Abstract
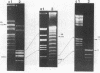
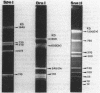
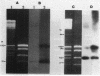
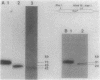
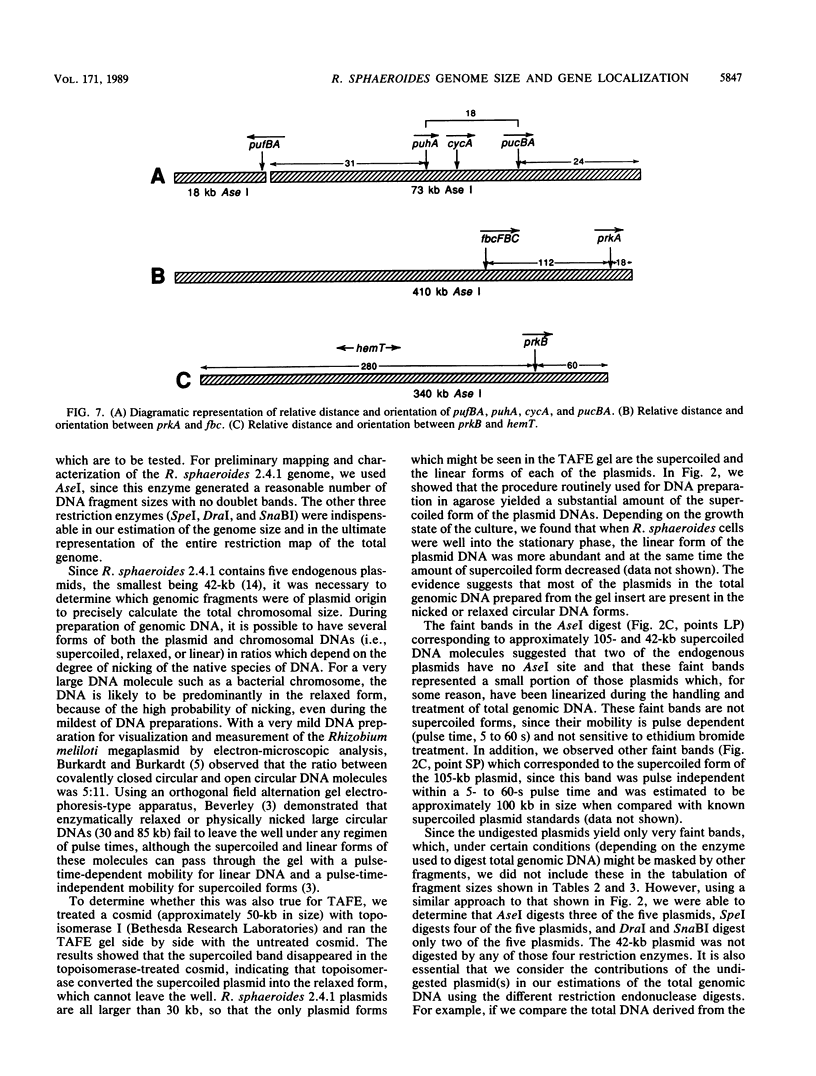
Four restriction endonucleases, AseI (5'-ATTAAT), SpeI (5'-ACTAGT), DraI (5'-TTTAAA), and SnaBI (5'-TACGTA), generated DNA fragments of suitable size distributions for mapping the genome of Rhodobacter sphaeroides by transverse alternating field electrophoresis. AseI produced 17 fragments, ranging in size from 3 to 1,105 kilobases (kb), SpeI yielded 16 fragments (12 to 1,645 kb), DraI yielded at least 25 fragments (6 to 800 kb), and SnaBI generated 10 fragments (12 to 1,225 kb). A total genome size of approximately 4,400 +/- 112 kb was determined by summing the fragment lengths in each of the digests generated by using the different restriction endonucleases. The total genomic DNA consisted of chromosomal DNA (3,960 +/- 112 kb) and the five endogenous plasmids (approximately 450 kb total) whose cognate DNA fragments have been unambiguously identified. A number of genes have been physically mapped to the AseI-generated restriction endonuclease fragments of total genomic DNA by Southern hybridization analysis with either homologous or heterologous specific gene probes or, in the case of several auxotrophic and pigment-biosynthetic mutants apparently generated by Tn5, a Tn5-specific probe. Other genes have been mapped by a comparison with wild-type patterns of the electrophoretic banding patterns of the AseI-digested genomic DNA derived from mutants generated by the insertion of either kanamycin or spectinomycin-streptomycin resistance cartridges. The relative orientations, distance, and location of the pufBALMX, puhA, cycA, and pucBA operons have also been determined, as have been the relative orientations between prkB and hemT and between prkA and the fbc operon.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Bourg G., Ramuz M., Pages M., Bellis M., Roizes G. DNA polymorphism in strains of the genus Brucella. J Bacteriol. 1988 Oct;170(10):4603–4607. doi: 10.1128/jb.170.10.4603-4607.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G., Friesen J. D. The nucleotide sequence of tufB and four nearby tRNA structural genes of Escherichia coli. Gene. 1980 Dec;12(1-2):33–39. doi: 10.1016/0378-1119(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Beverley S. M. Characterization of the 'unusual' mobility of large circular DNAs in pulsed field-gradient electrophoresis. Nucleic Acids Res. 1988 Feb 11;16(3):925–939. doi: 10.1093/nar/16.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkardt B., Burkardt H. J. Visualization and exact molecular weight determination of a Rhizobium meliloti megaplasmid. J Mol Biol. 1984 May 15;175(2):213–218. doi: 10.1016/0022-2836(84)90475-3. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., Hoger J. H., Kaplan S. Cloning and expression of the Rhodobacter sphaeroides reaction center H gene. J Bacteriol. 1986 Nov;168(2):953–961. doi: 10.1128/jb.168.2.953-961.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., McEwan A. G., Kaplan S. Cloning, DNA sequence, and expression of the Rhodobacter sphaeroides cytochrome c2 gene. J Bacteriol. 1986 Nov;168(2):962–972. doi: 10.1128/jb.168.2.962-972.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B., Gerardot C. J. Use of pulsed-field-gradient gel electrophoresis to construct a physical map of the Caulobacter crescentus genome. Gene. 1988 Sep 7;68(2):323–333. doi: 10.1016/0378-1119(88)90035-2. [DOI] [PubMed] [Google Scholar]
- Fornari C. S., Watkins M., Kaplan S. Plasmid distribution and analyses in Rhodopseudomonas sphaeroides. Plasmid. 1984 Jan;11(1):39–47. doi: 10.1016/0147-619x(84)90005-2. [DOI] [PubMed] [Google Scholar]
- Gabellini N., Harnisch U., McCarthy J. E., Hauska G., Sebald W. Cloning and expression of the fbc operon encoding the FeS protein, cytochrome b and cytochrome c1 from the Rhodopseudomonas sphaeroides b/c1 complex. EMBO J. 1985 Feb;4(2):549–553. doi: 10.1002/j.1460-2075.1985.tb03663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner K., Laas W., Patterson D. Fractionation of large mammalian DNA restriction fragments using vertical pulsed-field gradient gel electrophoresis. Somat Cell Mol Genet. 1986 Mar;12(2):185–195. doi: 10.1007/BF01560665. [DOI] [PubMed] [Google Scholar]
- Jin D. J., Gross C. A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988 Jul 5;202(1):45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- Kiley P. J., Donohue T. J., Havelka W. A., Kaplan S. DNA sequence and in vitro expression of the B875 light-harvesting polypeptides of Rhodobacter sphaeroides. J Bacteriol. 1987 Feb;169(2):742–750. doi: 10.1128/jb.169.2.742-750.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Cloning, DNA sequence, and expression of the Rhodobacter sphaeroides light-harvesting B800-850-alpha and B800-850-beta genes. J Bacteriol. 1987 Jul;169(7):3268–3275. doi: 10.1128/jb.169.7.3268-3275.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Molecular genetics of photosynthetic membrane biosynthesis in Rhodobacter sphaeroides. Microbiol Rev. 1988 Mar;52(1):50–69. doi: 10.1128/mr.52.1.50-69.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokjohn T. A., Miller R. V. Molecular cloning and characterization of the recA gene of Pseudomonas aeruginosa PAO. J Bacteriol. 1985 Aug;163(2):568–572. doi: 10.1128/jb.163.2.568-572.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. C., Kohara Y., Akiyama K., Smith C. L., Craigen W. J., Caskey C. T. Rapid and precise mapping of the Escherichia coli release factor genes by two physical approaches. J Bacteriol. 1988 Oct;170(10):4537–4541. doi: 10.1128/jb.170.10.4537-4541.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Smith H. O. Sizing of the Haemophilus influenzae Rd genome by pulsed-field agarose gel electrophoresis. J Bacteriol. 1988 Sep;170(9):4402–4405. doi: 10.1128/jb.170.9.4402-4405.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. K., Kiley P. J., Kaplan S. Posttranscriptional control of puc operon expression of B800-850 light-harvesting complex formation in Rhodobacter sphaeroides. J Bacteriol. 1989 Jun;171(6):3391–3405. doi: 10.1128/jb.171.6.3391-3405.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueking D. R., Fraley R. T., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Fate of "old" and "new" membrane. J Biol Chem. 1978 Jan 25;253(2):451–457. [PubMed] [Google Scholar]
- Meinhardt S. W., Kiley P. J., Kaplan S., Crofts A. R., Harayama S. Characterization of light-harvesting mutants of Rhodopseudomonas sphaeroides. I. Measurement of the efficiency of energy transfer from light-harvesting complexes to the reaction center. Arch Biochem Biophys. 1985 Jan;236(1):130–139. doi: 10.1016/0003-9861(85)90612-5. [DOI] [PubMed] [Google Scholar]
- Miller L., Kaplan S. Plasmid transfer and expression in Rhodopseudomonas sphaeroides. Arch Biochem Biophys. 1978 Apr 15;187(1):229–234. doi: 10.1016/0003-9861(78)90028-0. [DOI] [PubMed] [Google Scholar]
- Moore M. D., Kaplan S. Construction of TnphoA gene fusions in Rhodobacter sphaeroides: isolation and characterization of a respiratory mutant unable to utilize dimethyl sulfoxide as a terminal electron acceptor during anaerobic growth in the dark on glucose. J Bacteriol. 1989 Aug;171(8):4385–4394. doi: 10.1128/jb.171.8.4385-4394.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nano F. E., Kaplan S. Plasmid rearrangements in the photosynthetic bacterium Rhodopseudomonas sphaeroides. J Bacteriol. 1984 Jun;158(3):1094–1103. doi: 10.1128/jb.158.3.1094-1103.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Pemberton J. M., Bowen A. R. High-frequency chromosome transfer in Rhodopseudomonas sphaeroides promoted by broad-host-range plasmid RP1 carrying mercury transposon Tn501. J Bacteriol. 1981 Jul;147(1):110–117. doi: 10.1128/jb.147.1.110-117.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Pyle L. E., Corcoran L. N., Cocks B. G., Bergemann A. D., Whitley J. C., Finch L. R. Pulsed-field electrophoresis indicates larger-than-expected sizes for mycoplasma genomes. Nucleic Acids Res. 1988 Jul 11;16(13):6015–6025. doi: 10.1093/nar/16.13.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle L. E., Finch L. R. A physical map of the genome of Mycoplasma mycoides subspecies mycoides Y with some functional loci. Nucleic Acids Res. 1988 Jul 11;16(13):6027–6039. doi: 10.1093/nar/16.13.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Sistrom W. R. Transfer of chromosomal genes mediated by plasmid r68.45 in Rhodopseudomonas sphaeroides. J Bacteriol. 1977 Aug;131(2):526–532. doi: 10.1128/jb.131.2.526-532.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Cantor C. R. Purification, specific fragmentation, and separation of large DNA molecules. Methods Enzymol. 1987;155:449–467. doi: 10.1016/0076-6879(87)55030-3. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Kolodner R. D. Mapping of Escherichia coli chromosomal Tn5 and F insertions by pulsed field gel electrophoresis. Genetics. 1988 Jun;119(2):227–236. doi: 10.1093/genetics/119.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockett R. E., Donohue T. J., Varga A. R., Kaplan S. Control of photosynthetic membrane assembly in Rhodobacter sphaeroides mediated by puhA and flanking sequences. J Bacteriol. 1989 Jan;171(1):436–446. doi: 10.1128/jb.171.1.436-446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: presence of two unique circular chromosomes. J Bacteriol. 1989 Nov;171(11):5850–5859. doi: 10.1128/jb.171.11.5850-5859.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita F. R. Molecular and cellular regulation of autotrophic carbon dioxide fixation in microorganisms. Microbiol Rev. 1988 Jun;52(2):155–189. doi: 10.1128/mr.52.2.155-189.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai T. N., Moore M. D., Kaplan S. Cloning and characterization of the 5-aminolevulinate synthase gene(s) from Rhodobacter sphaeroides. Gene. 1988 Oct 15;70(1):139–151. doi: 10.1016/0378-1119(88)90112-6. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Shiina T., Takahashi H. Multiple principal sigma factor homologs in eubacteria: identification of the "rpoD box". Science. 1988 Nov 18;242(4881):1040–1042. doi: 10.1126/science.3194753. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Falk G., Walker J. E. Rhodopseudomonas blastica atp operon. Nucleotide sequence and transcription. J Mol Biol. 1984 Oct 25;179(2):185–214. doi: 10.1016/0022-2836(84)90465-0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Woisetschläger M., Hödl-Neuhofer A., Högenauer G. Localization of the kdsA gene with the aid of the physical map of the Escherichia coli chromosome. J Bacteriol. 1988 Nov;170(11):5382–5384. doi: 10.1128/jb.170.11.5382-5384.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel C. B. THE CULTURE, GENERAL PHYSIOLOGY, MORPHOLOGY, AND CLASSIFICATION OF THE NON-SULFUR PURPLE AND BROWN BACTERIA. Bacteriol Rev. 1944 Mar;8(1):1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]