Abstract
Mutations of the drop-dead gene in Drosophila melanogaster lead to striking early death of the adult animal. At different times, after emergence from the pupa, individual flies begin to stagger and, shortly thereafter, die. Anatomical examination reveals gross neuropathological lesions in the brain. The life span of flies mutant for the drop-dead gene is four to five times shorter than for normal adults. That raises the question whether loss of the normal gene product might set into motion a series of events typical of the normal aging process. We used molecular markers, the expression patterns of which, in normal flies, change with age in a manner that correlates with life span. In the drop-dead mutant, there is an acceleration in the temporal pattern of expression of these age-related markers.
Shortened life span has been observed due to various single gene mutations in both humans and flies. In Drosophila, the mutations Hyperkinetic1 (Hk1) and Shaker5 (Sh5), which affect nervous system activity (1), as well as mutations affecting antioxidant systems (catalase, superoxide dismutase), lead to shortened life span, thought to result from effects on energy metabolism and/or oxidative stress (2–8). In humans, the progeroid syndromes, such as Werner and Hutchison–Guilford, may be considered dramatic examples of rapid aging, although not all organ systems are involved. The shortened life span in Werner is about a factor of two; in Hutchison–Guilford it is of the same order as in drop-dead (9).
It has been shown that various genes are expressed in the adult fly in characteristic, reproducible, temporal patterns throughout its adult life (10–12). When the life span of the adult is altered, either by temperature or genetic means, the relative temporal patterns of expression of certain of these genes remain the same, but on a time scale changed in proportion to life span (10, 11). These expression patterns thus would appear to relate more closely to physiological than chronological age, providing a set of molecular biomarkers of the rate of “aging.”
If early death in the drop-dead (13–15) animal is due to a defect that abruptly interrupts the normal sequence of age-related gene expression, the temporal pattern of the molecular biomarkers should be initially normal, but prematurely truncated. If, however, mutations in the drop-dead gene result in acceleration of the normal scenario, the usual sequence of gene expression should occur, but proceed more rapidly. In this report, we demonstrate that, in drop-dead, the temporal patterns of expression of three different molecular biomarkers are accelerated.
MATERIALS AND METHODS
Fly Strains and Culture Conditions.
The drop-deadx3 allele isolated by Buchanan and Benzer (15) was used for the experiments described in this paper. Similar results were found with the drop-dead1 allele (13). wingless (wg) and engrailed (en) enhancer-trap stocks were obtained from J. Kassis (16). The 206 enhancer trap line, obtained from Tim Tully, contains a LacZ-expressing construct (17). Control flies were Canton-S-5, which is a stock homozygous for an X chromosome derived from a Canton-S strain and an FM7 balancer stock, as described in (18). All studies were done with F1 offspring from crosses in which virgin females of the phenotype drop-deadx3/FM7c or Canton-S-5 were mated to males carrying the wg, en, or 206 enhancer-trap chromosome; each F1 male examined possessed one copy of the wg, or the en, or the 206 enhancer-trap chromosome.
All flies were kept in plastic vials containing a standard cornmeal agar medium, with several grains of yeast added (19). Approximately 30 flies initially were in each vial; survivors were passed to fresh vials every 7 days. All flies were cultured in humidified, temperature-controlled environmental chambers at 25°C throughout development. Adult flies were collected, without anesthesia, within 2 hr of emergence from the pupal case. For the experiments using wg and en, flies were then placed in humidified, temperature-controlled environmental chambers set at 25°C . For 206, 18°C was used because of the rapidity of the rising curve of expression of that marker. Male and female flies were aged together in the same vials.
Life Span Analysis.
Life span studies were carried out using the methods of collection and culture noted above, except that the flies were passed into fresh vials every other day, at which time the numbers of dead males were recorded. Over 400 male flies were scored for each life span study. Life spans were defined as times to 1% survival, which were 14 and 50 days, respectively, for drop-dead wg and control wg animals, 14 and 60 days for drop-dead en and control en animals, and 35 and 150 days for drop-dead 206 and control 206 animals. The scaling factors for the three markers were 3.6, 4.3, and 4.2, respectively, for wg, en, and 206. Some differences may possibly be due to secondary variations in genetic background.
Whole-Mount 5-Bromo-4-Chloro-3-Indolyl β-d-Galactoside (X-Gal) Staining.
For each time point, at least 10 male fly heads were removed, fixed for 20 min in 1% glutaraldehyde in phosphate buffered saline (1 × PBS), and reacted with a standard X-Gal solution (GIBCO/BRL) (19). X-Gal reactions were performed at 37°C, 18 hr for the wg and 206 enhancer-trap lines, and 2 hr for the en line, which has a higher level of expression of β-galactosidase (β-gal) (10). After the X-Gal reaction, heads were rinsed twice with 1× PBS and stored in 70% glycerol in 1× PBS. The antennae were cut off and placed under coverslips on microscope slides. Video images or photographs were taken with a ×16 Leitz objective.
Quantitation of β-Gal Expression in Whole-Mount Adult Antennae.
Rather than the standard assay (20), we used the more sensitive X-Gal method to measure the amount of β-gal expressed in the combined third and fifth segments of the antenna. An optically based, computer-assisted video microscopy system that integrates the blue color into arbitrary units was used to quantify the X-Gal reaction (10, 21). Antennae from at least 10 animals were sampled at each time point, except for days 9 and 10 in the case of drop-dead wg (where only 2–4 antennae were available, see Fig. 2), and days 4 and 9 in drop-dead en (6 and 4 antennae, respectively, see Fig. 3). The reliability of the video microscopy system is such that repeated capture of the same image shows <2% variation.
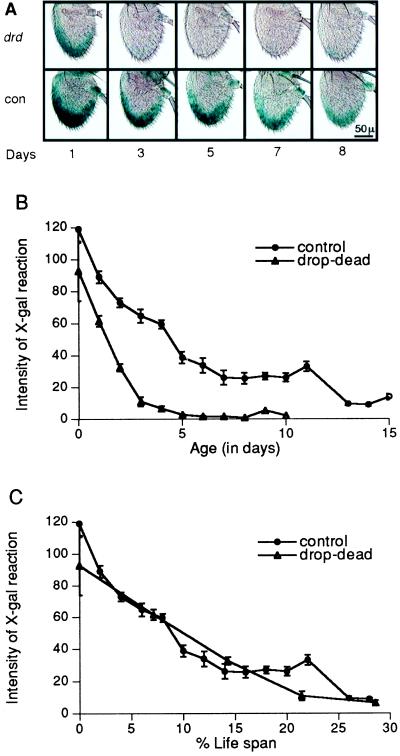
Figure 2.
Temporal patterns of expression of the wg gene in the antennae of adult drop-dead vs. control males. (A) Photomicrographs of whole-mount adult antennae from animals containing one autosomal copy of the wg enhancer-trap chromosome, with or without drop-dead on the X-chromosome, reacted with X-Gal to reveal blue staining in cells expressing β-gal. Each is a typical example from over 10 flies at each age after emergence of the adult. (B) Relative amount of β-gal expressed in the combined third and fifth antennal segments of drop-dead vs. control males at 25°C, plotted against chronological age. (C) Plotted against percent life span. (Bars = SEM.)
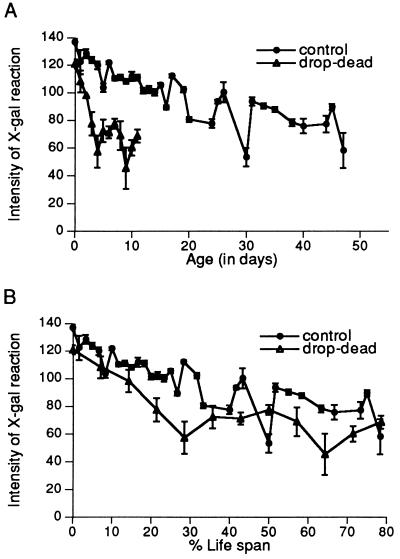
Figure 3.
Temporal patterns of expression of the en gene in the antennae of drop-dead vs. control males at 25°C. (A) Plotted against chronological age. (B) Plotted against percent life span. Each animal contained one autosomal copy of the en enhancer-trap chromosome, with or without drop-dead on the X-chromosome. (Bars = SEM.) Photographs not shown because the high level of expression in en makes it difficult to see differences in photographs that are clearly seen using the computer-assisted optical system.
RESULTS
Life Span of drop-dead Animals.
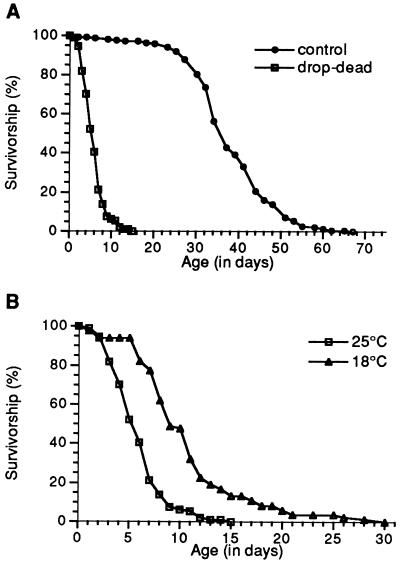
Fig. 1A shows survival curves for drop-dead mutant flies, compared with normal controls. While drop-dead flies live about five times shorter, the shapes of the survival curves are similar. As is true for normal flies (22), death in drop-dead occurs more rapidly at higher ambient temperature (Fig. 1B).
Figure 1.
Effect of drop-dead on life span. (A) Survival curves of adult male drop-dead and control flies at 25°C. (B) drop-dead males at 25°C vs. 18°C. All animals in this figure contain one autosomal copy of the en enhancer-trap P-element insert, with or without drop-dead on the X-chromosome.
Age-Related Markers.
We chose the three lacZ-marked (17, 23–25) genes wg (16), en (16), and 206 (10, 26), whose relative temporal patterns of expression of β-gal have been shown to correlate with life span at different ambient temperatures (18°C, 25°C, and 29°C). wg has previously been tested both vs. ambient temperature and in combination with the reduced life span mutants Hk1, Sh5, and superoxide dismutase (SODN1) (ref. 27 and B.R. and S.L.H., unpublished data). Genes en and 206 have so far been tested only vs. ambient temperature (ref. 27 and B.R. and S.L.H., unpublished data). In this paper, we examined all three in the background of the drop-dead mutation. Expression of β-gal was measured in the antenna as a function of chronological age at 25°C and at 18°C, using the method of Helfand et al. (10) based on the optical absorption of the blue X-Gal reaction product. Since experiments have shown that the enzyme decays within a few hours (26) this is a valid measure of current synthesis of β-gal.
Acceleration of Age-Related Markers in drop-dead Animals.
Effect of drop-dead on wg expression. The enhancer-trap line marking the wg gene normally shows a level of β-gal expression that declines from the time of eclosion, reaching a low value at around days 10–15 at 25°C (Fig. 2A) (ref. 27 and B.R. and S.L.H., unpublished data). Since the β-gal expression occurs in a sizable proportion of the antennal cells, and examination of serial sections revealed no decrease in cell count (26), it is unlikely that the decline can be attributed to cell degeneration. In a drop-dead background, the decline is accelerated, reaching its lowest level of expression by days 3–4 (Fig. 2 A and B). In Fig. 2C, where the data are replotted as percentage of life span rather than calendar time, the curves closely resemble one another.
Effect of drop-dead on en expression.
In the enhancer-trap line marking the en gene, β-gal expression in the antenna shows a complex temporal pattern characterized by oscillations superimposed on a general decline (27). In a drop-dead background, the pattern is similar, but accelerated (Fig. 3A). The temporal pattern of expression, normally extending over a period of some 2 months in the lacZ strain at 25°C, is compressed into the 2 weeks of life in the presence of the drop-dead mutation (Fig. 3B).
Effect of drop-dead on 206 expression.
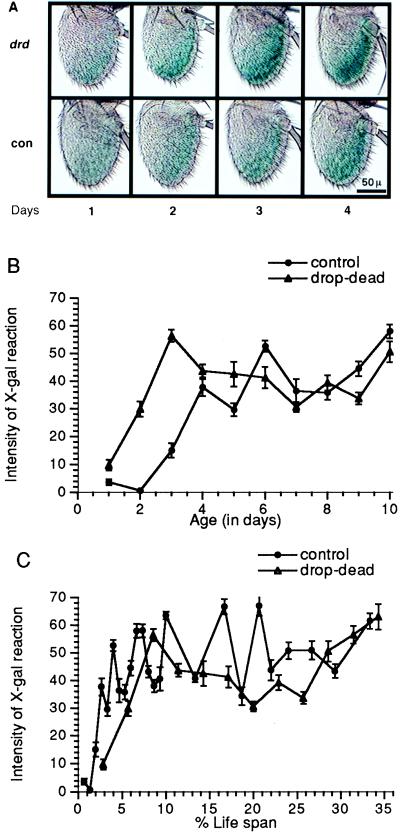
The third marker used was an enhancer-trap strain, designated 206 (10, 26), in which the amount of β-gal expression in the antenna normally increases rapidly in the first days of adult life. This rise was missed in a previous study (26) in which the earliest time point taken was at 10 days, at which time a plateau had already been reached. Because of the relatively fast time scale of expression of this marker, the assay of the effect of drop-dead was performed at 18°C to slow down the time course. This marker is especially pertinent since its increase with time counteracts possible concern that the β-gal-expressing cells might be degenerating with age. In the drop-dead background, β-gal expression in 206 was already strong on day 1, a plateau being attained by day 3 (Fig. 4B). At 18°C, the maximal life span of drop-dead in a 206 background at 18°C was about 40 days, as compared with about 155 days for 206 in the absence of the drop-dead mutation. When plotted with respect to percentage of life span, the temporal pattern of β-gal expression of the 206 marker in the drop-dead animals was comparable with the control animals (Fig. 4C).
Figure 4.
Temporal patterns of expression of the 206 enhancer trap-marked gene in the antennae of drop-dead vs. control males at 18°C. (A) Photomicrographs of whole-mount adult antennae reacted with X-Gal. Each is a typical example from over 10 flies at each age. (B) Relative amount of β-gal expressed in the antennae, plotted vs. chronological age. (C) Plotted vs. percent life span. Each animal contained one autosomal copy of the 206 enhancer-trap insertion, with or without drop-dead on the X-chromosome. (Bars = SEM.)
DISCUSSION
The finding that the temporal expression patterns of three different biomarkers, including one that rises with age, roughly scale in proportion to the life span of the drop-dead animals, suggests that, during its shortened life, the drop-dead fly undergoes, more rapidly, a series of events normally associated with advancing age.
It is important to note that such acceleration of the age-associated scenario by mutations in the drop-dead gene does not identify the normal gene as a longevity gene, per se. Hyperkinetic1 (Hk1), Shaker5 (Sh5), and SODN1 have similar effects on accelerating the choreography of the age-related markers (refs. 11 and 27 and B.R. and S.L.H., unpublished data), yet they all involve different physiological effects. Hk1 is defective in a beta subunit of the potassium channel (28), Sh5 is defective in an alpha subunit of the potassium channel (29–31), and SODN1 is defective in an enzyme (superoxide dismutase) (8). These clearly cannot all be “the aging gene,” yet mutations in all of them appear to speed up ongoing processes that, in normal flies, proceed with chronological time and culminate in death of the fly. It remains to identify the key physiological events in the scenario illuminated by the changing expression patterns of the marker genes.
Note, that in the search for genes conferring longevity, one of the difficulties is the long time one must wait to score survival. The rapidity of drop-dead’s demise offers the advantage that it can facilitate the search for such genes (Y.-J. Lin and S.B., unpublished data).
Acknowledgments
We are grateful to Joseph Jack, Deborah Foster, William Orr, Robert Reenan, Marvin Tanzer, and members of the Benzer laboratory for helpful discussions and critical reading of the manuscript. This work was supported by a fellowship to B.R. from The Donaghue Medical Research Foundation, the Sandoz Foundation for Gerontological Research, and the National Institute of Aging-supported Claude Pepper Older Americans Independence Center at the Travelers Center on Aging of the University of Connecticut Health Center, by grants to S.L.H. from the National Science Foundation (IBN-9122097), the American Federation for Aging Research, the Sandoz Foundation for Gerontological Research, and the Shock Aging Research Foundation. Support to S.B. was from the National Science Foundation (MCB 9408718), the National Institutes of Health (AG 12289 and EY 09278), the McKnight Foundation, and the James G. Boswell Foundation.
ABBREVIATIONS
- wg
wingless
- en
engrailed
- β-gal
β-galactosidase
- X-Gal
5-bromo-4-chloro-3-indolyl-β-d-galactoside
References
- 1.Kaplan W D, Trout W E. Genetics. 1969;61:399–409. doi: 10.1093/genetics/61.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griswold C M, Matthews A L, Bewley K E, Mahaffey J W. Genetics. 1993;134:781–788. doi: 10.1093/genetics/134.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orr W C, Sohal R S. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 4.Orr W C, Arnold L A, Sohal R S. Mech Ageing Dev. 1992;63:287–296. doi: 10.1016/0047-6374(92)90006-y. [DOI] [PubMed] [Google Scholar]
- 5.Sohal R, Agarwal A, Agarwal S, Orr W C. J Biol Chem. 1995;270:15671–15674. doi: 10.1074/jbc.270.26.15671. [DOI] [PubMed] [Google Scholar]
- 6.Sohal R S, Weindruch R. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trout W E, Kaplan W D. Exp Gerontol. 1970;5:83–92. doi: 10.1016/0531-5565(70)90033-1. [DOI] [PubMed] [Google Scholar]
- 8.Phillips J P, Campbell S D, Michaud D, Charbonneau M. Proc Natl Acad Sci USA. 1989;86:2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin G M. In: Genetic Effects on Aging II. Harrison D E, editor. Caldwell, NJ: Telford; 1990. pp. 493–520. [Google Scholar]
- 10.Helfand S L, Blake K J, Rogina B, Stracks M D, Centurion A, Naprta B. Genetics. 1995;140:549–555. doi: 10.1093/genetics/140.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogina B, Helfand S L. Genetics. 1995;141:1043–1048. doi: 10.1093/genetics/141.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler J C, Bieschke E T, Tower J. Proc Natl Acad Sci USA. 1995;92:10408–10412. doi: 10.1073/pnas.92.22.10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotta Y, Benzer S. Nature (London) 1972;240:527–535. doi: 10.1038/240527a0. [DOI] [PubMed] [Google Scholar]
- 14.Benzer S. J Am Med Assoc. 1971;218:1015–1022. [Google Scholar]
- 15.Buchanan R L, Benzer S. Neuron. 1993;10:839–850. doi: 10.1016/0896-6273(93)90200-b. [DOI] [PubMed] [Google Scholar]
- 16.Kassis J A, VanSickle E P, Sensabaugh S M. Genetics. 1991;128:751–761. doi: 10.1093/genetics/128.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bier E, Vassin H, Shepherd S, Lee K, McCall K, Barbel S, Ackerman L, Carretto L, Uemura T, Greel E, Jan L, Jan Y. Genes Dev. 1989;3:1273–1287. doi: 10.1101/gad.3.9.1273. [DOI] [PubMed] [Google Scholar]
- 18.Helfand S L, Carlson J. Proc Natl Acad USA. 1989;86:2908–2912. doi: 10.1073/pnas.86.8.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashburner, M. (1989) Drosophila: A Laboratory Handbook, eds. (Cold Spring Harbor Lab. Press, Plainview, NY).
- 20.Glaser R L, Lis J T. Mol Cell Biol. 1990;10:131–137. doi: 10.1128/mcb.10.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blake K, Rogina B, Centurion A, Helfand S L. Mech Dev. 1995;52:179–185. doi: 10.1016/0925-4773(95)00398-k. [DOI] [PubMed] [Google Scholar]
- 22.Miquel J, Lundgren P R, Bensch K G, Atlan H. Mech Ageing Dev. 1976;5:347–370. doi: 10.1016/0047-6374(76)90034-8. [DOI] [PubMed] [Google Scholar]
- 23.O’Kane K, Gehring W. Proc Natl Acad Sci USA. 1987;84:9123–9127. doi: 10.1073/pnas.84.24.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman M. Curr Biol. 1991;1:378–381. doi: 10.1016/0960-9822(91)90199-7. [DOI] [PubMed] [Google Scholar]
- 25.Bellen H J, O’Kane C J, Wilson C, Grossniklaus U, Pearson R K, Gehring W J. Genes Dev. 1989;3:1288–1300. doi: 10.1101/gad.3.9.1288. [DOI] [PubMed] [Google Scholar]
- 26.Helfand S L, Naprta B. Mech Dev. 1996;55:45–51. doi: 10.1016/0925-4773(95)00489-0. [DOI] [PubMed] [Google Scholar]
- 27.Rogina B, Helfand S L. Mech Dev. 1997;63:89–97. doi: 10.1016/s0925-4773(97)00033-6. [DOI] [PubMed] [Google Scholar]
- 28.Chouinard S W, Wilson G F, Schlimgen A K, Ganetzky B. Proc Natl Acad Sci USA. 1995;92:6763–6767. doi: 10.1073/pnas.92.15.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamb A, Iverson L E, Tanouye M A. Cell. 1987;50:405–13. doi: 10.1016/0092-8674(87)90494-6. [DOI] [PubMed] [Google Scholar]
- 30.Papazian D M, Schwarz T L, Tempel B L, Jan Y N, Jan L Y. Science. 1987;237:749–53. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- 31.Baumann A, Krah-Jentgens I, Muller R, Muller-Holtkamp Siedel, Kecskemethy N, Canal I, Ferrus A, Pongs O. EMBO J. 1987;6:3419–29. doi: 10.1002/j.1460-2075.1987.tb02665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]