Abstract
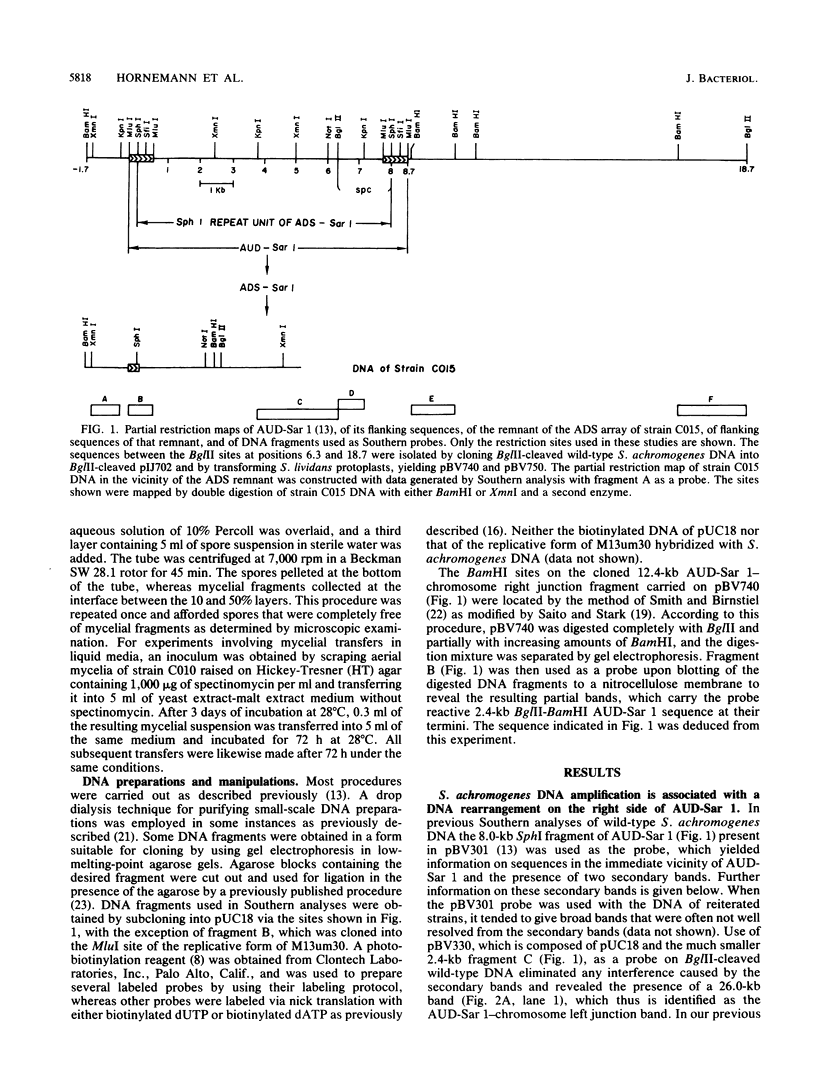
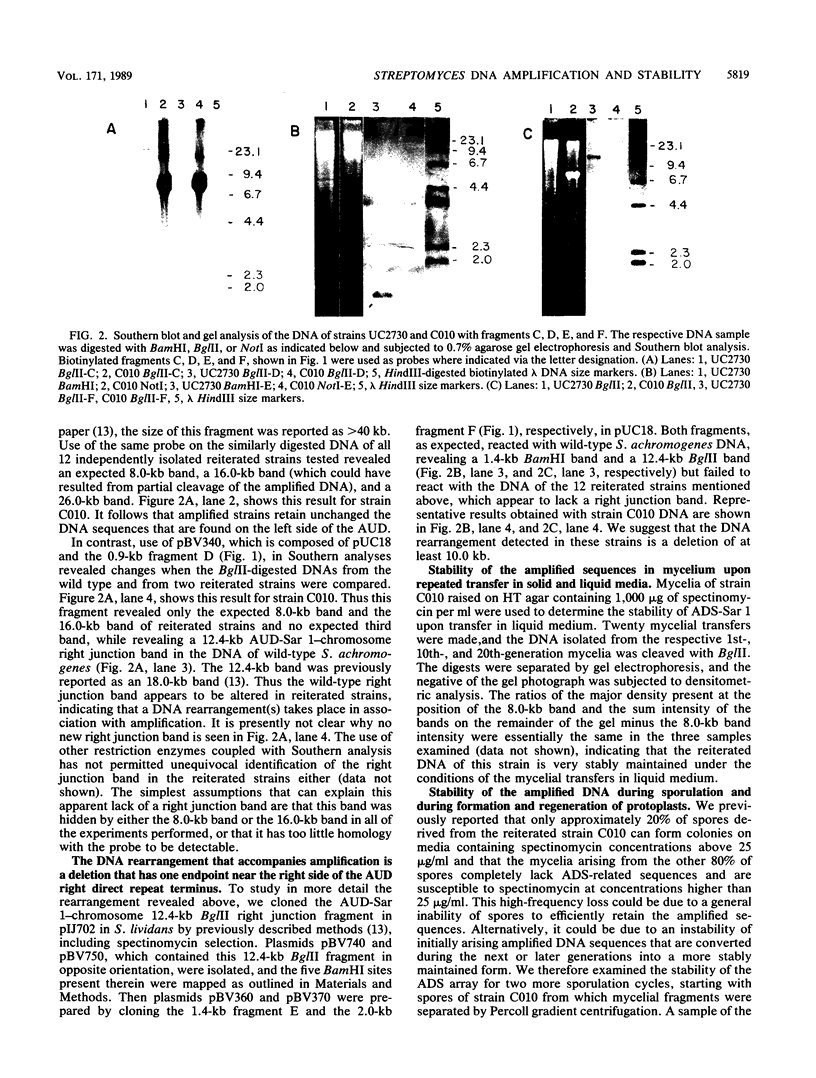
Southern blot analysis of BglII-digested DNA isolated from wild-type Streptomyces achromogenes, which harbors the 8.8-kilobase amplifiable unit of DNA, AUD-Sar 1, and of similarly digested DNA from 12 strains carrying an array of 200 to 300 tandem copies of a specific AUD-Sar 1-derived 8.0-kilobase DNA sequence, ADS-Sar 1, revealed the absence of the 12.4-kilobase BglII AUD-Sar 1-chromosome right junction band in the latter strains, whereas the corresponding 26.0-kilobase left junction band remained unaltered. Further Southern analyses indicated in all of the seven amplified strains tested the occurrence of a deletion of at least 10 kilobases of the DNA adjacent to the right side of the AUD. The deletion has one endpoint in the vicinity of the ADS array. Corroborating and expanding upon previously reported results, we found that the amplified DNA of strain C010 was stably maintained for at least 20 transfers when the transfers involved mycelia propagated in spectinomycin-free liquid medium. In contrast, when strain C010 was subjected separately to one cycle of protoplast formation and regeneration or to three cycles of spore germination, aerial mycelium formation, and sporulation on spectinomycin-free media, only approximately 20% of the protoplast regenerants and spores retained the reiterated DNA sequences and the ability subsequently to form colonies on media containing high levels of spectinomycin. Approximately 80% of these units completely deleted the reiterated DNA and left adjacent sequences and exhibited sensitivity to 25 micrograms of spectinomycin per ml. One among 24 protoplast-derived deletants apparently retained the left portion of the AUD-ADS left direct repeat plus left adjacent sequences.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbuchner J., Cullum J. Structure of an amplifiable DNA sequence in Streptomyces lividans 66. Mol Gen Genet. 1985;201(2):192–197. doi: 10.1007/BF00425659. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cullum J., Altenbuchner J., Flett F., Piendl W. DNA amplification and genetic instability in Streptomyces. Biotechnol Genet Eng Rev. 1986;4:59–78. doi: 10.1080/02648725.1986.10647823. [DOI] [PubMed] [Google Scholar]
- Dyson P., Schrempf H. Genetic instability and DNA amplification in Streptomyces lividans 66. J Bacteriol. 1987 Oct;169(10):4796–4803. doi: 10.1128/jb.169.10.4796-4803.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flett F., Cullum J. DNA deletions in spontaneous chloramphenicol-sensitive mutants of Streptomyces coelicolor A 3(2) and Streptomyces lividans 66. Mol Gen Genet. 1987 May;207(2-3):499–502. doi: 10.1007/BF00331621. [DOI] [PubMed] [Google Scholar]
- Forster A. C., McInnes J. L., Skingle D. C., Symons R. H. Non-radioactive hybridization probes prepared by the chemical labelling of DNA and RNA with a novel reagent, photobiotin. Nucleic Acids Res. 1985 Feb 11;13(3):745–761. doi: 10.1093/nar/13.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M., Hintermann G., Simonet J. M., Crameri R., Piret J., Hütter R. Certain chromosomal regions in Streptomyces glaucescens tend to carry amplifications and deletions. Mol Gen Genet. 1985;200(3):375–384. doi: 10.1007/BF00425720. [DOI] [PubMed] [Google Scholar]
- Hashimoto H., Rownd R. H. Transition of the R factor NR1 and Proteus mirabilis: level of drug resistance of nontransitioned and transitioned cells. J Bacteriol. 1975 Jul;123(1):56–68. doi: 10.1128/jb.123.1.56-68.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Kieser T., Wright H. M., Bibb M. J. Plasmids, recombination and chromosome mapping in Streptomyces lividans 66. J Gen Microbiol. 1983 Jul;129(7):2257–2269. doi: 10.1099/00221287-129-7-2257. [DOI] [PubMed] [Google Scholar]
- Hornemann U., Otto C. J., Hoffman G. G., Bertinuson A. C. Spectinomycin resistance and associated DNA amplification in Streptomyces achromogenes subsp. rubradiris. J Bacteriol. 1987 Jun;169(6):2360–2366. doi: 10.1128/jb.169.6.2360-2366.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa J., Koyama Y., Mizuno S., Hotta K. Mechanism of increased kanamycin-resistance generated by protoplast regeneration of Streptomyces griseus. II. Mutational gene alteration and gene amplification. J Antibiot (Tokyo) 1988 Jan;41(1):104–112. doi: 10.7164/antibiotics.41.104. [DOI] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Potekhin Ia A., Danilenko V. N. Determinant ustoichivosti k kanamitsinu Streptomyces rimosus: amplifikatsiia v sostave khromosomy i obratimaia geneticheskaia nestabil'nost'. Mol Biol (Mosk) 1985 May-Jun;19(3):805–817. [PubMed] [Google Scholar]
- Saito I., Stark G. R. Charomids: cosmid vectors for efficient cloning and mapping of large or small restriction fragments. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8664–8668. doi: 10.1073/pnas.83.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]