fine powder in sterile, baked mortars and pestles and then adding various extraction buffers. Embedding sandstone and samples of coalified plant material were likewise extracted and subjected to some of the same analytical techniques.
Some of the tissues were extracted according to Gurley et al. (33). An extraction buffer [0.3 M NaCl/5% glycerol/5 mM DTT/2 mM EDTA/1% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate)/6 M guanadine⋅HCl/100 mM Tris⋅HCl] was applied to powdered bone and incubated overnight at room temperature, then dialyzed against 10 mM Tris⋅HCl (pH 7.9) for use in HPLC analysis. For immunization studies, 1 ml of a guanidine–thiocyanate extraction buffer (38) was added to 1.5-ml Eppendorf tubes containing bone powder, and the tubes were rocked gently overnight at 50–60°C and then centrifuged at 14,000 × g for 5 min. The reddish brown supernatant was then drawn off and dialyzed overnight against either 30 mM PBS (pH 7.5) or 10 mM Tris⋅HCl (pH 7.9). All samples were concentrated to at least half-volumes using a Savant speed vac. For the remaining analyses, tissues were extracted in 1 N HCl overnight with gentle agitation. The extracts were precipitated as described below.
HPLC.
The material resulting from the extraction detailed above was concentrated on a Savant speed vac, and 20 ml was injected onto a phenyl analytical HPLC column (Vydac, Hesperia, CA). Extraction buffers and extracts of the surrounding sandstone sediments also were analyzed. Elution profiles were monitored at 410 nm using a Spectraflow 757 (Applied Biosystems) absorbance detector. Elution solvents were placed in two reservoirs: HPLC grade water in reservoir A and HPLC grade acetonitrile in reservoir B. Both solvents contained 0.1% trifluoroacetic acid. The gradient protocol was: 100% A for 5 min, ramp to 75% B at 55 min, remain at 75% B until 60 min, then ramp to 100% A at 62 min. The flow rate was 0.3 ml/min and sensitivity was 0.05 absorbance units.
Chemical Analyses.
Powdered trabecular tissues were extracted overnight with rotation in 10 volumes of 1 N HCl at 4°C. The extract was applied to a Sep-Pak C-18 column (Waters) for purification, and the eluate was lyophilized. The lyophilized pellet was treated by first adding 100 ml of methyl-t-butyl ether, then 0.5 ml of a solution consisting of 150 ml of 6 N HCl in 5 ml of acetone. The “protein” fraction precipitated out after a final addition of 5–10 ml of dilute (≈1 M) ammonium hydroxide and was stored at −20°C for subsequent amino acid analyses at San Diego State University (39). The remaining dark-brown/red supernatant was lyophilized and stored for spectroscopic analysis. Sandstone and plant extracts were precipitated similarly.
Spectroscopy.
The lyophilized supernatants were resolubilized in PBS and subjected to transmission UV/visible spectroscopy. Transmission spectra of solid samples of bone were measured through potassium bromide (KBr)-fused glass. Data were measured at room temperature on either a Hewlett–Packard Diode-array Spectrometer 8452 or a Perkin–Elmer Lambda-9 spectrometer.
NMR.
The lyophilized supernatant remaining after extraction and precipitation was subjected to high resolution, solution phase, proton NMR. A lyophilized sample of 40 mg was dissolved in 0.5 ml of D2O. NMR spectra were collected on a Bruker (Billerica, MA) model AC300 NMR spectrometer operating at 300 MHz proton frequency, using techniques appropriate for studying paramagnetic proteins (40). The data were collected as 8-K complex data points with a sweepwidth of 83333 Hz using a 5-ms pulse length and a total repetition rate of 100 ms. A total of 20,000 scans was collected, and the data were processed using a 20-Hz exponential line broadening window function. No absorbance at 410 nm was demonstrated in sandstone and plant extracts, so these samples were not subjected to NMR analyses.
Electron Spin Resonance.
Samples of bone from MOR 555 were extracted with 1 N HCl and precipitated as described. The precipitate and controls of pigeon met–hemoglobin (Sigma) and turkey hemoglobin (Sigma) powders were diluted or dissolved in PBS (pH 7.04), transferred to a 4-mm quartz tube and frozen in liquid nitrogen. A second sample was vacuum-degassed and treated with 40 ml of 0.1 M sodium nitrite and 40 ml of 0.1 M sodium dithionite in an attempt to nitrosylate an iron species in the extract.
Raman Spectroscopy.
Resonance enhancement of Raman scattering was augmented by surface-enhanced Raman scattering. This method was used to enhance the Raman scattering and because samples were inherently fluorescent, perhaps because of a small remnant of humic material. It was impossible to obtain Raman spectra from the bulk samples because of high background. Fluorescent emission was reduced, and the Raman spectrum was obtained by placing a dilute solution on a surface-enhanced Raman scattering active substrate. The Raman system and surface-enhanced Raman scattering substrate preparation are described elsewhere (41). In brief, the Raman spectra were obtained with an LN2-cooled charge-coupled device system comprised of a Kr+ ion laser (Spectra Physics 2025) operated at 647 nm, an HR320 spectrograph (ISA), and a CCD9000 charge-coupled device camera system from Photometrics (Tucson, AZ). The Raman scattering was collected perpendicular to excitation with an f/1.8 camera lens. The surface-enhanced Raman scattering substrate was prepared by etching a 99.9% silver foil (Aldrich) in 30% nitric acid for several seconds. All Raman samples were placed on the foils using dilute ethanolic solutions and the solvent was allowed to evaporate. The spectra were obtained with low laser power (less than 10 mW) and integration times of 10 s or less to avoid laser-induced damage.
Antiserum Production.
Trabecular tissues from MOR 555 were extracted with guanidinium–thiocyanate buffer and dialyzed, as described, as were samples of coalified plant and embedding sands. The bone extracts were concentrated and used for immunization studies. Although discrete polypeptides were not consistently visible using SDS/PAGE and Coomassie blue staining, protein–polypeptide concentrations of ≈0.4 mg/ml were supported by the Bradford assay. It has been suggested that endogenous proteins in fossil bone may be best demonstrated by raising antibodies to those protein fragments (42). Therefore, a total of 0.8 mg of whole bone extract was used to immunize two rats according to the following immunization schedule. On day 1, a preimmunization serum sample was obtained, and the primary immunization was carried out using an s.c. injection of a 0.12-mg sample in 1 ml of Freund’s complete adjuvant. Secondary immunizations were carried out with Freund’s incomplete adjuvant, injecting a 0.12-mg sample s.c. on day 28, a 0.08-mg sample i.m. on day 49, and a 0.08-mg sample s.c. on day 60. The animals were exsanguinated on day 72, and the antiserum was prepared.
ELISA.
Polystyrene 96-well ELISA plates (Corning) were coated with turkey hemoglobin (Sigma) by adding 0.1 ml per well of a 0.01 mg/ml protein solution in 0.2 M sodium carbonate–bicarbonate buffer (Pierce; pH 9.4) 1 h at room temperature. Wells were washed three times with a buffer consisting of PBS, BSA, and Tween 20 (pH 7.4) and then blocked with 0.25 ml of 10% BSA/PBS for 1 h at room temperature. Plant and sandstone extracts were diluted in binding buffer and added to wells in the same manner as the turkey hemoglobin. After washing, immune and pre-immune antisera were each added at a 1:10 dilution into 0.1 ml of blocking buffer and serially diluted out to 1:10240. The antisera were incubated with the target plates for 1 h at room temperature, followed by three washes with wash buffer. To each well, 0.1 ml of horseradish peroxidase conjugate anti-rat Ig (Amersham), diluted 1:1000 in blocking buffer, was added, followed by incubation for 1 h at room temperature. Positive reactions were visualized by adding 0.1 ml of 2,2′-azino-bis (3-ethylbenziothiazoline-y-sulfonic acid) diammonium salt (Pierce) for 30 min at room temperature. The color reaction was stopped by the addition of 0.05 ml of 1% SDS, and the absorbance of the substrate solution was measured at 405 nm.
To independently confirm the ELISA results, 20 ng of purified, commercially prepared hemoglobins from various taxa was electrophoretically separated on a 4–20% gradient SDS/PAGE gel and transferred to Immobilon-P membranes (Millipore). The membranes were blocked with 5% nonfat, dried milk in Tris-buffered saline (TBS; 20 mM Tris⋅HCl, pH 8.0/150 mM NaCl) for 1 h at room temperature. Blocking solution was removed, and the membranes were washed six times with TBS-T (TBS plus 0.1% Tween 20). Normal rat serum and the immune antisera generated from extracts of T. rex bone were diluted 1:100 with TBS-T with 5% milk and exposed to membranes for 3 h at room temperature. After washing, the membranes were incubated for 1 h in anti-rat secondary antibody coupled to horseradish peroxidase (Amersham), diluted 1:1000 in TBS-T plus 5% milk. After six TBS-T washes, antibody–antigen complexes were detected using enhanced chemiluminescence (Amersham).
RESULTS
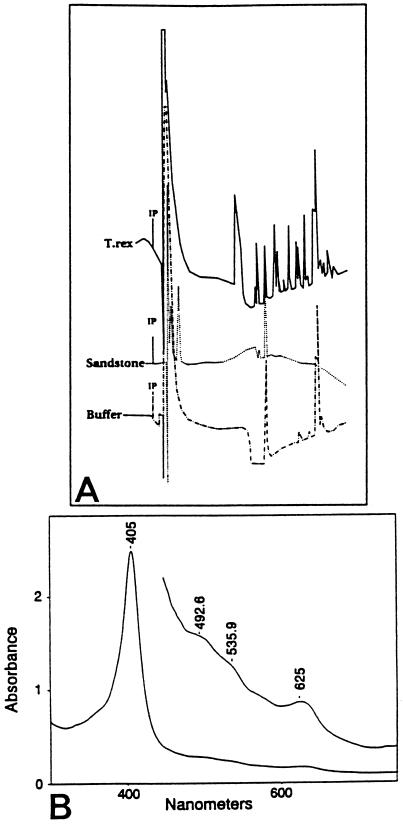
HPLC analysis of MOR 555 tissue extracts revealed the presence of several peaks with absorbance at 410 nm (Fig. 1A), the value at which hemoglobin and other heme-containing compounds absorb strongly. The peaks were observed in the bone extracts but not in the controls, indicating that the signals were derived solely from the bone and not from contaminating factors in the sediments or extraction buffers.
Figure 1.
(A) Reverse phase HPLC profiles of extracted tissues and controls; 20 μl of concentrated extract was injected onto a phenyl analytical column and monitored at 410 nm. From top to bottom, samples are: T. rex tissues in extraction buffers, sandstone matrix, and extraction buffers alone. IP, point of injection with time running to the right. (B) UV/visible absorbance spectrum of dinosaurian tissue extract, precipitated in HCl/acetone/ether as described. The large peak at 405 nm is within the range of variation seen for heme compounds and is characteristic of these compounds. The smaller peaks at the longer wavelengths (quasi-allowed, or Q-, bands) also are characteristic of these compounds. The Inset represents an expansion of the longer wavelengths of the tracing for visualization and identification of individual peaks in this region. Plant and sandstone samples, similarly extracted, showed no specific absorbance at this wavelength.
UV/visible spectroscopic analysis of extracts were variable, but in extracts exposed to the HCl/acetone/ether precipitation, a distinct peak in the region of 405–410 nm was clearly present (Fig. 1B). This peak is consistent with the absorbance characteristic of the Soret band of hemoglobin and other heme proteins (43). No Soret band was seen in extracts of either coalified plant material or embedding sandstone similarly extracted (data not shown). Additionally, an observed color change when the bone extracts were shifted from an oxidized to a reduced state is consistent with the presence of heme.
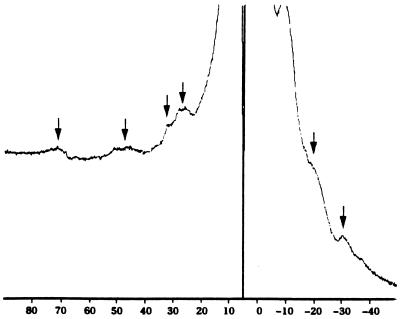
The proton NMR spectrum of the dinosaur extracts (Fig. 2) contains peaks upfield and downfield from the standard 0- to 10-ppm window characteristic of the resonances of protons in proteins and other organic molecules. Four broad resonances at 25.0, 29.0, 45.0, and 72.0 ppm, as well as three other peaks at −9.0, −20.0, and −30.0 ppm indicate the presence of a paramagnetic atom, such as those seen in various metalloproteins (44, 45). The spectrum is consistent with degraded heme proteins in the met (Fe3+) state (6, 45).
Figure 2.
High resolution, solution phase proton NMR profile obtained from extracts of dinosaur tissues precipitated in HCl/acetone/ether as described. For this spectrum, samples were filtered through a 5000 MW cut-off filter, and the higher molecular weight fraction was again filtered through a 30,000 molecular weight filter for better resolution before being subjected to NMR. The arrows indicate resonance in regions that are consistent with the presence of a paramagnetic compound such as heme.
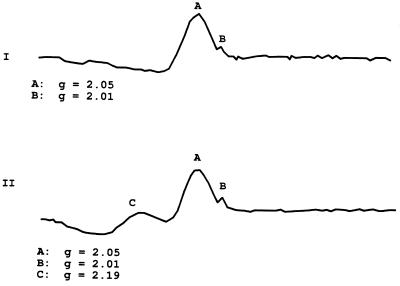
The profile obtained in ESR illustrates that the bone extracts are capable of chemical modification by the addition of a nitrosyl group. Paramagnetic nitric oxide molecules bind to the reduced iron in the solution, generating a new ESR signal. The sharp spike usually seen in hemoglobin and myoglobin proteins at g = 6 (12) is absent (Fig. 3), but the detection limits of the instrument used in this analysis are such that this signal would not be seen in a dilute sample, and indeed, the peak was not observed in a dilute solution of commercially prepared met–Hb.
Figure 3.
A, ESR profile of the HCl/acetone/ether precipitate and B, the same extract, vacuum degassed, flushed with nitrogen, and injected with 40 μl of anaerobic 0.1 M sodium nitrite followed by 40 μl of anaerobic 0.1 M sodium dithionite. Scan range, 1000 g; field set, 3240 g; modulation amplitude, 10 g; microwave power, 3.4 mW; microwave frequency, 9.132 GHz; receiver gain, 2.0 × 105; temperature, 77 K.
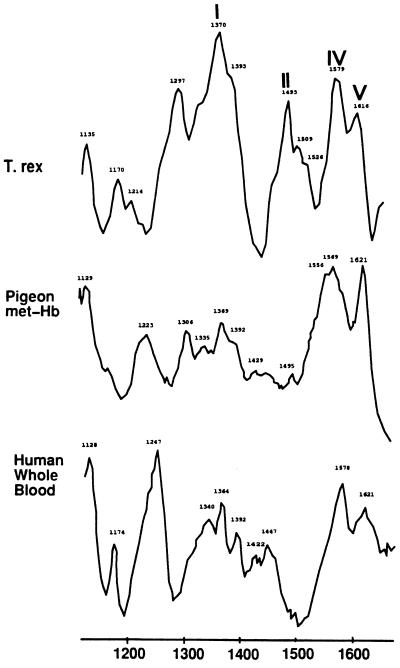
Resonance Raman spectroscopy elucidates the bonding environment of the iron and heme group and ligands bound to these structures (8). Raman analysis of hemoglobins generate specific and characteristic peaks identified as: marker band I, between 1340 and 1390 wavenumbers; band II, between 1470 and 1505; band III, between 1535 and 1575; band IV, between 1550 and 1590; band V, between 1605 and 1645; and band VI, between 1560 and 1600 (8). The location of marker band III is somewhat variable and may overlap with band IV. The ferric (Fe III) derivatives of heme for marker band I are shifted to a slightly higher region than those for the ferrous (Fe II) state. Fig. 4 illustrates resonance Raman spectra of MOR 555 extracts. The presence of peaks in the dinosaur extracts consistent with marker bands I, II, IV, and V are strongly suggestive of the heme prosthetic group.
Figure 4.
Raman profile of dinosaur extracts, precipitated in HCl/acetone/ether, as described. Human whole blood and commercially prepared pigeon met–hemoglobins are shown for comparison. Roman numerals indicate peaks that are mentioned in the literature as marker bands for heme compounds.
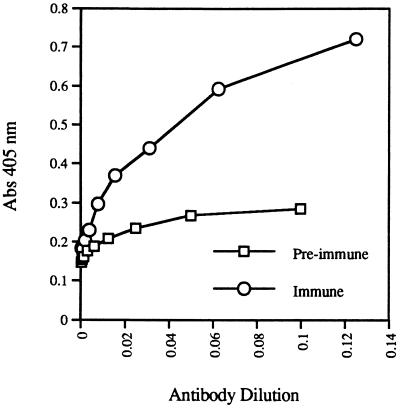
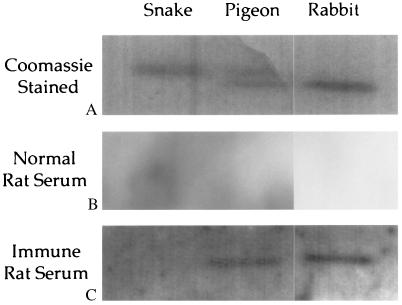
Hemoglobin polypeptides in the trabecular extract were confirmed immunologically. Immunization of two rats with the extracts resulted in the production of antibodies that specifically recognized avian hemoglobin. By ELISA, both immune antisera were found to recognize turkey hemoglobin whereas the preimmune sera did not (Fig. 5). Similar results were obtained using rabbit hemoglobin as the antigen in the ELISA (data not shown). The production of antibodies specific for hemoglobin in two individual rats injected with the trabecular extract is striking evidence for the presence of hemoglobin-derived peptides in the bone extract. The rat immune response against hemoglobin in the T. rex bone was further verified by immunoblot analysis (Fig. 6). The antiserum obtained from each rat recognized both pigeon and rabbit hemoglobins whereas a normal (nonimmunized) rat serum did not. Weak reactivity was observed against the turkey hemoglobin although the protein band was too faint to be reproduced photographically. That the antisera did not react with snake hemoglobin shows that the reactivity is specific and not artifact. This was further confirmed by the lack of reactivity between the antisera and samples of plant and sandstone that were extracted in the same manner as the bone tissues used to immunize the animals (data not shown). Because of the low anti-hemoglobin antibody titer in the sera, low dilution was necessary for detection of proteins, producing the nonspecific background observed in Fig. 6. Immunogenicity is not dependent on fully intact protein (42), and even very small peptides are immunogenic when complexed with larger organic molecules, so this is a highly sensitive method that maximizes the possibility of detecting small, specific, endogenous proteins in fossil bones, even after extensive degradation has occurred.
Figure 5.
Rats immunized with T. rex tissue extracts produce antibodies that recognize hemoglobin. Antisera drawn from rat IN9 before and after the immunization procedure were compared at increasing dilutions for reactivity against purified turkey hemoglobin by ELISA. The intensity of absorbance measured at 405 nm is directly proportional to the quantity of anti-hemoglobin antibodies present in each serum. No reactivity of the antisera with components of sandstone and plant extracts was noted.
Figure 6.
(A) Electrophoretic separation of commercially prepared hemoglobins stained with Coomassie blue. (B) Western blot of the hemoglobins in A exposed to rat preimmune sera. No reaction to any of the hemoglobins is visualized. (C) Positive and specific binding between dinosaur antisera and purified hemoglobins on Western blot. Background was high because of necessary low dilutions of the antisera. The reaction to turkey hemoglobin was measureable but could not be reproduced photographically. There was no reaction to the snake hemoglobin, either by immunoblot or ELISA, nor was there reactivity with plant or sandstone extracts. Protein-specific reactivity, which is variable in intensity, supports a true immune response rather than artifact.
DISCUSSION
Independent results from the spectroscopic, analytical, and immunochemical techniques used in this study support the existence of heme and hemoglobin breakdown products in extractions of trabecular tissues of the MOR 555 specimen of T. rex. Significant levels of d-enantiomers of individual amino acids (39) suggest that the source proteins are ancient. Considering the rapid burial and geological sequestration of the skeleton, as well as excellent microstructural preservation (39), the most likely source of these proteins is the once-living cells of the dinosaur. This is supported by the lack of signal from either embedding sandstone or plant material extracted in the same manner as the bone tissues in various analytical examinations. Extractions of the bone tissues absorbed light at 410 nm and demonstrated the presence of a Soret band, indicative of heme. Proton NMR studies are sensitive to the presence of paramagnetic ions, which can induce broadening of the signals and, in many cases, shifts in the resonance frequency to regions outside the normal proton spectral window (46). In the dinosaur extracts, contact shifted resonances both upfield and downfield are consistent with the presence of a paramagnetic compound such as heme. The proton arrangements of iron or copper complexes normally found in geologically derived minerals do not posses the aromatic delocalization found in porphyrins. Because solution phase NMR spectroscopy of inorganic or geologically deposited iron do not have contact-shifted resonances with this pattern (47, 48), observation of multiple resonances at different chemical shifts suggests the presence of biological paramagnetic molecules such as heme.
The use of ESR showed that this compound was able to be nitrosylated after treatment with nitrite and dithionite. This is also observed with modern hemoglobins. Resonance Raman yielded information regarding the oxidation state of the iron in the extracts. The iron was found in the more stable ferric, or Fe (III) met, form rather than the Fe (II) form of modern biological systems, as would be expected for heme of ancient origin. Resonance Raman also identified peaks within the range of variation for four of the six unique marker bands characteristic of the heme prosthetic group. Finally, the most revealing results were from the immunochemical studies, in which a component in the extracts of MORE 555 tissues elicited an immune response in rats specifically against hemoglobin. When considered as a whole, the results support the hypothesis that heme prosthetic groups and hemoglobin fragments were preserved in tissues of this Late Cretaceous dinosaur skeleton.
None of the analytical results obtained was completely identical to those noted experimentally or in the literature for modern hemoglobins. However, ancient molecules typically show variations in analytical profiles from their purified modern counterparts because of chemical modifications during degradation (49, 25). Geochemical interactions with biomolecules preserved in fossil bone over millions of years are to be expected, and the presence of additional, nonhemoglobin signals detected by the various physical methods is not unexpected given the highly degraded and diagenetically altered biological compounds in the bone.
The biochemical and biophysical data, when taken together with a repeated, strong, positive immunological reaction of antisera raised against the trabecular extract with hemoglobin antigens and with the specific results of Western blots, provide powerful support for the hypothesis that some form of heme as well as fragments of hemoglobin proteins are preserved within the dinosaur tissues. Heme proteins, including hemoglobin and myoglobin, are found in blood and muscle tissues of modern vertebrates. Indeed, it is estimated that each circulating mammalian red blood cell might contain ≈300 million molecules of hemoglobin, and in birds and other archosaurs the level is higher yet (2, 15). Myoglobin, a muscle protein closely related to hemoglobin, could be proposed as the source of the observed heme signals, given the sheer mass of muscle that surrounded the bones of this animal at one time. However, this study was conducted on trabecular bone taken from the endosteal cavities of bones. These tissues were rich in blood and blood forming tissues but were not in direct contact with muscle tissue.
The presence of heme-containing cytochrome proteins from a microbial source could also be offered as an explanation for the source of the signals because heme porphyrins of a presumed microbial source have been identified in ancient sediments (5, 50). However, cytochrome proteins are present at much lower concentrations in trabecular tissues than hemoglobins and are not immunologically cross-reactive with hemoglobin. Stable isotope analyses may ultimately elucidate the source of the heme signals (51).
There is, of course, abundant biogeochemistry of tetrapyrroles (5, 50, 51), but transmetallation reactions normally require reducing conditions at high temperatures and pressures. However, in addition to the lack of evidence for such diagenetic alteration in these tissues, the following experimental features are most consistent with the presence of an iron tetrapyrrole unit: (i) Magnesium and nickel tetrapyrrole groups are dimagnetic and therefore will not give rise to the observed ESR and NMR spectra; (ii) the observed Soret band in the UV/visible has higher energy than is typically found for vanadyl porphyrins; and (iii) vanadium, copper, magnesium, and nickel tetrapyrrole compounds will not generally nitrosylate under the conditions observed here. From these results, only iron, manganese, and cobalt are possible metal ligands, and, of these elements, only iron was identified by electron probe analyses (39).
Further application of immunological techniques to the study of fossil bones from extinct taxa may increase our understanding of these animals. Some possibilities suggested by this and other works include the identification of taxon-specific epitopes, which may provide a means to immunologically classify extinct organisms. This in turn would provide an independent test of morphologically derived phylogenetic trees. The generation of antisera from dinosaur extracts may provide a means for purifying and separating what proteins remain so that further analyses can be performed. Protein fragments purified in this manner could then be digested with specific proteases to yield uniform amino termini, a requirement for amino acid sequencing. This procedure is not possible with whole bone extracts of degraded proteins. Phylogenies based upon hemoglobin sequence data currently exist, and if such data can be recovered from fossil tissues, they may be used to resolve some of the phylogenetic and physiological questions still remaining for extinct organisms.
Acknowledgments
The expertise of many people in different fields was required for completion of this multidisciplinary project. The following people gave generously of their time, advice, and opinions, and we extend our gratitude to them: R. Avci, B. Breithaupt, S. Brumfield, T. Clack, D. Dooley, N. Equall, C. Johnson, M. McGuirl, M. Schmidt, J.Sears, R. Sharrock, and M. Teintze. Special thanks to W. D. Maxwell for editorial assistance. This research was supported by National Science Foundation Grant EAR-9311542.
ABBREVIATIONS
- NMR
nuclear magnetic resonance
- RR
resonance Raman spectroscopy
- ESR
electron spin resonance
- TBS
Tris-buffered saline
References
- 1.Perutz M F, Rossmann M G, Cullis A F, Muirhead H, Will G, North A C T. Nature (London) 1960;185:416–422. doi: 10.1038/185416a0. [DOI] [PubMed] [Google Scholar]
- 2.Dickerson R E, Geis I. Hemoglobin: Structure, Function, Evolution, and Pathology. Menlo Park, CA: Benjamin; 1983. [Google Scholar]
- 3.Riggs A F. Am Zool. 1991;31:535–545. [Google Scholar]
- 4.Gorr T, Kleinschmidt T. Am Sci. 1993;81:73–82. [Google Scholar]
- 5.Boreham C J, Fookes C J R, Popp B N, Hayes J M. Geochim Cosmochim Acta. 1989;53:2451–2455. doi: 10.1016/0016-7037(89)90368-2. [DOI] [PubMed] [Google Scholar]
- 6.Ho C, Russu I M. Methods Enzymol. 1981;76:275–312. doi: 10.1016/0076-6879(81)76128-7. [DOI] [PubMed] [Google Scholar]
- 7.Ho C, Perussi J R. Methods Enzymol. 1994;232:97–139. doi: 10.1016/0076-6879(94)32046-2. [DOI] [PubMed] [Google Scholar]
- 8.Asher S A. Methods Enzymol. 1981;76:371–413. doi: 10.1016/0076-6879(81)76132-9. [DOI] [PubMed] [Google Scholar]
- 9.Carey P R. Biochemical Applications of Raman and Resonance Raman Spectroscopies. New York: Academic; 1982. pp. 99–147. [Google Scholar]
- 10.Tu A T. Raman Spectroscopy in Biology: Principles and Applications. New York: Wiley; 1982. pp. 317–368. [Google Scholar]
- 11.Friedman J M. Methods Enzymol. 1994;232:205–231. doi: 10.1016/0076-6879(94)32049-7. [DOI] [PubMed] [Google Scholar]
- 12.Peisach J, Blumberg W E, Adler A. Ann NY Acad Sci. 1973;206:310–326. doi: 10.1111/j.1749-6632.1973.tb43219.x. [DOI] [PubMed] [Google Scholar]
- 13.Blumberg W E. Methods Enyzmol. 1981;76:312–329. doi: 10.1016/0076-6879(81)76129-9. [DOI] [PubMed] [Google Scholar]
- 14.Perutz M F. Mol Biol Evol. 1983;1:1–28. doi: 10.1093/oxfordjournals.molbev.a040299. [DOI] [PubMed] [Google Scholar]
- 15.Nikinmaa M. Vertebr. Red Blood Cells. Berlin: Springer; 1990. [Google Scholar]
- 16.Abbasi A, Weber R E, Braunitzer G, Goltenboth R. Biol Chem Hoppe Seyler. 1987;368:323–32. doi: 10.1515/bchm3.1987.368.1.323. [DOI] [PubMed] [Google Scholar]
- 17.Palmer G. In: The Iron Porphyrins, Part II. Lever A P B, Gray H B, editors. Reading, MA: Addison–Wesley; 1983. pp. 43–85. [Google Scholar]
- 18.Spiro T G. In: The Iron Porphyrins, Part II. Lever A P B, Gray H B, editors. Reading, MA: Addison–Wesley; 1983. pp. 89–152. [Google Scholar]
- 19.Curry G B. In: Paleobiology: A synthesis. Briggs D E G, Crowther P R, editors. Oxford: Blackwell Scientific; 1990. pp. 95–100. [Google Scholar]
- 20.Cattaneo C, Gelsthorpe K, Phillips P, Sokol R J. Nature (London) 1990;347:339. doi: 10.1038/347339a0. [DOI] [PubMed] [Google Scholar]
- 21.Smith P R, Wilson M T. J Archaeol Sci. 1990;17:255–268. [Google Scholar]
- 22.Ascenzi A, Brunori M, Citro G, Zito R. Proc Natl Acad Sci USA. 1985;82:7170–7172. doi: 10.1073/pnas.82.21.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loy T H. Science. 1983;220:1269–1270. doi: 10.1126/science.220.4603.1269. [DOI] [PubMed] [Google Scholar]
- 24.Loy T H. In: Archaeometry: Further Australasian Studies. Ambrose W R, Mummery J M J, editors. Canberra, Australia: Australian National Gallery; 1987. pp. 57–65. [Google Scholar]
- 25.Lowenstein J M. In: Molecular Evolution and the Fossil Record: Short Courses in Paleontology. Broadhead T W, editor. Paleontological Society; 1988. pp. 12–19. [Google Scholar]
- 26.Maat G J R. Int J Osteoarchaeol. 1991;1:209–214. [Google Scholar]
- 27.Maat G J R. Int J Osteoarchaeol. 1993;3:77–86. [Google Scholar]
- 28.Allison P A. In: Paleobiology: A Synthesis. Briggs D E G, Crowther P R, editors. Oxford: Blackwell Scientific; 1990. pp. 213–216. [Google Scholar]
- 29.Tuross N, Stathoplos L. Methods Enzymol. 1993;224:121–129. doi: 10.1016/0076-6879(93)24010-r. [DOI] [PubMed] [Google Scholar]
- 30.Richards M B, Sykes B C, Hedges R E M. J Arch Sci. 1995;22:291–299. [Google Scholar]
- 31.DeNiro M J, Weiner S. Geochim Cosmochim Acta. 1988;52:2415–2423. [Google Scholar]
- 32.Ambler R P, Daniel M. Phil Trans R Soc Lond B. 1991;333:381–389. doi: 10.1098/rstb.1991.0088. [DOI] [PubMed] [Google Scholar]
- 33.Weiner S, Lowenstam H A, Hood L. Proc Natl Acad Sci USA. 1976;73:2541–2545. doi: 10.1073/pnas.73.8.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurley L R, Valdez J G, Spall W D, Smith B F, Gillette D D. J Prot Chem. 1991;10:75–90. doi: 10.1007/BF01024658. [DOI] [PubMed] [Google Scholar]
- 35.Muyzer G, Sandberg P, Knapen M H J, Vermeer C, Collins M, Westbroek P. Geology. 1992;20:871–874. [Google Scholar]
- 36.Ostrom P H, Macko S A, Engel M H, Silfer J A, Russell D A. Org Geochem. 1993;16:1139–1144. [Google Scholar]
- 37.Barrick R E, Showers W J. Science. 1994;256:222–224. doi: 10.1126/science.265.5169.222. [DOI] [PubMed] [Google Scholar]
- 38.Hoss M, Paabo S. Nucleic Acids Res. 1993;21:3913–3914. doi: 10.1093/nar/21.16.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schweitzer, M. H., Johnson, C., Zocco, T. G., Horner, J. R. & Starkey, J. R. (1997) J. Vert. Paleontol., in press.
- 40.La Mar G N, De Ropp J S. In: Biological Magnetic Resonance. Beliner L J, Reuben J S, editors. Vol. 12. New York: Plenum; 1993. pp. 1–78. [Google Scholar]
- 41.Mullen K, Carron K. Anal Chem. 1994;66:478–483. [Google Scholar]
- 42.Child A M, Pollard A M. J Arch Sci. 1992;19:39–47. [Google Scholar]
- 43.Brown S B. In: An Introduction to Spectroscopy for Biochemists. Brown S B, editor. New York: Academic; 1980. pp. 14–40. , 135–145. [Google Scholar]
- 44.La Mar G N. In: NMR of Paramagnetic Molecules. La Mar G N, Horrocks W D, Holm R H, editors. New York: Academic; 1973. pp. 86–123. [Google Scholar]
- 45.Bertini I, Luchinat C. NMR of Paramagnetic Molecules in Biological Systems. Menlo Park, CA: Benjamin; 1986. [Google Scholar]
- 46.Cerdonio M, Morante S, Vitale S. Methods Enzymol. 1981;76:354–371. doi: 10.1016/0076-6879(81)76131-7. [DOI] [PubMed] [Google Scholar]
- 47.Hatcher P G, Rowan R, Mattingly M A. Org Geochem. 1980;2:77–85. [Google Scholar]
- 48.Stuermer D H, Payne J R. Geochim Cosmochim Acta. 1976;40:1109–1114. [Google Scholar]
- 49.Eglinton G, Logan G. Phil Trans R Soc Lond B. 1991;333:315–328. doi: 10.1098/rstb.1991.0081. [DOI] [PubMed] [Google Scholar]
- 50.Bonnett R, Burke P J, Czechowski F, Reszka A. Org Geochem. 1984;6:177–182. [Google Scholar]
- 51.Ocampo R, Callot H J, Albrecht P. Naturwissenschaften. 1989;76:419–421. doi: 10.1007/BF00366165. [DOI] [PubMed] [Google Scholar]