Abstract
Science educators agree that an undergraduate research experience is critical for students who are considering graduate school or research careers. The process of researching a topic in the primary literature, designing experiments, implementing those experiments, and analyzing the results is essential in developing the analytical skills necessary to become a true scientist. Because training undergraduates who will only be in the laboratory for a short period is time consuming for faculty mentors, many students are unable to find appropriate research opportunities. We hypothesized that we could effectively mentor several students simultaneously, using a method that is a hybrid of traditional undergraduate research and a traditional laboratory course. This article describes a paradigm for mentored undergraduate research in molecular microbiology where students have ownership of their individual projects, but the projects are done in parallel, enabling the faculty mentor to guide multiple students efficiently.
INTRODUCTION
Performing research greatly enhances the undergraduate educational experience (Lopatto, 2004; Seymour et al., 2004). The process of researching a topic in the primary literature, designing experiments, implementing those experiments, and analyzing the results is critical in developing the analytical skills necessary to become a scientist. Students also benefit from undergraduate research experiences in increased undergraduate graduation rates (Nagda et al., 1998), increased rates of pursuit of graduate education (Kremer and Bringle, 1990; Hathaway et al., 2002), and increased interest in science careers (Fitzsimmons and Associates, 1990). The benefits of undergraduate research exist for Caucasian and underrepresented ethnic groups alike (Lopatto, 2004).
There are several hurdles, however, in implementing this for all the undergraduates who would benefit. Molecular biology, in particular, is notoriously time consuming to teach to undergraduates in the research lab. Prior molecular biology laboratory course work eases the process, but even then, only the strongest of students are able to independently implement the knowledge and skills in novel research projects that vary significantly from the experiments performed in lab courses. In both research-intensive universities and undergraduate institutions, faculty members often do not have enough time to mentor a large number of undergraduate researchers individually.
A common method of introducing undergraduates to research in research-intensive universities has them work with a graduate student on a small part of the graduate student's research project. While this has significant benefits, often the undergraduate acts more in the role of a technician than a researcher and is unable or not encouraged to take full responsibility for the project. Another frequently used method gives undergraduates a dedicated task that they repeat for various members of the lab. In this case, the undergraduate becomes an expert in one technique but does not experience the entire research process or take ownership of a specific project. A feeling of project ownership is an essential feature of ideal undergraduate research (Lopatto, 2003). In cases where a faculty member does personally mentor undergraduate students in independent projects, the amount of student training involved is significant, and so only one or two undergraduates can often be taken on at a time.
We implemented a method for mentored undergraduate research that gives students individual projects, but the projects are done in parallel, enabling the faculty mentor to guide multiple students efficiently. We piloted this method with four undergraduates involved in research projects over a 10-wk period; students were engaged in research 15 h/wk. The method could be used in a small liberal arts college or a research-intensive university setting.
Our students focused on genes putatively involved in iron utilization in the plant pathogen Xanthomonas campestris pathovar vesicatoria, strain 85-10 (Thieme et al., 2005; Xcv 85-10), but this model could be used with any microorganism with a sequenced and annotated genome. The goal of the students' projects was to create a gene knockout, to test the phenotype of the mutant versus the wild-type strain in appropriate nutrition and biochemical assays, and then to test the pathogenesis on the host pepper plant.
Desired Student Learning Outcomes
The students who participated in this research had prior exposure to theory and practice of basic molecular biology techniques. The hope was to expand on these skills and enable students to implement them independently. On completion of this research experience, students should be able to
generate and understand an experimental design;
read and understand primary journal articles and scientific review articles;
describe the process of creating bacterial genetic knockouts;
use databases to research a topic and identify relevant journal articles;
use molecular biology databases including microbesonline.org and GenBank;
perform BLAST searches and interpret the results;
design PCR primers using Vector NTI software (Invitrogen, Carlsbad, CA);
perform multiple sequence alignments using Vector NTI software;
independently execute a number of molecular biology techniques including PCR, TA cloning, and gel electrophoresis;
and prepare and present scientific data to a scientific audience.
METHODS
Selection of Student Participants
The prerequisite for student enrollment was a molecular biology lab course. Enrollment was not open, but the first four students with the required prerequisite and whose schedule permitted were allowed to register. Three of the students took a course in the Biotechnology Program that the faculty mentor taught as the prerequisite, and one took an upper-level biochemistry course. The students all had skills in DNA gel electrophoresis, ligation, transformation, and setting up small enzymatic reactions such as restriction digestions and PCR. The student who had taken the biochemistry course instead of the biotechnology course was slightly less prepared for this experience because of a lesser focus on DNA manipulation in that course.
It is relevant to note that all four students were of “average” academic ability, with GPAs in the “B” to “C” range. Two of the students were chemical engineering majors, one had a dual major of chemical engineering and biochemistry, and one was a biomedical engineering student.
Staffing
This laboratory experience took place in the Biotechnology Education Facility, which is dedicated 100% to teaching efforts. The faculty mentor was the main point of contact for the participants and was always available to the students, with the exception of 1 wk. This project did not utilize teaching assistants. A laboratory manager was present in the facility, and she ordered reagents and also demonstrated the use of certain pieces of equipment. She also was available for technical assistance when the mentor was out of the office. Her total time spent directly assisting undergraduates over the 10-wk period was on the order of 8 h; she of course was present as a lab member and interacted with the students on a daily basis as any lab member would do. A teaching postdoctoral fellow was also present in the lab and mentored other undergraduates in unrelated research projects. She did not spend significant time directly assisting the students in this project, but she did help two of the students with multisequence alignments on a day that the regular mentor was out of the office. Her total time working directly with the students was about 2 h over the 10-wk period.
The Student Experience
Students committed 15 h/wk for 10 wk to this research project. The overall goal was to study aspects of the molecular mechanisms of iron utilization in the plant pathogen X. campestris pathovar vesicatoria, strain 85-10, by creating gene knockouts in open reading frames hypothesized to be involved in iron acquisition.
I preselected a number of appropriate putative iron acquisition genes. The criteria for preselection of the putative iron acquisition genes was first using microbesonline.org to search the X. campestris pathovar vesicatoria strain 85-10 genome using various search terms including “tonB,” “siderophore,” and “iron.” Special attention was given to genes that appeared in contiguous clusters within the genome sequence. For example, a putative siderophore receptor that was located in proximity to putative siderophore biosynthesis genes (but not predicted in the same operon) was selected over a putative receptor that was alone. The final criterion was putting the sequences of interest through the BLASTn algorithm to confirm relatedness to other siderophore uptake and synthesis genes in other bacterial species. Students were only given a MicrobesOnline sequence identification number at the start of the experience; they were not given MicrobesOnline predictions, BLAST results, or actual DNA sequence. Gathering this data independently was part of the learning process.
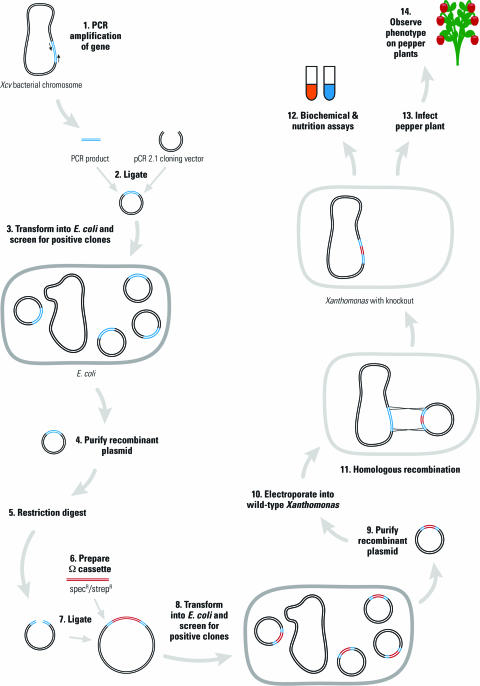
A strategy for creating gene knockouts in noncoliform Gram-negative bacteria was outlined for the students in the first week of the experience. Figure 1 shows the flow chart of the generalized experimental design framework. Students designed their own experimental protocols and strategies within this general scaffold.
Figure 1.
Flow chart of general experimental design. The numbered steps of the experimental procedure are as follows: 1) PCR-amplify the putative gene; 2) Ligate the PCR product into the TA cloning vector, pCR2.1; 3) Transform into E. coli and screen for transformants harboring the plasmid containing the gene of interest; 4) Purify recombinant plasmid; 5) Perform a restriction digest in order to linearize the resulting plasmid; 6) Prepare the omega cassette by restriction digestion and gel purification; 7) Ligate the omega cassette into the digested plasmid from step 5; 8) Transform into E. coli and screen for transformants harboring plasmids containing the omega cassette; 9) Purify this recombinant plasmid; 10) Electroporate into wild-type Xcv 85-10; 11) Allow homologous recombination to occur in vivo; 12) Perform biochemical and nutrition assays on wild-type versus mutant Xcv; 13) Infect pepper plants; and 14) Observe pathogenicity phenotype of mutant versus wild-type on host pepper plant.
Each student randomly chose a preselected MicrobesOnline identification number. As a group, I taught the students how to use microbesonline.org (Alm et al., 2005), BLAST (Altschul et al., 1997; National Center for Biotechnology Information, 2006), literature databases, and Vector NTI (particularly the primer design, restriction analysis, and multisequence alignment tools). Tools of Vector NTI were taught throughout the experience, proximal in time to when they would be necessary. Students then used these tools independently to draw hypotheses about their individual genes. They used MicrobesOnline to obtain their DNA sequence and to predict operons and clusters; performed BLAST searches to find homologous genes in other organisms in order to draw hypotheses about the function of their own gene; and researched primary literature of related genes in other organisms.
Students then individually planned their specific cloning and mutagenesis strategies and proceeded with the “wet” science. The steps below were planned and implemented independently by students, unless indicated otherwise.
They first designed PCR primers using the Vector NTI software, ordered primers commercially, and PCR-amplified the putative gene using taq DNA polymerase. Using DNA gel electrophoresis, they confirmed that a single PCR product was formed, and if not, optimized PCR conditions or gel-purified the correct product.
Next, they ligated the PCR product into the TA cloning vector, pCR2.1, then transformed the ligation mixture into Escherichia coli, and plated it onto selective (kanamycin) medium containing Xgal for blue/white screening. Students chose to use the cloning vector pCR2.1 because of its ease of use, because it was available in the lab, and because it is unable to replicate in Xanthomonads. Its inability to replicate in Xcv allows it to act as a suicide vector later in the process. Students then screened for the desired clone by blue/white screening, restriction analysis of its plasmid, and finally commercial DNA sequencing.
Using the Vector NTI software, students then identified a restriction site that would cut the putative gene, but not the cloning vector. The restriction site/enzyme chosen either needed to leave blunt ends (preferable) or to have 5′ overhangs. Students performed the restriction digest and tested for complete digestion by gel electrophoresis. If the student chose a restriction endonuclease that left the resulting ends with 5′ overhangs, she or he treated them with the Klenow fragment of DNA polymerase to create blunt ends.
The next step was the preparation of the omega cassette. The omega cassette is a gene that confers spectinomycin and streptomycin resistance in a wide range of bacteria. Students digested the plasmid pHP45Ω (Prentki and Krisch, 1984) with the restriction enzyme SmaI, which leaves blunt ends, and then gel-purified the 2-kb omega cassette. Because this step was common for all the students, the two students who were ahead prepared extra to share with the others.
Students then ligated the omega cassette into the digested plasmid containing their cloned gene. This was transformed into E. coli, and transformants were selected on medium containing spectinomycin and then screened by both PCR and restriction analysis. The positive clones are the “knockout constructs.”
The knockout constructs were purified and transformed by electroporation into wild-type Xcv 85-10, and homologous recombination was allowed to occur in vivo. Spectinomycin-resistant colonies were screened by PCR, but unfortunately none had the desired insertion. We have previously observed that Xcv can spontaneously mutate to gain spectinomycin resistance when plated on selective medium, even without any transformation/genetic manipulation. Xanthomonads, like pseudomonads, are highly adaptable organisms and are able to spontaneously obtain resistance to a wide variety of antibiotics. We used spectinomycin even though there was a chance of spontaneous resistance, both because our lab has been successful using spectinomycin resistance in Xcv for the same purpose in the past and because other antibiotics we tested (including streptomycin, kanamycin, and chloramphenicol) led to more frequent rates of spontaneous resistance. On the basis of previous experience, if the electroporation and homologous recombination were successful, we would have expected a higher number of true transformants than of spontaneous mutants.
Students planned to perform biochemical and nutrition assays on wild-type versus mutant Xcv and then infect pepper plants and observe the pathogenicity phenotype of mutant versus wild-type on host pepper plants. Because none of the students achieved the Xanthomonas knockout, these experiments could not be done. The student who was studying the siderophore biosynthesis gene was able to do the CAS (chrome azural S) assay, an assay that tests for the presence of siderophores. She performed the assay on spent medium of wild-type Xcv 85-10 grown to stationary phase in both iron-rich and iron-depleted conditions and showed that a siderophore was made when the bacteria were grown in iron-depleted conditions, but not in iron-rich conditions. This indicated that the organism does, indeed, produce a siderophore and that its production is iron-regulated.
Two of the four students achieved all of the molecular construction steps and attempted electroporation of the final plasmid construct into the wild-type Xanthomonas strain (Figure 1, steps 10 and 11). Unfortunately, the few attempts at electroporation and homologous recombination did not succeed before the summer session ended. These two students continued working on the optimization of the electroporation method as their schedules allowed during the fall semester. An alternate approach to move the knockout construct into Xcv, triparental mating, will be attempted by new students.
The other two students had greater difficulty with their experiments. Both of those students were able to clone their genes and were in the process of creating the plasmid knockout construct (Figure 1, step 8) when the summer session ended. The students who were delayed because of difficulties gained knowledge from their experiments through the process of troubleshooting.
One student had considerable difficulty in the initial PCR amplification of his gene. He did succeed after designing new primers and multiple optimization attempts and then successfully performed the TA cloning before the experience ended. That particular student is now a pro in primer design and PCR optimization strategies. The other student who had difficulties was notably the student who did not have the full-length molecular biology course. His errors were mainly in the planning of his experiments. He lost significant time because of poor choices of the restriction endonuclease to be used to cut his cloned gene in order to ligate in the omega cassette. For example, he first chose an enzyme that also cut the vector and then he chose an enzyme that could only cut nonmethylated DNA. Rather than choosing another appropriate available restriction site, he chose to subclone the plasmid into a dam− (methylase minus) E. coli strain and use his enzyme choice. He gained valuable knowledge of DNA methylation going this route, but he also fell further behind because of it.
Students also had the opportunity to assist with a 2-d hands-on biotechnology workshop I taught at a neighboring historically minority university, North Carolina Central University (Durham, NC). The workshop for incoming honors freshmen focused on PCR and gel electrophoresis. Three of the four of my undergraduates volunteered as teaching assistants. Although the freshmen at NC Central had a great experience, I think it was my own students who benefited the most. My students gained so much confidence by guiding the less experienced students in their first entrée into molecular biology.
RESULTS
Assessment
Quantitative Student Self-Assessment.
Approximately 1 mo after students completed their research experiences, and after grades were given, they completed an anonymous questionnaire. Note that students answered both the “before” and “after” portions of the questionnaire retrospectively. All four students completed the questionnaire.
The survey below reads exactly as that provided to the students, but with results entered and a “mean increase” field added to each question. The “before” field corresponds to the mean self-assessment score of the four students for the retrospective before assessment. The “after” field corresponds to the mean self-assessment score after completion of the experience. In both fields, the parenthetical numbers represent the numerical range given by all four students for that question. “Mean increase” refers to the mean score increase from before to after. The rating scale for before and after was 1–5, with 5 being the highest rating and 1 the lowest.
* * * * *
Survey directions: For each skill, please rate your level of knowledge or competence, both before and after your research experience (summer 2006). The ratings are as follows:
1 = no knowledge or competence
2 = little knowledge or competence
3 = moderate knowledge or competence
4 = a good deal of knowledge or competence
5 = excellent knowledge and/or competence
1. Ability to read and understand primary journal articles and scientific review articles
Before 2.5 (2–3); After 4.5 (4–5); Mean increase 2
2. Ability to find relevant primary journal articles and scientific review articles
Before 2.5 (1–3); After 4.25 (3–5); Mean increase 1.75
3. Competency in understanding experimental design
Before 2.25 (2–3); After 4.5 (4–5); Mean increase 2.25
4. Competency in generating an experimental design
Before 1.5 (1–2); After 4 (3–5); Mean increase 2.5
5. Understanding of the process of creating bacterial genetic knockouts
Before 1.5 (1–3); After 4.75 (4–5); Mean increase 3.25
6. Proficiency in using microbesonline.org
Before 1 (1); After 4 (3–5); Mean increase 3
7. Proficiency in using GenBank and performing BLAST searches
Before 1.5 (1–2); After 4 (3–5); Mean increase 2.5
8. Proficiency in performing multiple sequence alignments
Before 1 (1); After 3.75 (3–5); Mean increase 2.75
9. Proficiency in designing PCR primers
Before 2 (1–3); After 4.5 (4–5); Mean increase 2.5
10. Proficiency in performing PCR
Before 2.5 (2–3); After 4.75 (4–5); Mean increase 2.25
11. Proficiency in and knowledge of TA cloning
Before 1.25 (1–2); After 4.25 (4–5); Mean increase 3
12. Proficiency in DNA gel electrophoresis
Before 3.5 (3–4); After 4.75 (4–5); Mean increase 1.25
13. Ability to prepare and present a poster for a scientific audience
Before 1.25 (1–2); After 4.25 (4–5); Mean increase 3
14. List additional skills you gained during this experience
* * * * *
Each student reflecting on the experience reported a greater level of confidence in every area after research compared with before research. The areas that had smaller differences, such as gel electrophoresis, were areas where the students had significant experience in prior course work, as indicated by the relatively high “before” score.
The following are responses to Question 14: “List additional skills you gained during this experience”
“Preparation of many things needed in lab (i.e., agarose and various plates) and use of most equipment. To me, going to NC Central was an invaluable as well as unforgettable experience. It gave me the opportunity to share my knowledge with peers and explaining concepts helped me feel confident with the things I learned. This experience has sparked my interest in graduate school and I am excited about participating in any other undergraduate research opportunities.”
“I found the general microbiology experience in the laboratory very informative. It was my first experience making agar, or even making agarose gel. The start-to-finish attitude throughout the experience forced me to understand a lot of the things that I had not truly learned in other biotech courses, but had just skimmed through.”
“I gained a better understanding of all aspects of cloning and I hope to continue researching in my future endeavors.”
Qualitative Instructor Assessment of Students.
The students met the majority of the student learning outcomes. All four of the students met all of the technical objectives, which included the following:
using databases to search for relevant journal articles;
using molecular biology databases including microbesonline.org and GenBank to find gene sequences;
performing BLASTP and BLASTN algorithms and interpreting the results;
designing PCR primers using Vector NTI software;
performing multiple sequence alignments using Vector NTI software;
and independently executing a number of molecular biology techniques including PCR, TA cloning, and gel electrophoresis.
Of the higher-level objectives, students achieved differing levels of competence. All displayed a solid understanding of the process of creating bacterial genetic knockouts. All were able to understand the overall experimental design of their project. In observing students planning their own individual experiments, only two of the four truly demonstrated a high degree of competency in generating his or her own experimental design scheme completely independently. In the case of the other two, it is difficult to decipher whether they lacked confidence to trust their own ideas or whether lack of self-motivation played a role. Both students had the mental capacity to be able to achieve this goal. One of the students continued her project throughout the school year and has improved in this area quite a bit, probably because of incremental successes boosting her confidence. In their self-assessment, all the students believed they were capable of designing their own experiments.
In terms of reading primary literature, students varied in their ability to gather pertinent information, understand, analyze, and critique papers, but all improved significantly over the course of the research experience. Undoubtedly, a portion of the improvement was due to a greater understanding of the field of research; they had a greater framework of knowledge toward the end of the experience. They also gained greater skill in reading papers. They learned that there are different reasons to read different papers; sometimes one needs to pore over the details of the Materials and Methods section, and sometimes it is appropriate to just skim it over and focus on the Results and Discussion sections.
All of the students successfully prepared and professionally presented posters based on their research at the North Carolina State University Annual Undergraduate Summer Research Symposium, meeting the final learning objective.
The Mentor Experience
The time burden to the mentor was reduced as compared with mentoring students in nonparallel projects. The instructor was able to present the common background of the projects to all of the students at one time, in a small group setting, rather than individually. Time was also saved by teaching students computer software, experimental techniques, and equipment usage as a group. This method differs from students learning these tools in a class and then doing the research after, because these small “workshops” were integrated into the research experience, in close proximity in time to when the students were ready to implement their new skills in their projects. Moreover, because the concept of all the projects was the same, students were able to assist each other.
Although the “parallel project” method did save the mentor time compared with mentoring diverse projects, it took more effort per student compared with teaching a laboratory course. Some students got ahead while some lagged behind, and so some individual training of students had to take place, especially toward the end of the summer experience. Students also occasionally needed individual help with troubleshooting experiments. Additionally, projects would have diverged considerably at the point where phenotypes of mutants could be analyzed. At that point, students would have needed more individual mentoring, but their increased skills in reading scientific literature should help to offset this. Finally, because a research mentor gets to know students deeply on a personal level and is able to judge students' abilities, students want and deserve individual career guidance. This is a commitment that every research mentor has to be able to provide to each student on an ongoing basis.
In terms of total faculty mentor time, there was a “fixed cost” for the first student, and each student above that “cost” an extra 10–25%, depending on student ability and technical problems. Therefore, as a conservative estimate, I was able to mentor four students using this technique of parallel projects for slightly less than the time cost it would have taken to mentor two students in nonparallel projects. As a comparison, my teaching postdoctoral fellow was concurrently mentoring two nonparallel undergraduate research projects and spent slightly more time than I did with the four.
For this project, enrollment was limited to four students because this was an experimental offering. Retrospectively, this model worked very well, and enrollment could easily be increased to eight, but more than that would be difficult to manage with only one mentor. The number could be increased even more if teaching assistants were utilized, but a close relationship with a faculty member is part of what makes an undergraduate research experience so special, and some of that would be lost with an increase in size above 8 or 12. Certainly, the quality of the learning experience could still be very high with a larger class size and teaching assistants.
DISCUSSION
The goals of this research model were twofold: first, to provide students with a quality research experience that would allow them to feel ownership of their projects and meet the learning outcomes; and second, to ease the time burden on faculty in order to allow them to take on additional students. Engaging students in parallel, yet independent projects met both goals.
Students mastered or significantly improved skills and knowledge in each of the areas measured, including all of the student learning outcomes. This was assessed by methods of both student self-assessment and instructor evaluation.
The time burden to the mentor was reduced significantly by presenting the common background information, computer software, experimental techniques, and equipment usage in a small group setting, rather than one on one. Students did still require a level of individual mentoring for troubleshooting experiments and working out problems, so the mentor's time per student was higher than for a traditional laboratory course.
Although this work does have elements of a discovery-oriented laboratory course, the students truly are engaged in novel research because the experiments are untested and the outcomes are unknown. The final outcomes of the individual research projects could be publishable when completed, probably after several “generations” of students. The experimental design described could be used in a discovery-oriented laboratory course. The strategy could be modified to allow students to work together in teams. Each team would have a single gene to work with, or the whole class could work on a single gene. In either case, these arrangements would allow for greater numbers of students.
Implementing this paradigm of parallel yet independent undergraduate research experiences will be beneficial to any institution that serves undergraduates but is struggling to give large numbers of students a quality experience. The particular model of experimentation described here can be easily modified to make use of virtually any microbe for which the genome has been sequenced.
One unavoidable shortcoming of this method is that student projects will naturally diverge as the research progresses. In many cases, students will be more experienced and therefore more independent as this occurs, needing less individual attention. In other cases, new, inexperienced students will likely need to pick up projects. Because of this latter scenario, if the mentor wants to pursue one or more of the projects more deeply, she or he may not be able to use the parallel projects model continuously but may need to alternate years of using this method, with years of traditional mentoring of fewer students. If multiple faculty members subscribed to this method, however, the overall capacity of a department to mentor undergraduate students could increase significantly.
ACKNOWLEDGMENTS
I thank Jerry Minsavage at the University of Florida for Xcv 85-10. I also thank my lab manager, Melissa Cox, who spent time helping students with various technical aspects of their projects; Joanna Miller, who answered student questions when I could not be there; Cavell Brownie for statistical consulting; and Dwayne Purper for graphic design assistance, and I especially thank the four students who conducted the research projects. The NCSU Biotechnology Education Facility funded all supplies and reagents and provided instrumentation and computers for the projects.
Footnotes
Conflict of interest: The faculty mentor is also the author of this work.
REFERENCES
- Alm E., Huang K., Price M., Koche R., Keller K., Dubchak I., Arkin A. The MicrobesOnline Web site for comparative genomics. Genome Res. 2005;15:1015–1022. doi: 10.1101/gr.3844805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Madden T., Schäffer A., Zhang J., Zhang Z., Miller W., Lipman D. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons S. J., Associates A. A Preliminary Evaluation of the Research Experiences for Undergraduates (REU) Program of the National Science Foundation Program Evaluation Staff, Office of Budget and Control. Washington, DC: National Science Foundation; 1990. [Google Scholar]
- Hathaway R. S., Nagda B.R.A., Gregerman S. R. The relationship of undergraduate research participation to graduate and professional education pursuit: an empirical study. J. Coll. Student Dev. 2002;43:614–631. [Google Scholar]
- Kremer J. F., Bringle R. G. The effects of an intensive research experience on the careers of talented undergraduates. J. Res. Dev. Educ. 1990;24:1–5. [Google Scholar]
- Lopatto D. Survey of Undergraduate Research Experiences (SURE): first findings. Life Sci. Educ. 2004;3:270–277. doi: 10.1187/cbe.04-07-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatto D. The essential features of undergraduate research. Council Undergraduate Res. Quart. 2003;23:139–142. [Google Scholar]
- Nagda B. A., Gregerman S. R., Jonides J., von Hippel W., Lerner J. S. Undergraduate student-faculty research partnerships affect student retention. Rev. Higher Educ. 1998;22:55–72. [Google Scholar]
- National Center for Biotechnology Information. [accessed May-August 2006];NCBI Home Page. 2006 www.ncbi.nlm.nih.gov.
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Seymour E., Hunter A. B., Laursen S. L., DeAntoni T. Establishing the benefits of research experiences for undergraduates in the sciences: first findings from a three-year study. Sci. Educ. 2004;88:493–534. [Google Scholar]
- Thieme F., Koebnik R., Bekel T., Berger C., Boch J., Büttner D., Caldana C., Gaigalat L., Goesmann A., Kay S. Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J. Bacteriol. 2005;187:7254–7266. doi: 10.1128/JB.187.21.7254-7266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]