Abstract
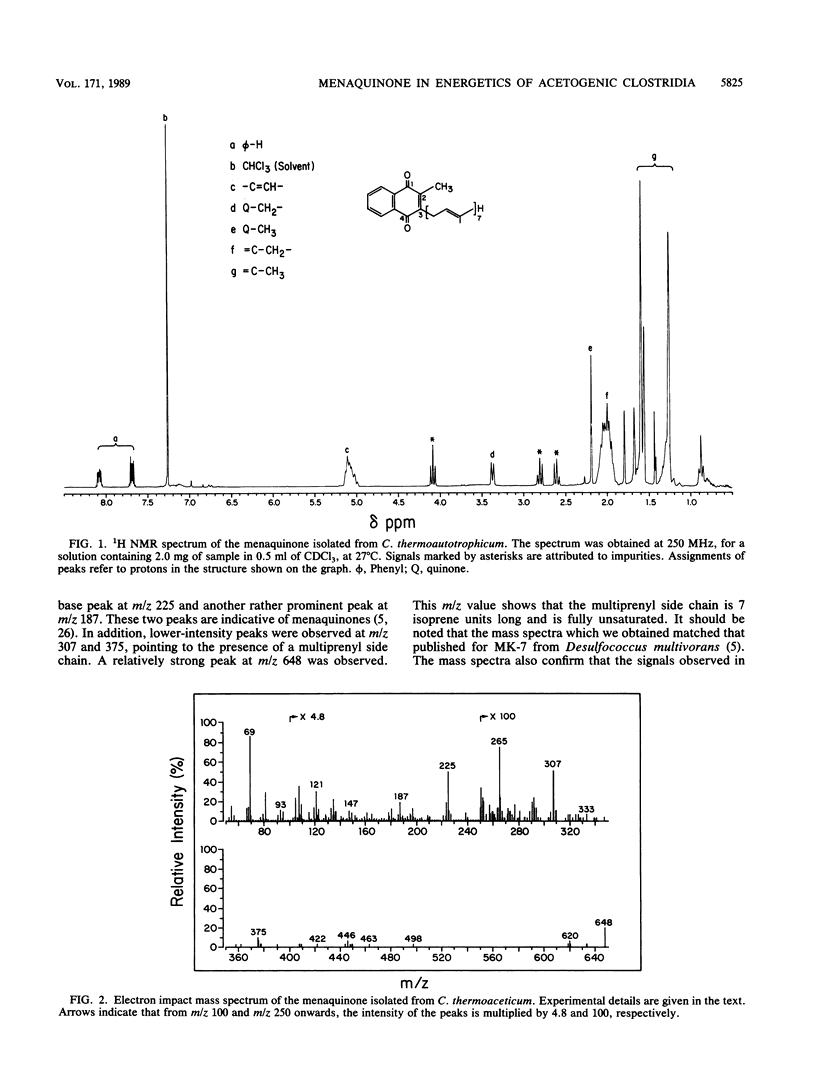
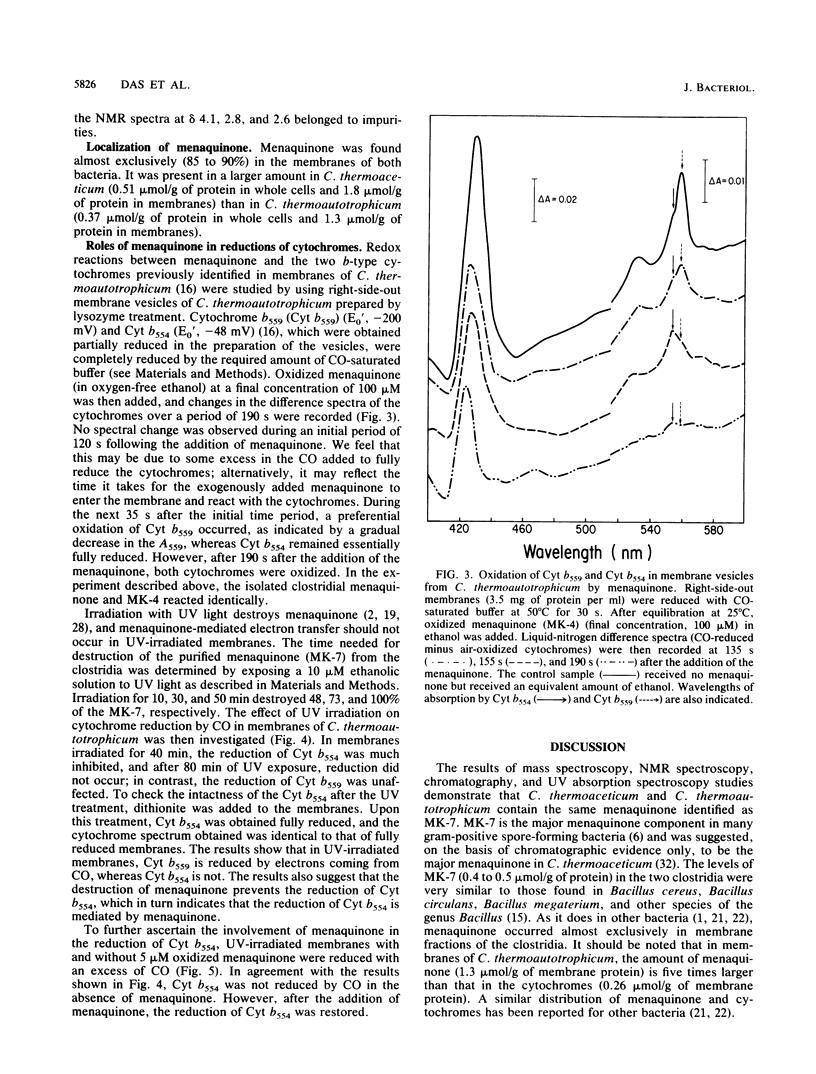
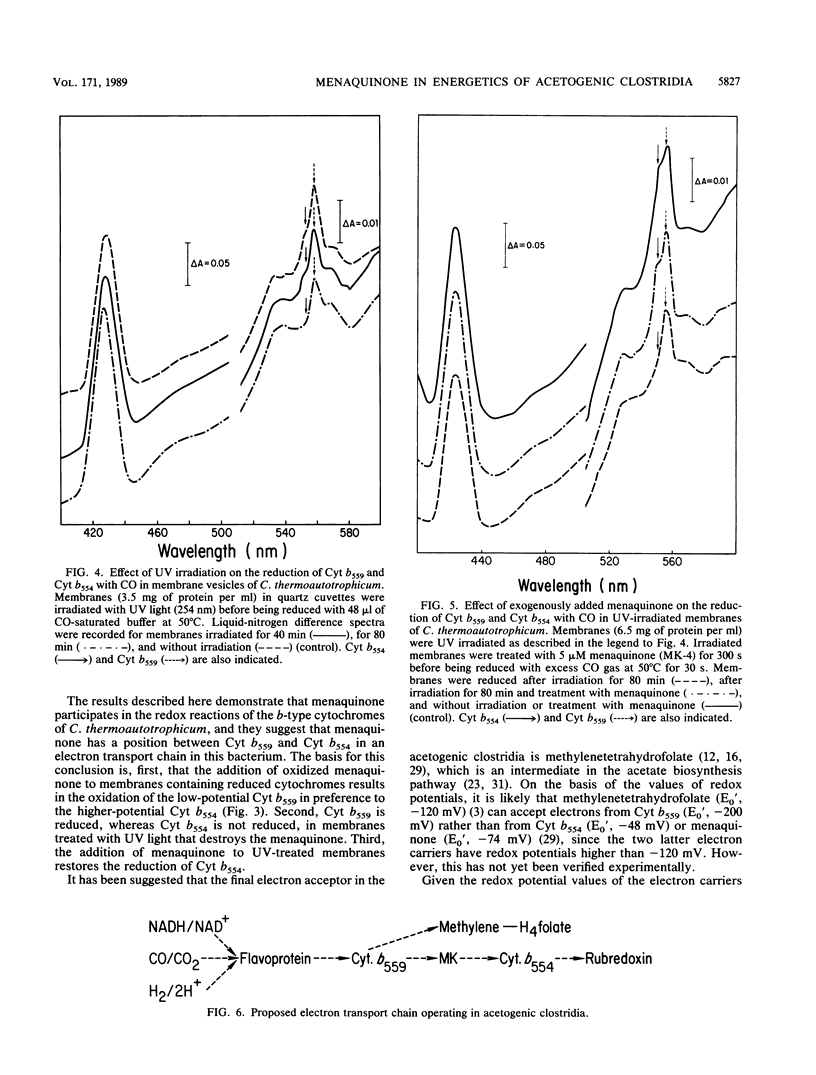
Clostridium thermoaceticum and Clostridium thermoautotrophicum contain the same menaquinone. Its structure, determined by thin-layer chromatography, UV absorption spectroscopy, mass spectrometry, and nuclear magnetic resonance spectroscopy, was found to be MK-7 (2-methyl-3-heptaprenyl-1,4-naphthoquinone). The menaquinone is located in the cytoplasmic membranes and is involved in redox reactions of two b-type cytochromes present in the clostridia. These reactions were studied with right-side-out membranes prepared from C. thermoautotrophicum by using CO as an electron donor. In intact membranes, both cytochromes were reduced, whereas after inactivation of the menaquinone by exposure of the membranes to UV irradiation, reduction of the low-potential cytochrome (Eo', -200 mV) but not of the high-potential cytochrome (Eo', -48 mV) occurred. The reduction of the high-potential cytochrome in UV-irradiated membranes was restored following the addition of oxidized menaquinone and with an excess of CO. The addition of oxidized menaquinone to reduced membranes resulted initially in a preferential oxidation of the low-potential cytochrome. The results obtained indicate that the menaquinone acts between the two b-type cytochromes in an electron transport chain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP D. H., KING H. K. Ubiquinone and vitamin K in bacteria. 2. Intracellular distribution in Escherichia coli and Micrococcus lysodeikticus. Biochem J. 1962 Dec;85:550–554. doi: 10.1042/bj0850550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. E., Ljungdahl L. G. Purification and properties of 5,10-methylenetetrahydrofolate reductase, an iron-sulfur flavoprotein from Clostridium formicoaceticum. J Biol Chem. 1984 Sep 10;259(17):10845–10849. [PubMed] [Google Scholar]
- Clark J. E., Ragsdale S. W., Ljungdahl L. G., Wiegel J. Levels of enzymes involved in the synthesis of acetate from CO2 in Clostridium thermoautotrophicum. J Bacteriol. 1982 Jul;151(1):507–509. doi: 10.1128/jb.151.1.507-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. D., Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev. 1981 Jun;45(2):316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. D., Pirouz T., Goodfellow M., Minnikin D. E. Distribution of menaquinones in actinomycetes and corynebacteria. J Gen Microbiol. 1977 Jun;100(2):221–230. doi: 10.1099/00221287-100-2-221. [DOI] [PubMed] [Google Scholar]
- Elliott J. I., Brewer J. M. The inactivation of yeast enolase by 2,3-butanedione. Arch Biochem Biophys. 1978 Sep;190(1):351–357. doi: 10.1016/0003-9861(78)90285-0. [DOI] [PubMed] [Google Scholar]
- Elliott J. I., Ljungdahl L. G. Isolation and characterization of an Fe,-S8 ferredoxin (ferredoxin II) from Clostridium thermoaceticum. J Bacteriol. 1982 Jul;151(1):328–333. doi: 10.1128/jb.151.1.328-333.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand S. K., Taber H. W. Changes in menaquinone concentration during growth and early sporulation in Bacillus subtilis. J Bacteriol. 1974 Jan;117(1):324–326. doi: 10.1128/jb.117.1.324-326.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald M., Andreesen J. R., LeGall J., Ljungdahl L. G. Presence of cytochrome and menaquinone in Clostridium formicoaceticum and Clostridium thermoaceticum. J Bacteriol. 1975 Apr;122(1):325–328. doi: 10.1128/jb.122.1.325-328.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Schairer H. U. Electron-transport chains of Escherichia coli. Reconstitution of respiration in a 5-aminolaevulinic acid-requiring mutant. Eur J Biochem. 1973 May;35(1):34–45. doi: 10.1111/j.1432-1033.1973.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Hess A., Holländer R., Mannheim W. Lipoquinones of some spore-forming rods, lactic-acid bacteria and actinomycetes. J Gen Microbiol. 1979 Nov;115(1):247–252. doi: 10.1099/00221287-115-1-247. [DOI] [PubMed] [Google Scholar]
- Hugenholtz J., Ivey D. M., Ljungdahl L. G. Carbon monoxide-driven electron transport in Clostridium thermoautotrophicum membranes. J Bacteriol. 1987 Dec;169(12):5845–5847. doi: 10.1128/jb.169.12.5845-5847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz J., Ljungdahl L. G. Electron transport and electrochemical proton gradient in membrane vesicles of Clostridium thermoautotrophicum. J Bacteriol. 1989 May;171(5):2873–2875. doi: 10.1128/jb.171.5.2873-2875.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey D. M., Ljungdahl L. G. Purification and characterization of the F1-ATPase from Clostridium thermoaceticum. J Bacteriol. 1986 Jan;165(1):252–257. doi: 10.1128/jb.165.1.252-257.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Doyle R. J., Kubitschek H. E. Inactivation of membrane transport in Escherichia coli by near-ultraviolet light. J Bacteriol. 1976 Apr;126(1):140–146. doi: 10.1128/jb.126.1.140-146.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A., Dadák V. On the role of quinones in bacterial electron transport. The respiratory system of Bacillus megaterium. Eur J Biochem. 1969 Dec;11(2):328–340. doi: 10.1111/j.1432-1033.1969.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Kröger A. Fumarate as terminal acceptor of phosphorylative electron transport. Biochim Biophys Acta. 1978 Oct 23;505(2):129–145. doi: 10.1016/0304-4173(78)90010-1. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G., Andreesen J. R. Formate dehydrogenase, a selenium--tungsten enzyme from Clostridium thermoaceticum. Methods Enzymol. 1978;53:360–372. doi: 10.1016/s0076-6879(78)53042-5. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Sommer P., Kofler M. Physicochemical properties and methods of analysis of phylloquinones, menaquinones, ubiquinones, plastoquinones, menadione, and related compounds. Vitam Horm. 1966;24:349–399. doi: 10.1016/s0083-6729(08)60211-3. [DOI] [PubMed] [Google Scholar]
- Sone N. Isolation of a novel menaquinone with a partly hydrogenated side chain from Propionibacterium arabinosum. J Biochem. 1974 Jul;76(1):133–136. doi: 10.1093/oxfordjournals.jbchem.a130537. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. S., Ljungdahl L. G., Dervartanian D. V., Watt G. D. Isolation and characterization of two rubredoxins from Clostridium thermoaceticum. Biochim Biophys Acta. 1980 Mar 7;590(1):24–33. doi: 10.1016/0005-2728(80)90143-7. [DOI] [PubMed] [Google Scholar]



