Abstract
Inbred strains of mice have served as valuable models for studying genetic susceptibility to drug addiction, an alternative to genetically modified mouse models. This is the first study comparing amphetamine (AMPH) effects on locomotor stimulation and dopamine efflux between two inbred strains of mice C57BL/6J and 129S2/SvHsd, frequently used as background strains for production of genetically engineered mice. There were no significant differences in basal locomotor activity and basal dopamine levels between the two strains. However, C57BL/6J mice showed greater AMPH-stimulated locomotor activity and AMPH-induced striatal dopamine efflux than 129S2/SvHsd mice. The differential AMPH effects could not be explained by differences in presynaptic dopamine components such as surface and total dopamine transporter (DAT) expression levels, striatal dopamine contents, and DAT activity. C57BL/6J and 129S2/SvHsd mice are excellent models for future identification of genetic, molecular, and behavioral components related to individual vulnerability to AMPH addiction. This study emphasizes the importance of mouse strain selections in the production of genetically modified mice for investigating phenotypes and mechanisms of psychostimulants.
Keywords: amphetamine, mouse strain, locomotor activity, dopamine efflux, dopamine transporter
1. Introduction
Amphetamine (AMPH) is an addictive and a widely abused illicit drug. Although AMPH has high affinities for the dopamine, norepinephrine, and serotonin transporters, the dopamine transporter (DAT) is associated with the stimulating and rewarding properties of AMPH (Koob and Nestler, 1997; Wise and Bozarth, 1987). As both a substrate and an inhibitor of DAT, AMPH binds to DAT, stimulating dopamine efflux and inhibiting dopamine uptake, respectively, leading to an increased level of extracellular dopamine. The increased extracellular dopamine level contributes to AMPH-stimulated locomotor behavior (Koob and Nestler, 1997; Wise and Bozarth, 1987). However, the molecular determinants of AMPH-induced dopamine efflux through DAT have not been determined despite continued progress (for reviews see Gnegy, 2003; Sulzer et al., 2005).
Genetically modified mice have provided us valuable information of specific genes in drug abuse. These genetic manipulations, however, may complicate phenotypes resulting from developmental adaptations in the long-term presence of altered genes, requiring caution in interpreting results using these mice. Another potential confound of genetically modified mice is the fact that the backgrounds of the mice used for production show a wide span of genetic variation (e.g., Simpson et al., 1997), raising the question of whether the phenotypes of congenic mice in response to drugs result from the specific gene mutation or simply background traits. The most commonly used background strains in the production of knock-out, knock-in or transgenic animals are substrains of C57BL/6 and 129 mice. Given that behavioral and biochemical phenotypes vary significantly among strains of mice in response to psychostimulants (for review see Puglisi-Allegra and Cabib, 1997), characterization of the background strains used in genetically engineered mice is a prerequisite for appropriately concluding phenotypes of mutant mice.
Alternatively, investigation of inbred mouse strains displaying differential behavioral and neurochemical phenotypes in response to psychostimulants is another useful approach for identifying the genetic basis underlying the phenotypes (e.g. He and Shippenberg, 2000; Kuzmin and Johansson, 2000; Schlussman et al., 1998, 2003). Individual liability to drug addiction has been well documented in humans (Piazza et al., 1989; Siegel, 1984). Inbred mouse strains have been used as valuable models for studying susceptibility to drug addiction given their wide span of responses to commonly abused drugs. Comparisons of responses of divergent mouse strains to psychostimulants such as cocaine and AMPH might provide insights into potential genetic factors for drug addiction.
Mouse strain differences in cocaine effects on locomotor activity, stereotypy, and reward between C57BL/6 and 129 substrains have been extensively studied. In general, C57BL/6 mice are more sensitive to cocaine-stimulated locomotor and stereotypy sensitization than 129 substrains (e.g., Schlussman et al., 1998, 2003; Zhang et al., 2002). C57BL/6 mice are able to develop cocaine-induced conditioned place preference (Cunningham et al., 1999; Miner, 1997) and self-administer cocaine (Fuchs et al., 2003; Griffin and Middaugh 2003; Highfield et al., 2003; Kuzmin and Johansson, 2000) while substrains of 129 mice do not (Kuzmin and Johansson, 2000; Miner, 1997).
There have been comparatively few studies on mouse strain differences in AMPH-induced behavioral and neurochemical alterations. Although there are strong similarities between cocaine- and AMPH-mediated behaviors, the stimulated behavioral patterns (e.g., Antoniou et al., 1998) and molecular components (Briegleb et al., 2004; George et al., 1991; He and Shippenberg, 2003) involved in behavioral stimulation are not exactly the same. Furthermore, dopamine efflux induced by psychostimulants has not been extensively examined in different strains of mice. Therefore, the main goal of the study was to extend the characterization of mouse strain differences in AMPH-induced locomotor stimulation and dopamine efflux into other lines of inbred mice. C57BL/6J and 129S2/SvHsd mice were chosen based on their commonality used in gene targeting for generation of mutant animals. Presynaptic dopaminergic components likely involved in AMPH-stimulated efflux were compared between the two mouse strains.
2. Materials and Methods
2.1. Animals
Adult female C57BL/6J (Jackson Laboratory, Bar Harbor, ME) and 129S2/SvHsd (Harlan, Indianapolis, IN) mice aged 8-10 weeks were group housed before experiments. Mice had free access to standard Purina rodent chow and water and were maintained in a temperature- and humidity-controlled environment on a 12-h dark/light cycle with lights on at 7:00 AM. All animal use procedures complied with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health, and were approved by the University of Michigan Committee on the Use and Care of Animals.
2.2. Transmitter implantation and locomotor activity measurement
A radiotransmitter (Mini Mitter Co., Bend, OR) was implanted into the peritoneal cavity of each mouse. Surgery was performed under xylazine (10 mg/kg) and ketamine (100 mg/kg). The transmitter-produced locomotor activity signals (both horizontal and vertical locomotion) were sent to a receiver placed underneath the mouse home cage (model ER-4000 Receiver, Mini Mitter Co., Bend, Oregon). Signals from the receivers were sent to a computer, and data were collected and processed by the Vital view data acquisition system (Mini Mitter Co.). Locomotion is expressed in gross activity counts. This measurement does not differentiate stereotyped behavior such as licking or sniffing from locomotion.
Animals were individually housed after transmitter implants and allowed to recover for 7 days. One day before behavioral testing, animals in their home cages, with food and water ad libitum, were placed on the top of the transmitter receivers for habituation to the testing environment. On the next day, baseline locomotor data were collected 30 min prior to the saline treatment and behavioral recording continued for 3 hours. On the third day, AMPH (2.5 or 5 mg/kg, i.p.) was given to mice following the baseline locomotor data collection. Locomotor activity data were summed over 3 hours. Mice were visually monitored for at least 30 min following injection of AMPH to determine whether mice expressed stereotyped behavior. No significantly increased stereotyped behavior was noted in either mouse strain following either dose of AMPH compared to the saline treatment. A two-way analysis of variance (ANOVA) (mouse strain × AMPH dose) using Prism 3.0 (GraphPad, San Diego, CA) was performed to analyze main effects of AMPH treatment, mouse strain, and their interaction effects followed by post-hoc analysis with Bonferroni test. The strain difference in the basal locomotor activity was analyzed by a two-way ANOVA with repeated measures (strain × time).
2.3. AMPH-induced striatal dopamine efflux
Mice were sacrificed by cervical dislocation and the brains were rapidly removed. Striata were dissected, sliced, weighed, and placed in chambers of a Brandel perfusion apparatus (Brandel SF-12, Gaithersburg, MD) onto Whatman GF/B filter disks. Dorsal and ventral striatum were dissected together to provide enough material to measure dopamine efflux from one mouse. The chambers were perfused at 37 °C with oxygenated Krebs-Ringer buffer (KRB) containing 24.9 mM NaHCO3, 1.2 mM KH2PO4, 145 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 10 mM glucose, 0.25 mM ascorbic acid, and 50 μM pargyline at a rate of 100 μl/min. After 60 min of perfusion, perfusates were collected at 5-minute intervals for a total of 13 fractions. At fraction number 4, 10 μM AMPH dissolved in KRB was introduced to the tissues for 5 min followed by perfusion with KRB for the rest of experiment. Perfusates were collected into vials with a final concentration of 50 mM HClO4, 25 μM sodium bisulfate, 25 μM EDTA, and 10 nM dihydroxybenzylamine (DHBA) as an internal standard. Dopamine was measured by high performance liquid chromatography (HPLC) with electrochemical detection, and expressed as pmole dopamine per milligram wet weight tissue per 5 min. A two-way ANOVA with repeated measures over time intervals was performed to analyze strain differences in the basal dopamine level and AMPH-induced dopamine efflux followed by post-hoc analysis with Bonferroni test using SYSTAT (SPSS Science, Chicago, IL).
2.4. Striatal dopamine content
Dopamine was extracted from mouse dorsal and ventral striata and analyzed by HPLC. Briefly, drug naive mice were sacrificed by cervical dislocation and striata were homogenized in 0.1 M perchloric acid containing 10 nM DHBA. Homogenates were centrifuged for 10 min at 10,000 × g. The supernatants were filtered through 0.2 μm filters and dopamine was analyzed using HPLC with electrochemical detection. Protein assays were performed using a Bio-Rad Dc protein assay kit (BioRad Laboratories, Herculase, CA). Mouse strain differences in striatal dopamine content were analyzed by student’s t-test.
2.5. Synaptosomal dopamine uptake
Dopamine uptake was measured in mouse synaptosomes freshly prepared from dorsal and ventral striata (Johnson et al., 2005). The uptake assay was conducted in KRB containing 30 nM [3H]dopamine (23.5 Ci/mmol) (PerkinElmer, Boston, MA), various concentrations of unlabeled dopamine ranging from 100 nM to 10 μM, and 150 μM ascorbic acid. The uptake assay was initiated by the addition of aliquots of the synaptosomes (50 -100 μg) in the presence of increasing concentrations of unlabeled dopamine followed by incubation for 3 min at 37°C.
Non-specific uptake of [3H]dopamine was determined by incubation of a parallel set of samples in the presence of 200 μM cocaine. The assay was terminated by the addition of 5 ml ice-cold KRB, filtration, and two 5 ml washes with the same buffer. The radioactivity on the filters was determined by a Beckman liquid scintillation counter. Dopamine binding affinity for DAT (Km) and dopamine uptake velocity (Vmax) values were determined by nonlinear regression analysis using Prism 3.0. Mouse strain differences in dopamine uptake kinetics were analyzed by student’s t-test.
2.6. Biotinylation of striatal surface DAT
Surface DAT expression in dorsal and ventral striata was determined in synaptosomes using sulfo-NHS-SS-biotin (Pierce, Rockford, IL) and purified by streptavidin beads (Pierce) as described previously (Johnson et al., 2005). One hundred percent of the biotinylated fraction and one-tenth of the total lysate fractions were resolved by SDS-PAGE and immunoblotted using 1:1000 dilution of a DAT antibody mAb16 against the N-terminal of DAT, a generous gift from Dr. Roxanne Vaughn (Department of Biochemistry, University of ND). The membranes were further blotted with a goat anti-mouse horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) with a 1:5,000 dilution. Bands were visualized using the Enhanced Chemiluminescence detection method (Amersham Corp, Piscataway, NJ). Quantification of the bands was performed by the Scion Image software, and strain differences were analyzed by student’s t-test.
2.7. Drugs
All drugs used were from Sigma-Aldrich (St. Louis, MO) except those specified. AMPH sulphate was dissolved in 0.9% saline and was injected at a volume of 10 mg/kg.
3. Results
3.1. Baseline and AMPH-induced locomotor activity in C57BL/6J and 129S2/SvHsd mice
To reduce novelty-induced locomotor stimulation, the locomotor activity of the mice was conducted in their home cages. A two-way ANOVA with repeated measures over time intervals did not reveal statistical mouse strain difference in baseline locomotor activity (data not shown). However, AMPH administration induced a significant difference in locomotor stimulation between mouse strains as indicated in Fig. 1. Locomotor activity was plotted as the sum of locomotor counts during the 3 hours after AMPH or saline injection (N = 5-6 for each experimental group). A two-way ANOVA (strain × dose) showed significant main effects of dose [F(2,26) = 58.13, P< .01] and strain [F(1,26) = 20.95, P< .01], and a significant strain × dose interaction effect [F(2,26) = 5.44, P=.01]. As indicated in Fig. 1, AMPH stimulated locomotor activity from both C57BL/6J and 129S2/SvHsd mice in a dose-dependent manner but C57BL/6J mice showed greater stimulation by AMPH than 129S2/SvHsd mice at both the 2.5 and 5 mg/kg AMPH dose (P<0.05 and P<0.01, respectively).
Fig.1.
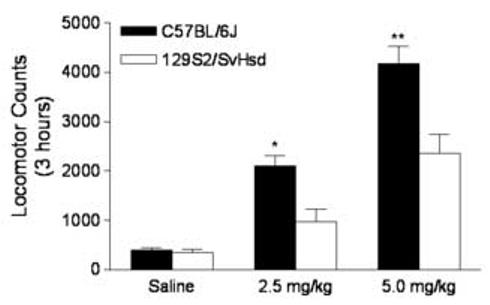
Comparisons of AMPH-induced locomotor activity between C57BL/6J and 129S2/SvHsd mice. Mice were given either saline or AMPH treatments (2.5 or 5 mg/kg, i.p.). Locomotor counts were summed over 3 hours following AMPH or saline injection. Data are represented as mean ± SEM. *, P<0.05 as compared to 129S2/SvHsd mice at the dose of 2.5 mg/kg; **, P<0.01 as compared to 129S2/SvHsd mice at the dose of 5 mg/kg.
3.2. AMPH-induced striatal dopamine efflux in C57BL/6J and 129S2/SvHsd mice
The striatal basal dopamine level and AMPH-induced dopamine efflux are shown in Fig. 2. The basal level of dopamine was calculated by averaging 3 fractions of perfusates prior to the AMPH treatment. The basal levels of striatal dopamine (pmole/mg wet weight/5 min) for C57BL/6J and 129S2/SvHsd were 0.19 ± 0.06 (N=10) and 0.17 ± 0.07 (N=7), respectively, which were not statistically different (p>0.05, t-test).
Fig. 2.
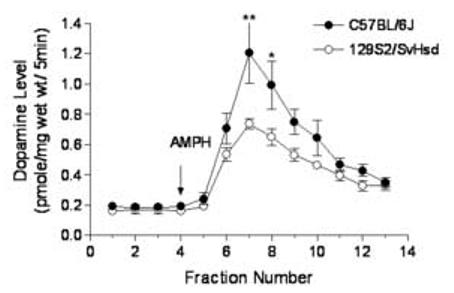
Comparisons of the basal dopamine level and AMPH-induced dopamine efflux in striatal slices between C57BL/6J and 129S2/SvHsd mice. Data are represented as mean ± SEM. The AMPH treatment (10 μM, 5 min) was given at the fraction #4 indicated by the arrow. *, P<0.05 as compared to 129S2/SvHsd mice; **, P<0.01 as compared to 129S2/SvHsd mice.
AMPH induced significant dopamine efflux from both strains of mice compared to their basal dopamine levels as indicated in Fig. 2. However, a two-way ANOVA with repeated measures indicated significant effects of strain [F(1,16) = 4.573, P<.05] and treatment [F(12, 192) = 35.919, P<0.01], and a significant strain × treatment interaction [F(12, 192) = 2.524, P<.01]. As shown in Fig. 2, AMPH (10 μM, 5 min exposure) induced a greater dopamine efflux from striata of C57BL/6J mice than from that of 129S2/SvHsd. The AMPH-stimulated dopamine peak level from 129S2/SvHsd mice was only 61.23% of that from C57BL/6J mice (P<0.01).
3.3. Striatal dopamine content in C57BL/6J and 129S2/SvHsd mice
Striatal dopamine content was analyzed in both strains of mice. The total dopamine concentrations (nmole/mg protein) for C57BL/6J and 129S2/SvHsd mice were 0.34 ± 0.03 and 0.46 ± 0.06, respectively (N = 5). There was no significant difference between mouse strains (p>0.05, t-test) although 129S2/SvHsd showed a slightly higher level of dopamine.
3.4. Striatal surface DAT in C57BL/6J and 129S2/SvHsd mice
Striatal surface expression of DAT was analyzed by biotinylating striatal synaptosomal surface proteins in both C57BL/6J and 129S2/SvHsd mice. Fig. 3A shows a representative western blot of DAT surface and total expression levels in a C57BL/6J and a 129S2/SvHsd mouse. There was no significant difference in the expression of biotinylated surface DAT between the two strains (p>0.05, t-test, N=4) as indicated in Fig. 3B. In addition, total DAT expression levels of both mouse strains analyzed from the striatal lysates did not reveal a significant strain difference either (Fig. 3B).
Fig. 3.
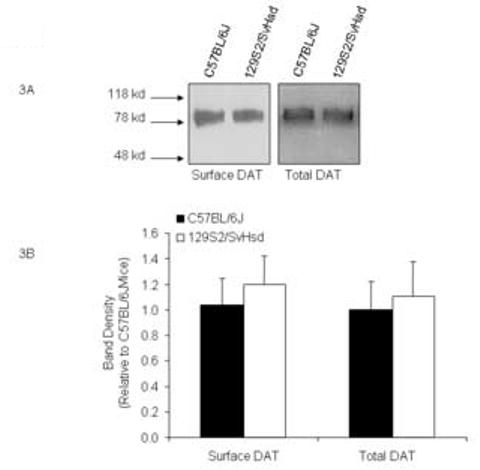
Comparisons of striatal surface and total DAT expression levels between C57BL/6J and 129S2/SvHsd mice. A representative western blot of DAT surface and total expression levels for a C57BL/6J and a 129S2/SvHsd mouse (Fig. 3A) and a statistical summary of quantitation of band densities (Fig. 3B) are shown. Data are represented as mean ± SEM.
3.5. Striatal DAT activity in C57BL/6 and 129S2/SvHsd mice
Dopamine transporter activity was measured in striatal synaptosomes from both mouse strains (N=5). Dopamine affinities (Km, μM) for DAT of C57BL/6J and 129S2/SvHsd mice were 0.35 ± 0.09 and 0.14 ± 0.06, respectively, while dopamine uptake velocities (pmole/mg protein/min) were 10.58 ± 0.69 and 8.22 ± 0.76 for C57BL/6 and 129S2/SvHsd mice, respectively (Fig. 4). There were no significant differences in either DAT affinity for dopamine or uptake velocity between the mouse strains (p>0.05, t-test).
Fig. 4.
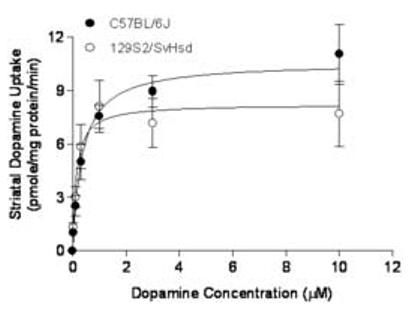
Comparisons of striatal dopamine uptake kinetics (Km and Vmax) in synaptosomes between C57BL/6J and 129S2/SvHsd mice. Data are represented as mean ± SEM.
4. Discussion
Our data demonstrate that C57BL/6J and 129S2/SvHsd mice differ in AMPH-stimulated locomotor activity in vivo and AMPH-induced dopamine efflux in vitro. These results add to a growing body of evidence delineating behavioral and biochemical differences of inbred strains of mice resulting from genetic variations. This is the first study comparing AMPH-stimulated behavioral activity and AMPH-stimulated dopamine efflux in the two commonly used inbred mouse strains.
There was a significant difference in AMPH-induced locomotor stimulation between the two mouse strains. C57BL/6J mice showed greater locomotor activity than 129S2/SvHsd mice upon the AMPH treatment (2.5 and 5 mg/kg). Our result is in contrast to the report that C57BL/6J and 129/SvJ mice do not differ in locomotor stimulation by the cocaine treatment (Miner, 1997). Although different substrains of 129 mice used might attribute greatly to this discrepancy, it is also possible that cocaine and AMPH might act on different sites to stimulate locomotor activity, and thus elicit different behavioral patterns as suggested by George and his colleagues (1991).
Moreover, we did not observe the difference in basal locomotor activity between C57BL/6J and 129S2/SvHsd mice which has been reported between C57BL/6J and 129/J mice (Miner, 1997). In addition to different substrains of mice used in these studies, our behavioral tests were performed in the home cages as opposed to a habituated testing environment described in Miner studies (1997). The novelty of the testing environment is known for influencing the basal (Galani et al., 2001) and drug-stimulated locomotor activity (Carey et al., 2005) in rodents. Furthermore, it has been reported that habituation to a novel environment in rodents is determined by specific genes (for review see Leussis and Bolivar, 2006). Taken together, these results emphasize the importance of the mouse substrain and the environment in influencing behavioral phenotypes.
There was no difference in basal locomotor activity between C57BL/6J and 129S2/SvHsd mice, which parallels the lack of significant difference in basal dopamine levels between these two mouse strains. Our result is also consistent with the report of no difference in basal locomotor activity between C57BL/6J and 129Sv/ter mice (He and Shippenberg, 2000). The significantly lower AMPH-stimulated locomotor activity expressed in 129S2/SvHsd mice as compared to C57BL/6J mice also parallels the significantly lower AMPH-induced striatal dopamine efflux in 129S2/SvHsd mice than in C57BL/6J mice. AMPH exerts its effect by binding to DAT and being transported into the terminals, resulting in dopamine efflux. Therefore, the surface DAT expression level, the brain dopamine content, and dopamine uptake kinetics are likely influencing factors of AMPH-induced dopamine release and locomotor stimulation. However, the differential dopamine efflux induced by AMPH between the two mouse strains could not be accounted for by these factors because the two strains did not differ in any of these measurements.
Since we did not observe the mouse strain difference in the basal level of dopamine efflux and locomotor activity, the difference in AMPH-induced dopamine efflux and locomotor stimulation between the strains might be due to dissimilar pharmacokinetics of AMPH in the two strains. However, evidence suggests that brain levels of AMPH need not be directly related to AMPH stimulated locomotor activity. For instance, C57BL/6J mice show a higher level of AMPH-induced total distance traveled as compared to A/J mice yet have a lower brain AMPH content when treated with the same dose of AMPH (Torkamanzehi et al., 2006). Furthermore, a congenic strain of A/J mice has an equivalent brain level of AMPH to the A/J parental strain, but exhibits significantly more AMPH-stimulated locomotion (Torkamanzehi et al., 2006). Similarly, altered pharmacokinetics of AMPH between male and female rats did not underlie the marked difference in their behavioral and neurochemical responsiveness to AMPH (Robinson, 1984).
The difference in the AMPH-induced locomotor activity and dopamine efflux between the two mouse strains might be due to differential presynaptic regulation of DAT that affects AMPH-induced dopamine efflux. Phosphorylation of DAT is a prerequisite for AMPH-induced dopamine efflux (Khoshbouei et al., 2004). Protein kinase C (PKC) has been shown to contribute to AMPH-stimulated dopamine efflux (Cowell et al., 2000; Johnson et al., 2005; Kantor and Gnegy, 1998) and AMPH-stimulated locomotor activity (Browman et al., 1998). Striatal PKC activities might differ between the two mouse strains, which would have an impact on DAT phosphorylation, dopamine efflux, and locomotor activity. Different expressions of PKC isoforms between mouse strains have been reported. For instance, C57BL/6J and DBA2 mice exhibit differences in hippocampal PKC gamma expression levels and activity, which has been associated with differential learning ability between mouse strains (Matsuyama et al., 1997). Whether C57BL/6J and 129S2/SvHsd differ in striatal expression levels of PKC isoforms and importantly their functional activity for DAT phosphorylation requires further investigation.
Finally, there might be mouse strain differences in norepinephrine transporter (NET) function that contribute to the differential AMPH-stimulated dopamine efflux and locomotor activity between C57BL/6J and 129S2/SvHsd mice. AMPH is potent in releasing dopamine from the prefrontal cortex through NET (Shoblock et al., 2004). It warrants further investigation the possible role of NET in AMPH effect between mouse strains. Despite the potential role of norepinephrine in the AMPH-mediated behavior, our results have demonstrated a basic difference in dopamine efflux in the striatum in response to a challenge with amphetamine in the C57BL/6J and 129S2/SvHsd mice.
In summary, our data indicate that C57BL/6J and 129S2/SvHsd mice show significantly different locomotor stimulation and dopamine efflux induced by AMPH treatments. The higher AMPH-induced dopamine efflux in C57BL/6J mice than in 129S2/SvHsd mice might underlie their greater AMPH-stimulated locomotor activity. The two mouse strains are useful models for future elucidation of genetic, molecular, and behavioral components that contribute to AMPH effects. Additionally, our data emphasize the importance for selection of mouse lines in the production of genetically modified mice for investigating mechanisms of psychostimulants.
Acknowledgements
The authors would like to thank Dr. Emily Jutkiewicz and Conor Daining for their assistance with the animals. This study was supported by National Institute of Health grant DA11697.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antoniou K, Kafetzopoulos E, Papadopoulou-Daifoti Z, Hyphantis T, Marselos M. D-amphetamine, cocaine and caffeine: a comparative study of acute effects on locomotor activity and behavioural patterns in rats. Neurosci Biobehav Rev. 1998;23:189–96. doi: 10.1016/s0149-7634(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Briegleb Sk, Gulley JM, Hoover BR, Zahniser NR. Individual differences in cocaine- and amphetamine-induced activation of male Sprague-Dawley rats: contribution of the dopamine transporter. Neuropsychopharmacology. 2004;29:2168–79. doi: 10.1038/sj.npp.1300536. [DOI] [PubMed] [Google Scholar]
- Browman KE, Kantor L, Richardson S, Badiani A, Robinson TE, Gnegy ME. Injection of the protein kinase C inhibitor Ro31-8220 into the nucleus accumbens attenuates the acute response to amphetamine: tissue and behavioral studies. Brain Res. 1998;814:112–9. doi: 10.1016/s0006-8993(98)01040-3. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E. Acute and chronic cocaine behavioral effects in novel versus familiar environments: open-field familiarity differentiates cocaine locomotor stimulant effects from cocaine emotional behavioral effects. Behav Brain Res. 2005;158:321–30. doi: 10.1016/j.bbr.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Kantor L, Hewlett GH, Frey KA, Gnegy ME. Dopamine transporter antagonists block phorbol ester-induced dopamine release and dopamine transporter phosphorylation in striatal synaptosomes. Eur J Pharmacol. 2000;389:59–65. doi: 10.1016/s0014-2999(99)00828-6. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Dickinson SD, Grahame NJ, Okorn DM, McMullin CS. Genetic differences in cocaine-induced conditioned place preference in mice depend on conditioning trial duration. Psychopharmacology (Berl) 1999;146:73–80. doi: 10.1007/s002130051090. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, See RE, Middaugh LD. Conditioned stimulus-induced reinstatement of extinguished cocaine seeking in C57BL/6 mice: a mouse model of drug relapse. Brain Res. 2003;973:99–106. doi: 10.1016/s0006-8993(03)02560-5. [DOI] [PubMed] [Google Scholar]
- Galani R, Duconseille E, Bildstein O, Cassel JC. Effects of room and cage familiarity on locomotor activity measures in rats. Physiol Behav. 2001;74:1–4. doi: 10.1016/s0031-9384(01)00509-1. [DOI] [PubMed] [Google Scholar]
- George FR, Porrino LJ, Ritz MC, Goldberg SR. Inbred rat strain comparisons indicate different sites of action for cocaine and amphetamine locomotor stimulant effects. Psychopharmacology (Berl) 1991;104:457–62. doi: 10.1007/BF02245649. [DOI] [PubMed] [Google Scholar]
- Gnegy ME. The effect of phosphorylation on amphetamine-mediated outward transport. Eur J Pharmacol. 2003;479:83–91. doi: 10.1016/j.ejphar.2003.08.059. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Middaugh LD. Acquisition of lever pressing for cocaine in C57BL/6J mice: effects of prior Pavlovian conditioning. Pharmacol Biochem Behav. 2003;76:543–549. doi: 10.1016/j.pbb.2003.09.010. [DOI] [PubMed] [Google Scholar]
- He M, Shippenberg TS. Strain differences in basal and cocaine-evoked dopamine dynamics in mouse striatum. J Pharmacol Exp Ther. 2000;293:121–7. [PubMed] [Google Scholar]
- Highfield DA, Mead AN, Grimm JW, Rocha BA, Shaham Y. Reinstatement of cocaine seeking in 129X1/SvJ mice: effects of cocaine priming, cocaine cues and food deprivation. Psychopharmacology (Berl) 2002;161:417–424. doi: 10.1007/s00213-002-1047-9. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. Regulation of amphetamine-stimulated dopamineefflux by protein kinase C beta. J Biol Chem. 2005;280:10914–9. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- Kantor L, Gnegy ME. Protein kinase C inhibitors block amphetamine-mediated dopamine release in rat striatal slices. J Pharmacol Exp Ther. 1998;284:592–8. [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, et al. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:387–93. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–97. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B. Reinforcing and neurochemical effects of cocaine: differences among C57, DBA, and 129 mice. Pharmacol Biochem Behav. 2000;65:399–406. doi: 10.1016/s0091-3057(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev. 2006;30:1045–64. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Namgung U, Routtenberg A. Long-term potentiation persistence greater in C57BL/6 than DBA/2 mice: predicted on basis of protein kinase C levels and learning performance. Brain Res. 1997;763:127–30. doi: 10.1016/s0006-8993(97)00444-7. [DOI] [PubMed] [Google Scholar]
- Miner LL. Cocaine reward and locomotor activity in C57BL/6J and 129/SvJ mice and their F1 cross. Pharmacol Biochem Behav. 1997;58:25–30. doi: 10.1016/s0091-3057(96)00465-0. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Cabib S. Psychopharmacology of dopamine: the contribution of comparative studies in inbred strains of mice. Prog Neurobiol. 1997;51:637–61. doi: 10.1016/s0301-0082(97)00008-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE. Behavioral sensitization: characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacology (Berl) 1984;84:466–75. doi: 10.1007/BF00431451. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Ho A, Zhou Y, Curtis AE, Kreek MJ. Effects of ‘binge’ pattern cocaine on stereotypy and locomotor activity in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 1998;60:593–9. doi: 10.1016/s0091-3057(98)00047-1. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Kane S, Stewart CL, Ho A, Kreek MJ. Locomotion, stereotype, and dopamine D1 receptors after chronic “binge” cocaine in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 2003;75:123–31. doi: 10.1016/s0091-3057(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Maisonneuve IM, Glick SD. Differential interactions of desipramine with amphetamine and methamphetamine: Evidence that amphetamine releases dopamine from noradrenergic neurons in the medial preferontal cortex. Neurochem Res. 2004;29:1437–1442. doi: 10.1023/b:nere.0000026409.76261.f3. [DOI] [PubMed] [Google Scholar]
- Siegel RK. Changing patterns of cocaine use: longitudinal observations, consequences, and treatment. NIDA Res Monogr. 1984;50:92–110. [PubMed] [Google Scholar]
- Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–33. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Torkamanzehi A, Boksa P, Ayoubi M, Fortier ME, Ng Ying Kin NM, Skamene E, et al. Identification of informative strains and provisional QTL mapping of amphetamine (AMPH)-induced locomotion in recombinant congenic strains (RCS) of mice. Behav Genet. 2006;36:903–13. doi: 10.1007/s10519-006-9078-3. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Zhang Y, Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Conditioned place preference after single doses or “binge” cocaine in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 2002;73:655–62. doi: 10.1016/s0091-3057(02)00859-6. [DOI] [PubMed] [Google Scholar]


