Abstract
Introduction
Prenatal exposure to organic methylmercury (MeHg) from seafood consumption has been reported to increase children’s blood pressure (BP). A report from the Faroe Islands noted significantly increased diastolic and systolic BP in 7 year old children as prenatal MeHg exposure increased. The Faroese diet includes sea mammals that contain MeHg, cadmium, and other pollutants. We examined this relationship in the Seychelles Islands to determine if it was present in a society exposed primarily to MeHg from consuming ocean fish.
Methods
We obtained BP at ages 12 and 15 years on children with known prenatal MeHg exposure enrolled in the Seychelles Child Development Study (SCDS). We examined the association between prenatal MeHg exposure and BP using longitudinal models and linear regression adjusted for relevant covariates.
Results
Blood pressure at both ages was associated with BMI, height and maternal hypertension during pregnancy as expected. No association between prenatal MeHg exposure and BP was present in girls at either age or in either sex at age 12 years. At age 15 years diastolic BP in boys increased with increasing prenatal MeHg exposure, while systolic BP was unaffected.
Summary
It is unclear whether the association between prenatal MeHg exposure and diastolic BP seen in 15 year old boys is of biological significance or if it is a chance finding. However, the finding is intriguing and deserves further study.
Keywords: Methylmercury, blood pressure, Seychelles Child Development Study, BMI
Introduction
Prenatal exposure to methyl mercury (MeHg) from seafood consumption has been proposed as a factor that increases blood pressure (BP) in children (Sorensen et al, 1999). An epidemiologic study of toxic exposures in the Faroe Islands reported an association between prenatal MeHg exposure and children’s BP at age 7 years. This association was not present when these children were evaluated at age 14 years (Grandjean et al, 2004). At both ages the children’s BP was within the normal range. This possible association is important since some chronic disease are thought to have a prenatal onset and factors that increase blood pressure increase the risk of developing hypertension and thus cardiac, renal and cerebrovascular complications (Gillman, 2005; Law et al, 1993).
There are reasons to suspect that prenatal MeHg exposure might affect BP. Postnatal poisoning of experimental animals with MeHg is associated with hypertension (Wakita, 1987). Hypertension is a reported complication of postnatal poisoning by other forms of mercury (Hg), such as inorganic, elemental, and phenyl (Warkany, 1996); (Torres et al, 2000); (Al-Damluji, 1976). Hypertension has occasionally been reported with adult Hg poisoning (Hook et al, 1954; Hunter and Russell, 1954). However, blood pressure elevations were not a prominent part of the clinical picture following the extensive poisonings in Iraq or Minamata (Harada, 1968; Jalili and Abbasi, 1961; Bakir et al, 1973). In addition, several studies have examined the relationship between concurrent Hg exposure and BP in adults and found no evidence of an association (Dorea et al, 2005); (Mozaffarian et al, 2005). However one study found an adverse association between concurrent Hg exposure and systolic BP in women, only among non-fish eaters (Vupputuri et al, 2005).
Prenatal exposure to MeHg occurs primarily as a result of maternal fish consumption. Since fish is a primary source of protein for millions of people worldwide, exposure to small amounts of MeHg is widespread. In Japan high levels of MeHg and other pollutants were reported in fish as a result of industrial contamination. One incident occurred in the 1950’s in Minamata, Japan and the other in the 1960s in Niigata, Japan (Tsubaki and Irukayama, 1977; Tsubaki and Takahashi, 1986). In both cases, waste water containing pollutants from a chemical plant was discharged directly into the local waters. At Minamata Hg levels in marine organisms were reported to be as high as 37.5 ppm (Harada, 1995). That level is nearly 100 times higher than levels normally present in ocean fish. At Minamata, the Hg levels in the hair of local residents ranged up to 705 ppm, a value over 700 times higher than the average value in the US. Although hypertension occurred at a higher than usual rate in Minamata, no relationship to MeHg exposure was established (Tamashiro et al 1984). Also, in a study of prenatal MeHg poisoning in Japan (congenital Minamata disease) elevated BP was not present (Oka et al, 2003).
In contrast to Minamata, the ocean surrounding the Seychelles has only background levels of mercury. Hg levels in fish average 0.3 ppm, a level generally similar to those of commercial fish in the US. Women of childbearing age in the Seychelles consume fish on average with 12 meals per week and their hair Hg levels average 6.9 ppm (range 1–27, SD 4.5) (Myers et al, 2003). Seychellois women do not consume sea mammals. In comparison, Hg levels among US women of childbearing age are generally lower. US women have an average of 1 ppb Hg in blood, with hair Hg levels generally below 1 ppm (Vupputuri et al, 2005).(Schober et al, 2003).
If prenatal MeHg exposure from fish consumption does increase children’s BP, it would have important public health consequences. Consequently, we examined this relationship in the main cohort of the Seychelles Child Development Study (SCDS).
Methods
The SCDS is a prospective, observational, double blind investigation of the relationship between prenatal MeHg exposure from maternal fish consumption and children’s neurodevelopment (Myers et al, 2003; Davidson et al, 1998). The main cohort of 779 mother child pairs was enrolled in 1989–1990 when the children were 6 months old. Prenatal MeHg exposure was measured as total mercury (Hg) in maternal hair growing during pregnancy, a value that is known to correlate with infant brain levels (Cernichiari et al, 1995). The maternal, child and family characteristics of this cohort have been reported previously (Shamlaye et al, 1995, Davidson et al, 1998, Myers et al, 2003). Institutional review committees in the Ministry of Health, Republic of Seychelles and at the University of Rochester approved the study. All parents provided informed consent. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Evaluations of BP
BP was not part of the prospective design of the study, but was available from routine school health examinations. School health nurses conduct routine examinations in Kindergarten and grades 4, 7 and 10. In grades 7 and 10 (ages 12 and 15 years respectively) weight, height and BP are measured. Participation in this voluntary program was 86% in 2001 and 80% in 2002. A previous study using data from this program reported a strong association between BMI and both systolic and diastolic BP among the Seychellois children in four school grades (Chiolero et al, 2006). The Ministry of Health maintains a database of screening data from which we extracted the children’s data for those subjects who are part of the SCDS.
Blood pressure was measured by trained school nurses using a validated, automated sphygmomanometer (Omron, HEM 711AC) (O’Brien et al, 2001). The nurses used a cuff (pediatric, standard or large) whose bladder encircled at least 80% of the arm circumference and did not cover the antecubital fossa. The children rested several minutes prior to the BP measurement and were seated with their arm comfortably resting on a table. Duplicate BP readings were obtained on the right arm with an interval of at least one minute between readings. For analysis the average of the duplicate values was used. Weight was measured to 0.1 kg precision without shoes or heavy garments, using precision electronic scales (Seca 870, Hamburg, G). Height was measured with fixed stadiometers to 0.5 cm precision. Body mass index (BMI) was calculated as weight (kg) divided by height (meters) squared.
Exclusions
At age 12 years, 650 subjects had measurements of both diastolic and systolic BP. Five subjects were excluded due to missing covariates and one for an implausible height. At age 15 years, 568 subjects had both BP measurements. Seven subjects were excluded due to missing covariates, one because the weight was implausible, and one who was over 18 years of age.
Statistical Methods
The outcomes for all models were the mean diastolic and mean systolic BP, fit in separate models. The primary analyses consisted of linear longitudinal models with age at testing coded as 0 (12 years) or 1 (15 years). These primary models included an age at testing by MeHg interaction to test the hypothesis that MeHg affects BP differently at the two different ages. Models without a significant interaction term were next run without the interaction term. For any model in which the age at testing by MeHg interaction term was significant, linear regression models were then fit separately for each age. As a subsidiary analysis, the primary longitudinal models were re-run including a sex by age interaction to account for different growth rates among males and females during adolescence. For any model in which the overall MeHg effect was significant, two further subsidiary analyses were fit: (1) a sex-specific model to test whether the MeHg effect was present for both sexes; and (2) an additive model that allowed for a nonlinear relationship between BP and each continuous covariate. A further subsidiary model was fit for the age-specific linear regression models, which included a random effect for school to allow for BP variability across locations. Finally, models reported in detail in tables in this paper were also rerun (1) with adjustment for SES; and (2) in separate models, without covariates that might also be considered endpoints, namely BMI, height, birthweight, and maternal hypertension. SES has been found to be an important predictor of childhood BP in some studies (Colhoun et al 1998), but SES was missing for over 10% of our sample.
Model assumptions for linear regression were checked using standard regression diagnostics (Weisberg, 2005). When the assumptions held, they were not further checked in the longitudinal models. We examined the effects of outliers and influential points (Weisberg, 2005) by fitting linear regression models with and without these points.
Covariates
All models were adjusted for the following covariates: sex, prenatal MeHg exposure, maternal hypertension during pregnancy that required medical treatment, birth weight, age at testing, body mass index (BMI) and height. Maternal hypertension during pregnancy was only recorded as a dichotomous (yes/no) variable. We adjusted for these covariates because each is known or believed to be associated with childhood BP, and associations between these covariates and childhood BP have been reported in the literature (Chiolero et al 2006, Falkner et al 2006, Hardy et al 2006, Sorensen et al 1999) . Other covariates that have been associated with other outcomes in the SCDS, such as mother’s IQ, were not included because there was no reason to believe that they would affect childhood BP.
Results
Complete data were available on 644 children (313 boys and 331 girls) at age 12 years and 559 children (267 boys and 292 girls) at age 15 years. For the longitudinal models there was complete data at both ages on 524 children (244 boys and 280 girls). The means and ranges of the BP measurements and covariates are presented in Table 1.
Table 1.
Summary statistics
| Age at Testing | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 12 years | 15 years | ||||||||
| Units | Boys (n=313) | Girls (n=331) | Boys (n=267) | Girls (n=292) | |||||
| Mean or proportion | Range | Mean or proportion | Range | Mean or proportion | Range | Mean or proportion | Range | ||
| Prenatal MeHg exposure* | ppm | 6.6 | 0.5–23.1 | 7.0 | 0.7–26.7 | 6.5 | 0.5–20.0 | 7.0 | 0.8–26.7 |
| Birth weight | kg | 3.3 | 1.5–5.3 | 3.1 | 1.7–4.6 | 3.3 | 1.9–5.3 | 3.1 | 1.7–5.0 |
| BMI | kg/m2 | 18.5 | 12.4–31.1 | 19.7 | 12.7–36.5 | 20.0 | 13.2–35.4 | 21.2 | 12.9–37.6 |
| Height | cm | 153 | 131–178 | 155 | 130–174 | 169 | 141–187 | 161 | 144–181 |
| Age at testing | years | 12.7 | 11.7–13.4 | 12.6 | 11.6–13.4 | 15.4 | 14.8–16.2 | 15.4 | 14.7–16.3 |
| Blood pressure | |||||||||
| Diastolic | mmHg | 63.7 | 40–99 | 67.2 | 45–96 | 66.7 | 40–93 | 68.8 | 53–92 |
| Systolic | mmHg | 104 | 75–154 | 107 | 78–144 | 112 | 83–153 | 107 | 79–143 |
| Maternal hypertension | % | 6.7 | 7.6 | 7.9 | 8.2 | ||||
Prenatal MeHg exposure (MeHg) was measured as the concentration of total mercury (Hg/g or ppm) in maternal hair growing during pregnancy, see methods section.
BMI = body mass index, ppm = parts per million, mmHg = millimeters mercury.
At age 12 years the correlation between the duplicate systolic readings and the duplicate diastolic readings was 0.76 and 0.57 respectively while at age 15 years they were 0.76 and 0.67 respectively. As noted previously, the average of the duplicate readings was used for analysis. The Pearson correlations between average systolic and average diastolic BP at each age and between BP and MeHg are presented in Table 2. Of note is the change in sign and increased magnitude of the correlation between MeHg and diastolic BP (column 6) at age 15 versus at age 12, particularly among boys.
Table 2.
Pearson correlations between average blood pressure measurements (mm Hg) at a single age and between two ages, and correlations between average BP and prenatal MeHg exposure. BP measurements are the average of duplicate readings.
| N | Systolic BP and Diastolic BP | Diastolic BP (2 ages) | Systolic BP (2 ages) | Diastolic BP and MeHg | Systolic BP and MeHg | |
|---|---|---|---|---|---|---|
| Both sexes: | ||||||
| 12 years | 644 | 0.64 | - | - | −0.02 | 0.02 |
| 15 years | 559 | 0.50 | - | - | 0.10 | 0.03 |
| Across years | 524 | - | 0.32 | 0.34 | - | - |
| Boys: | ||||||
| 12 years | 313 | 0.59 | - | - | −0.03 | 0.03 |
| 15 years | 267 | 0.55 | - | 0.17 | 0.06 | |
| Across years | 244 | - | 0.22 | 0.34 | - | - |
| Girls: | ||||||
| 12 years | 331 | 0.69 | - | - | −0.03 | 0 |
| 15 years | 292 | 0.55 | - | - | 0.04 | 0.04 |
| Across years | 280 | - | 0.39 | 0.40 | - | - |
BP=blood pressure, MeHg = methylmercury. Column 3 of this table gives the correlations between average diastolic and average systolic BP at a single age (12 years or 15 years, as indicated in column 1). Correlations between measurements at two different ages are given in columns 4 and 5. For example, column 4 gives the correlation between diastolic BP at age 12 and diastolic BP at age 15.
Primary Analysis
The prenatal MeHg by age interaction was significant in the longitudinal model for diastolic BP (p=0.02), but not systolic BP (Table 3). Increased MeHg exposure was associated with a greater diastolic BP at age 15 years (p=0.02), but not at age 12 years. There was no association between MeHg and systolic BP overall or at either age. As expected, BMI, height, and maternal hypertension were significant predictors of both BP measures (Table 3). Taller children, those with a higher BMI, and those whose mothers had been treated for hypertension during pregnancy had significantly higher diastolic and systolic BP on average than their counterparts. In addition, boys had significantly lower diastolic BP than girls. In this adjusted model, measurements at age 15 years were associated with a significantly lower diastolic and systolic BP than measurements at age 12 *. Results from the age-specific linear regression models were consistent with results from the longitudinal model. Prenatal MeHg exposure was a significant predictor only in the model for 15-year diastolic BP. In this model, for each ppm rise in MeHg, diastolic BP was estimated to rise by 0.18 mm Hg.
Table 3.
Results of the longitudinal models based on data at both 12 and 15 years of age. All covariates are continuous except sex, maternal hypertension during pregnancy and age at which BP was measured. The age-specific slopes for MeHg (last two lines) were obtained from refitting the same model with the MeHg by age interaction reparameterized.
| Diastolic BP (mm Hg) | Systolic BP (mm Hg) | |||
|---|---|---|---|---|
| Variable | Slope (SE) | p | Slope (SE) | p |
| Sex (boy) | −2.25 (.50) | <0.001 | 1.17 (.68) | 0.08 |
| MeHg (ppm) | −0.03 (.07) | 0.62 | 0.07 (.09) | 0.46 |
| Birth weight (kg) | −1.13 (.49) | 0.02 | −0.82 (.67) | 0.22 |
| BMI (kg/m2) | 0.71 (.06) | <0.001 | 0.89 (.08) | <0.001 |
| Height (cm) | 0.16 (.03) | <0.001 | 0.45 (.04) | <0.001 |
| Maternal hypertension (yes) | 2.58 (.91) | <0.01 | 5.59 (1.24) | <0.001 |
| Age at testing (15 years) | −2.07 (.78) | 0.01 | −2.60 (1.01) | 0.01 |
| MeHg * age | 0.20 (.09) | 0.02 | 0.03 (.11) | 0.79 |
| MeHg | ||||
| 12 years | −0.03 (.07) | 0.62 | 0.07 (.09) | 0.46 |
| 15 years | 0.17 (.07) | 0.02 | 0.10 (0.10) | 0.32 |
BP=blood pressure, SE = standard error, MeHg=methylmercury, ppm=parts per million, BMI=body mass index.
Model assumptions appeared to be met in all models, except for some nonlinearities **. There were no influential observations as judged by Cook’s distance (Weisberg, 2005). Most models had 2–4 outliers, observations whose studentized residual was >3 or < −3. The regression coefficients were essentially unchanged when outliers were removed.
Subsidiary Analyses
The age by sex interaction was significant only for systolic BP. Boys showed a greater increase in systolic BP from age 12 to age 15 than girls. Including this interaction had little effect on the coefficients or SEs for MeHg and was not considered further. When the primary longitudinal model was adjusted for SES, the coefficient for MeHg changed from −0.03 (p=.62) to −0.05 (p=.46) for diastolic BP, and from 0.07 (p=.46) to 0.06 (p=.49) for systolic BP. The coefficient for SES was −0.04 (p=.14) for diastolic BP, and 0.02 (p=.60) for systolic BP. When the primary longitudinal models were rerun without variables that could be considered endpoints, for both diastolic and systolic BP the resulting coefficients and p-values for the remaining terms were similar to what they had been in the original models. In particular, the coefficient for the MeHg by age interaction changed from 0.20 (p=0.02) to 0.22 (p=0.02) for diastolic BP, and from 0.03 (p=0.79) to 0.05 (p=0.68) for systolic BP.
The additive model for 15-year diastolic BP showed a significant nonlinear relationship between MeHg and BP. Under this model, diastolic BP is predicted to increase for exposures up to about 10 ppm of MeHg, followed by a leveling off of the effect.
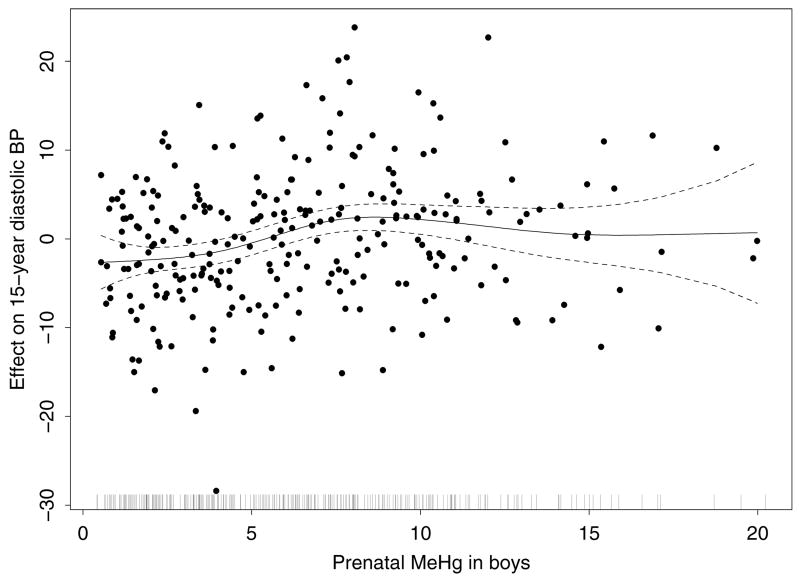
To further examine the association between prenatal MeHg exposure and diastolic BP at age 15 years, we fit separate linear regression models by sex. The overall effect of prenatal MeHg on diastolic BP was significant for boys (p=0.003) but not for girls (p=0.59) (Table 4). For boys, diastolic BP was predicted to increase by 0.36 mm Hg per 1 ppm increase in prenatal MeHg exposure. These coefficients and p-values were virtually unchanged when we did not adjust for BMI, height, maternal hypertension, and birthweight, and were very similar when we adjusted for SES. SES was not an important predictor for 15-year diastolic BP for either sex (p=.98 for girls, and p=.33 for boys). A similar pattern of nonlinearity between MeHg and diastolic BP as noted earlier for both sexes was present for boys only (Figure 1).
Table 4.
Linear regression models for 15-year diastolic blood pressure (mm Hg) by sex. All covariates are continuous variables except maternal hypertension during pregnancy.
| Girls | Boys | |||
|---|---|---|---|---|
| Variable | Slope (SE) | p | Slope (SE) | p |
| MeHg (ppm) | 0.05 (.10) | 0.59 | 0.36 (.12) | 0.003 |
| Birth weight (kg) | −1.60 (.90) | 0.08 | −1.21 (1.00) | 0.23 |
| BMI (kg/m2) | 0.55 (.10) | <0.001 | 0.63 (.16) | <0.001 |
| Height (cm) | 0.12 (.07) | 0.08 | 0.11 (.07) | 0.08 |
| Maternal hypertension (yes) | 4.11 (1.59) | 0.01 | −0.16 (1.82) | 0.93 |
| Age at testing (years) | 1.45 (1.80) | 0.42 | 1.38 (1.96) | 0.48 |
MeHg=methylmercury, ppm=parts per million, BMI=body mass index
Figure 1.
Partial residual plot from the additive model of diastolic BP among boys at age 15, showing the effect of the smoothed term for prenatal MeHg levels. The model also adjusts for maternal hypertension during pregnancy, and smoothed terms for birth weight, BMI, height, and age. The dashed lines are 2 times the pointwise standard error curves, and the vertical marks along the bottom show the distribution of MeHg.
The linear regression models for 15-year diastolic BP were rerun with a random school effect. Schools which were represented by fewer than 10 boys or girls in this sample were pooled into an “other” category. There were then 10 school categories. The intraclass correlations for these models were 6% (girls) and 10% (boys), indicating that no more than 10% of the total variability in 15-year diastolic BP could be explained by location. Schools in which the adjusted average BP was significantly above or below the average for boys or girls were examined further. These school effects were not consistent across the two sexes, suggesting that no single school had consistently higher or consistently lower BP readings than expected.
To investigate whether the different effects of MeHg on diastolic BP among boys at the two ages was due to different subjects at each age, we fit models adjusting for the primary covariates using only the 244 boys with BP measurements at both ages. Consistent with the previous results, in these models prenatal MeHg was not associated with diastolic BP elevations at age 12 (p=0.79), but there was an adverse association at age 15 (p=0.002).
Discussion
We found one relationship between BP and prenatal MeHg exposure from fish consumption in the SCDS main cohort. Prenatal MeHg exposure was associated with increased diastolic BP in boys at age 15 years. We found no associations between prenatal MeHg exposure in girls or in systolic BP in either sex at either age. There is no biological reason to expect an association only for diastolic pressure, only in boys or only at age 15 years. The single finding of an adverse relationship between MeHg and diastolic BP in males at age 15 warrants further study, but does not suggest a consistent association between MeHg and BP.
Our results are generally consistent with results from the Faroe Islands at a comparable age. They found no associations between prenatal MeHg exposure and BP at age 14 years (Grandjean et al, 2004). Our subjects did not have BP measured at earlier ages when an adverse association was reported in the Faroes study. However, the shape of the relationship present for 15 year diastolic BP in males is similar to the relationship they reported between prenatal MeHg exposure and BP at age 7 years. In their study, prenatal exposures between 1 and 10 ug/L (ppg) were associated with higher BP, but the association did not continue at exposures above 10 ug/L (Sorenson et al, 1999).
Differences in the results of the SCDS and the Faroe Islands study could be due to differences in diet and in exposure to pollutants. In the Seychelles, fish consumption accounts for the MeHg exposure while in the Faroe Islands most of the Hg exposure is from consumption of pilot whale meat. Pilot whale meat contains higher Hg levels than most fish (up to 3.3 ppm with half being MeHg) and also has high cadmium levels (Anderson et al, 1987). Cadmium has been linked to increases in BP (Perry and Erlanger, 1974). In the Faroe Islands whale blubber is also consumed and it contains contaminants such as polychlorinated biphenyls (PCBs) (Julshamn et al, 1987). Although the BP changes in the Faroes study were attributed to prenatal MeHg exposure, the contribution of other pollutants could not be excluded.
The presence of several associations previously reported as risk factors that increase children’s BP is reassuring and suggests the data is robust enough to find known relationships. For example there was a strong and generally linear relationship between both diastolic and systolic BP and BMI, height and maternal hypertension requiring medication during pregnancy at both ages in both sexes. In addition, taller children and those with a higher BMI had on average higher BP than their shorter and lighter counterparts, and there was an adverse association between children’s BP and maternal hypertension requiring medication during pregnancy. These findings also suggest that autonomic heart functions in the Seychelles children are similar to those from developed nations. The study has weakness including that the BP measurement was taken during a routine school physical evaluation. Measuring BP was not part of the original prospective study design and only part of the cohort was examined. Although the measurement of BP was carried out in multiple sites by different nurses, the measurement of BP was standardized and automated. This standardization is likely to minimize or eliminate any examiner-to-examiner variability in BP measurements, and we found no evidence that location consistently influenced BP readings. BP measurements are known to vary non-trivially around their average values and can be influenced by many factors such as measurement technique, emotional stress, and other circumstances. This would have the effect of increasing the model uncertainty and thus making it difficult to detect meaningful associations, especially if they are small. Future studies could benefit from obtaining more than two BP readings so as to reduce the measurement error.
In summary, we found only one association present between prenatal MeHg exposure and BP. Males at age 15 showed a small increase in BP as prenatal exposure increased. No other associations between prenatal MeHg exposure and BP were present. This finding is intriguing, but its biological significance, if any, is not clear.
Acknowledgments
This study was supported in part by grant 5-R01-ES008442 from the National Institutes of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), and in part by grant 1 UL1 RR024160-01 from the National Center for Research Resources (NCRR), NIH.. The work of Dr. Thurston, Ms. Georger, and Dr. Clarkson was supported in part by grant number K22 ES011027, training grant number 2 T 32 ES007271, and center grant number ES01247 respectively, also from the NIEHS. The study contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NCRR, or NIH.
Footnotes
Both average diastolic and average systolic BP were higher at age 15 than at age 12. However the age at testing indicator variable was correlated with the time-dependent covariates, most notably with height (correlation = 0.57). Consequently, the sign of the coefficient for the age at testing variable switched from positive in an unadjusted model, to negative in the adjusted model reported here.
We tested for nonlinearity between each continuous variable and the outcome using the approximate chi-squared test for the nonlinear contribution of the nonparametric terms (Chambers and Hastie, 1993) from additive models fit in Splus (Venables and Ripley, 1994). Results from these models showed that there was a significant departure from linearity between age and diastolic BP at age 12 (p<0.001), but not age 15. At age 12 there was a linear increase in BP up to about age 12.3 years, followed by an approximately constant relationship above that age. Because this relationship was so highly non-linear, age in the 12 year diastolic BP model was categorized as above or below 12.3 years. The relationship between 15-year diastolic BP and MeHg was significantly non-linear, for both sexes together (p=.013) and for boys only (p=.021). No other covariates in the 15-year diastolic BP models showed a significant departure from linearity. All covariates in the 12-year diastolic BP model (with both sexes together) were approximately linearly related to the outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Al-Damluji SF. Intoxication due to alkylmercury-treated seed-1971–72 outbreak in Iraq: clinical aspects. Bul World Health Organiz. 1976;53(supplement):65–81. [PMC free article] [PubMed] [Google Scholar]
- Andersen A, Julshamn K, Ringdal O, Morkore J. Trace elements intake in the Faroe Islands. II. Intake of mercury and other elements by consumption of pilot whales (Globicephalus meleanus) Sci Total Environ. 1987;65:63–68. doi: 10.1016/0048-9697(87)90161-6. [DOI] [PubMed] [Google Scholar]
- Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, Al-Rawi NY, Tikrita S, Dahahir HI, Clarkson TW, Smith JC, Doherty RA. Methylmercury poisoning in Iraq. Science. 1973;181:230–41. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Cernichiari E, Brewer R, Myers GJ, Marsh DO, Lapham LW, Cox C, Shamlaye CF, Berlin M, Davidson PW, Clarkson TW. Monitoring methylmercury during pregnancy: maternal hair predicts fetal brain exposure. Neurotoxicology. 1995;16:705–10. [PubMed] [Google Scholar]
- Chambers JM, Hastie TJ. Statistical Models. S. London: Chapman and Hall; 1993. [Google Scholar]
- Chiolero A, Madeleine G, Gabriel A, Burnier M, Paccaud F, Bovet P. Prevalence of elevated blood pressure and association with overweight in children of a rapidly developing country. J Hum Hypertens. 2007;21:120–27. doi: 10.1038/sj.jhh.1002125. [DOI] [PubMed] [Google Scholar]
- Colhoun HM, Hemingway H, Poulter NR. Socio-economic status and blood pressure: an overview analysis. J Hum Hypertens. 1998;12:91–110. doi: 10.1038/sj.jhh.1000558. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, Cernichiari E, Needham L, Choi A, Wang Y, Berlin M, Clarkson TW. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998;280:701–07. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- Dorea JG, De Souza JR, Rodrigues P, Ferrari I, Barbosa AC. Hair mercury (signature of fish consumption) and cardiovascular risk in Munduruku and Kayabi Indians of Amazonia. Environ Res. 2005;97:209–19. doi: 10.1016/j.envres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Falkner B, Gidding SS, Ramirez-Garnica G, Wiltrout SA, West D, Rappaport EB. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatrics. 2006;148:195–200. doi: 10.1016/j.jpeds.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Gillman MW. Developmental origins of health and disease. N Eng J Med. 2005;353(17):1848–50. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Murata K, Budtz-Jorgensen E, Weihe P. Cardiac autonomic activity in methylmercury neurotoxicity: 14-year follow-up of a Faroese birth cohort. J Pediatrics. 2004;144:169–76. doi: 10.1016/j.jpeds.2003.10.058. [DOI] [PubMed] [Google Scholar]
- Harada M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25(1):1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- Harada Y. Study Group of Minimata Disease. Kumamoto University; Kumamoto, Japan: 1968. Congenital (or Fetal) Minimata Disease; pp. 93–117. [Google Scholar]
- Hardy R, Sovio U, King VJ, Skidmore PM, Helmsdal G, Olsen SF, Emmett PM, Wadsworth ME, Jarvelin MR EURO-BLCS Study Group. Birthweight and blood pressure in five European birth cohort studies: an investigation of confounding factors. European J Public Health. 2006;16:21–30. doi: 10.1093/eurpub/cki171. [DOI] [PubMed] [Google Scholar]
- Hook O, Lundgren KD, Swensson A. On alkyl mercury poisoning; with a description of two cases. Acta Med Scand. 1954;150:131–37. [PubMed] [Google Scholar]
- Hunter D, Russell DS. Focal cerebellar and cerebellar atrophy in a human subject due to organic mercury compounds. J Neurochem. 1954;17:235–41. doi: 10.1136/jnnp.17.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili MA, Abbasi AH. Poisoning by Ethyl Mercury Toluene Sulphonanilide. Brit J Indrl Med. 1961;18:303–08. doi: 10.1136/oem.18.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julshamn K, Andersen A, Ringdal O, Morkore J. Trace elements intake in the Faroe Islands. I. Element levels in edible parts of pilot whales (Globicephalus meleanus) Sci Total Environ. 1987;65:53–62. doi: 10.1016/0048-9697(87)90160-4. [DOI] [PubMed] [Google Scholar]
- Law CM, de Swiet M, Osmond C, Fayers PM, Barker DJP, Cruddas AM, Fall CHD. Initiation of hypertension in utero and its amplification throughout life. BMJ. 1993;306:24–27. doi: 10.1136/bmj.306.6869.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, Sloane-Reeves J, Wilding GE, Kost J, Huang LS, Clarkson TW. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–92. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112:1945–52. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- O'Brien E, Waeber B, Parati G, Staessen J, Myers MG. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ. 2001;322:531–36. doi: 10.1136/bmj.322.7285.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Matsukura M, Okamoto M, Harada N, Kitano T, Miike T, Futatsuka M. Autonomic nervous functions in fetal type Minamata disease patients: Assessment of heart rate variability. Tohoku J Expl Med. 2003;198:215–21. doi: 10.1620/tjem.198.215. [DOI] [PubMed] [Google Scholar]
- Perry HM, Erlanger MW. Metal-induced hypertension following chronic feeding of low doses of cadmium and mercury. J Lab Clin Med. 1974;83(4):541–47. [PubMed] [Google Scholar]
- Schober SE, Sinks TH, Jones RL, Bolger PM, McDowell M, Osterloh J, Garrett ES, Canady RA, Dillon CF, Sun Y, Joseph CB, Mahaffey KR. Blood mercury levels in US children and women of childbearing age, 1999–2000. JAMA. 2003;289:1667–74. doi: 10.1001/jama.289.13.1667. [DOI] [PubMed] [Google Scholar]
- Shamlaye C, Marsh DO, Myers GJ, Cox C, Davidson PW, Choisy O, et al. The Seychelles child development study on neurodevelopmental outcomes in children following in utero exposure to methylmercury from a maternal fish diet: background and demographics. Neurotoxicology. 1995;16(4):597–12. [PubMed] [Google Scholar]
- Sorensen N, Murata K, Budtz-Jorgensen E, Weihe P, Grandjean P. Prenatal methylmercury exposure as a cardiovascular risk factor at seven years of age. Epidemiology. 1999;10:370–75. [PubMed] [Google Scholar]
- Tamashiro H, Akagi H, Arakaki M, Futatsuka M, Roht LH. Causes of death in Minamata disease: analysis of death certificates. Int Arch Occup Environ Health. 1984;54(2):135–46. doi: 10.1007/BF00378516. [DOI] [PubMed] [Google Scholar]
- Torres AD, Rai AN, Hardiek ML. Mercury intoxication and arterial hypertension: report of two patients and review of the literature. Pediatrics. 2000;105:E34. doi: 10.1542/peds.105.3.e34. [DOI] [PubMed] [Google Scholar]
- Tsubaki T, Irukayama K. Minamata disease: Methylmercury poisoning in Minamata and Niigata, Japan. New York: Elsevier Scientific Publishing Company; 1977. pp. 1–317. [Google Scholar]
- Tsubaki T, Takahashi H. Recent advances in Minamata disease studies: Methylmercury poisoning in Minamata Japan. Tokyo: Kodansha Ltd; 1986. pp. 1–214. [Google Scholar]
- Venables WN, Ripley BD. Modern Applied Statistics with S-plus. New York: Springer-Verlag; 1994. [Google Scholar]
- Vupputuri S, Longnecker MP, Daniels JL, Guo X, Sandler DP. Blood mercury level and blood pressure among US women: results from the National Health and Nutrition Examination Survey 1999–2000. Environ Res. 2005;97:195–200. doi: 10.1016/j.envres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Wakita Y. Hypertension induced by methyl mercury in rats. Toxicol Appl Pharmacol. 1987;89:144–47. doi: 10.1016/0041-008x(87)90185-2. [DOI] [PubMed] [Google Scholar]
- Warkany J. Acrodynia-postmortem of a disease. Am J Dis Child. 1996;112:147–56. [PubMed] [Google Scholar]
- Weisberg S. Applied Linear Regression. New York: Wiley; 2005. [Google Scholar]