Abstract
Objective:
Interferon-α2 (IFN-α) injections may be capable of triggering depression in some individuals. The first objective was to further characterize this depression, and secondly to examine whether pre-treatment temperament was correlated with subsequent vulnerability to IFN-α.
Methods:
Twenty-three initially euthymic adults undergoing year-long PEG-IFN-α treatment for hepatitis C were evaluated at baseline and then prospectively monitored using both the Structured Clinical Interview for DSM-IV (SCID) and self-report questionnaires.
Results:
A major depressive episode developed within three months in 39%. Principal component analysis of the change in self-report scores after one month of treatment demonstrated three orthogonal factors: (i) a specific increase in depression as manifested in the Beck Depression Inventory (BDI) and Hospital Anxiety and Depression Scale (HADS), (ii) an increase in hostility and anxiety, (iii) and a generalized combination of worse symptoms including somatic symptoms on the Symptom Check List (SCL-90). BDI at one month was predicted by baseline BDI (r=0.76, p=0.004). Hostility at one month was predicted by low baseline agreeableness (r=0.75, p=0.01). Controlling for baseline BDI scores, categorical major depression was predicted by combined high baseline neuroticism and low agreeableness (combined r=0.66, p=0.03).
Conclusion:
These initial results (i) support the depressogenic nature of IFN-α treatment in a subset of vulnerable individuals, (ii) indicate that some individuals are also independently vulnerable to worsened hostility, and (iii) suggest that it may be possible to clinically predict these vulnerabilities in initially euthymic subjects.
Keywords: Interferon, Personality, Hepatitis, Depression, Principal Component Analysis
Introduction
IFN-α is the principal treatment for hepatitis C (HCV), with about 2.3% of adults in the U.S. being exposed to the virus [1]. However, exogenous interferon-α2 (IFN-α) can potentially trigger major depression (MDD) in a subset of patients [2-4] similar to idiopathic MDD [5-8].
One hypothesized possibility is that endogenous IFN-α may be correspondingly involved in the etiology of primary MDD [9-17]. Examining the variable ‘psychiatric effects triggered by exogenous IFN-α treatment’ may therefore offer a way to prospectively explore variable vulnerability to MDD. However, the nature of the psychiatric syndrome induced by exogenous IFN-α requires further characterization.
In addition, prophylactic therapies are being considered for the prevention of IFN-α-induced depression [18-20]. Although the treatment of pre-existing depression prior to initiating IFN-α is recommended [5, 19, 21-24], the suitability of prophylaxis for euthymic individuals is less certain [25, 26]. Therefore, to characterize the psychiatric symptoms induced by IFN-α and to preliminarily assess potential personality vulnerability factors, we prospectively assessed a set of euthymic individuals before and during IFN-α treatment.
Methods
Participants
Twenty-three euthymic patients (aged 20 to 58; mean = 45; 52% male) initiated combination pegylated interferon-α2 (IFN-α) and oral ribavirin treatment for HCV. To specifically determine IFN-α's effects and minimize concurrent confounding variables, we excluded (a) active mood, anxiety, psychotic, or drug/alcohol use disorders within the past six months; (b) medications that could obscure the relationship between mood and IFN-α such as antidepressants, anticonvulsants, antipsychotics, or alcohol; (c) co-morbid neurological or immunological disorders. Four subjects had hypertension and one had diabetes, but were otherwise without other major co-morbidities. The study was approved by the UPMC Institutional Review Board.
Measures
Prior to initiating IFN-α, participants were evaluated with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) by a single interviewer (FEL), and completed the Neuroticism, Extraversion, Openness – Five Factor Inventory (NEO-FFI), the Psychosocial Adjustment to Illness Scale (PAIS), the Barratt Impulsiveness Scale (BIS-11), the Beck Depression Inventory-II (BDI), the Hospital Anxiety and Depression subscales (HD and HA), and the Symptom Checklist-90 (SCL-90) for subscales related to somatic symptoms (SCL-s), obsession-compulsion (SCL-oc), interpersonal sensitivity (SCL-is), depression (SCL-d), anxiety (SCL-a), and hostility (SCL-h). Monthly measures of the BDI, HDS, HAS, and SCL-90 subscales were obtained after initiating IFN-α, and the SCID-I updated monthly.
Participants who developed a major depressive episode (MDE) or concerns about lethality were provided immediate treatment. Psychiatric treatment effectively censored further relevant data regarding the development of interferon-triggered mood symptoms. Consequently, the primary outcome was symptoms after one month of IFN-α, prior to any confounding psychiatric intervention.
Statistics
A. Changes from baseline to month one were assessed with paired t-tests (employing Bonferroni corrections). B. Questionnaire difference scores (month one – baseline) were subjected to principal component analysis (PCA) with varimax rotation, selecting factors with Eigenvalues >1. Appropriateness of PCA was confirmed with a Kaiser-Meyer-Olkin test of sampling adequacy (0.66) and Bartlett's test of sphericity (Χ2(36) = 84.1; P<0.0005). C. BDI and SCL-hostility scores at month one were assessed using univariate regression of pre-treatment variables (employing Bonferroni corrections). Categorical MDD (coded as zero or one), controlling for pre-treatment BDI, was similarly examined. A subsequent multivariate regression for categorical MDD was then implemented, which included any pre-treatment variables that were initially correlated with MDD (p<0.1 not corrected for multiple testing) during univariate testing. All tests used SPSS v13.0. Means +/− standard deviation are reported unless otherwise indicated.
Results
A. Psychiatric symptoms after interferon-α
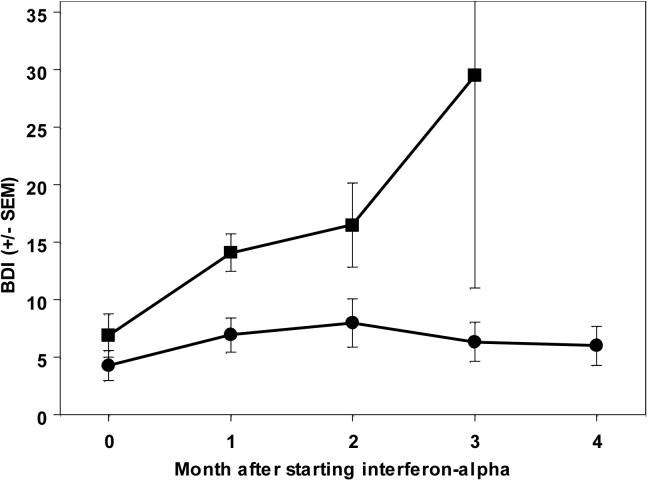
Categorical MDD developed in 9/23 subjects. Of these, 33% (3/9) had a history of a past major depressive episode and 55% were male; of those who did not develop MDD, 36% (5/14) had a past history and 50% were male; all non-significant differences. Overall, the BDI score increased from 4.6 +/− 4.4 at baseline to 9.7 +/− 6.3 after one month of IFN-α treatment (t(22)=5.4; p<0.0005), increasing more in participants who developed categorical MDD (Figure 1).
Figure 1.
IFN-α increases depression symptoms (mean +/− SEM) in subjects developing MDD (squares), and to a much lesser degree in subjects not developing categorical MDD (circles).
The HADS depression subscale (HD) also increased from 2.6 +/− 3.2 to 3.9 +/− 3.6 (t(21)=2.9; p=0.04) as did the hostility (SCL-h) score, from 0.2 +/− 0.25 to 0.58 +/− 0.55 (t(17)=3.0; p=0.04). There were non-significant trends for the other scores to increase: HA from 4.3 +/− 3.4 to 9.5+/− 2.9; SCL-s from 0.57 +/− 0.46 to 0.79 +/− 0.52; SCL-is from 0.32 +/− 0.44 to 0.4 +/− 0.44; SCL-d from 0.48 +/− 5.2 to 0.62 +/− 0.42; and SCL-a from 0.21 +/− 0.34 to 0.31 +/− 0.30.
B. PCA
After one month of interferon-α treatment, three orthogonal components were identified (Table 1). The first component loaded primarily on items from the SCL-90, including somatic symptoms, interpersonal sensitivity symptoms and non-specific depression symptoms. The second component loaded primarily on the anxiety and hostility components of the SCL-90. The third component principally related to the BDI and HADS.
Table 1.
Results of Principal Component Analysis
| Factor 1 | Factor 2 | Factor 3 | |
| Beck Depression Inventory | .31 | .15 | .75 |
| HADS depression subscale | .32 | −.13 | .74 |
| HADS anxiety subscale | −.15 | .22 | .84 |
| SCL-90 hostility subscale | .34 | .87 | .21 |
| SCL-90 anxiety subscale | .03 | .90 | .00 |
| SCL90 somatic subscale | .49 | .77 | .07 |
| SCL-90 obsessive-compulsive subscale | .79 | .49 | −.01 |
| SCL-90 interpersonal sensitivity subscale | .80 | .10 | .28 |
| SCL-90 depression subscale | .87 | .26 | .17 |
C. Risk for developing psychiatric symptoms.
As BDI and hostility (SCL-h) loaded onto distinct factors, vulnerability to these two distinct effects was assessed for eight pre-treatment self-reports: neuroticism (mean = 14.7 +/− 5.6), extraversion (28.4 +/− 5.9), openness (25.8 +/− 5.8), agreeableness (33.4+/− 4.8), conscientiousness (32.8 +/− 6.8), total PAIS (18.7 +/− 15.1), BIS (29.0 +/− 13.5), and pre-treatment BDI.
Pre-treatment BDI predicted BDI at one month (r=0.76, p=0.004), which was not predicted by pre-treatment NEO-FFI factors, total PAIS, nor BIS. Hostility (SCL-h) at one month negatively correlated with pre-treatment NEO agreeableness (r=0.75, p=0.01), but not with pre-treatment hostility or the other baseline self-reports. Controlling for pre-treatment BDI, both baseline neuroticism and agreeableness trended toward predicting categorical MDD (uncorrected p=0.095 and p=0.057, respectively) in the initial univariate analyses. When subsequently combined in the multivariate analysis, and continuing to control for pre-treatment BDI, the combination of pre-treatment high neuroticism and low agreeableness predicted MDD with statistical significance (combined r=0.66, p=0.028).
Discussion
Thirty-nine percent of euthymic subjects developed MDD, consistent with a growing literature [3, 7, 27-47], although this literature is complicated by retrospective, non-standardized diagnoses, and/or the present of active baseline mood disorder or antidepressants [23]. Notably, this study was specifically designed to prospectively assess IFN-α's effects while avoiding confounding biases from active neuropsychiatric illness and/or co-prescribed psychoactive medications.
Three orthogonal sets of symptoms were worsened following IFN-α: depression (BDI and HADS), hostility and anxiety (subscales within the SCL-90), and general symptoms on other SCL-90 subscales. This is consistent with a prior observation that IFN-induced depression-specific symptoms are distinct from general somatic and fatigue symptoms [8]. IFN-induced “MDD” may, in fact, potentially reflect a combination of three different phenomena (depression-specific, anxiety/hostility, and general psychiatric/somatic complaints).
The only factor predicting BDI at month one was the baseline BDI, which was consistent with multiple prior reports [23, 48], and which highlights the importance of controlling for baseline BDI in our assessments. Personality styles have been proposed to increase vulnerability to depression, although there have been many methodological problems [49]; for example, depression or subsyndromal depression can contaminate measures of personality such as neuroticism. In our prospective study, the development of a categorical DSM-IV MDE was predicted by a combination of high neuroticism and low agreeableness, when controlling for baseline BDI. The low pre-treatment agreeableness appeared to be related to the development of increased hostility on IFN-α. This supports the hypothesis that personality can independently influence vulnerability, even to a biologic trigger such as IFN-α.
Surprisingly, a past history of MDE did not predict vulnerability to IFN-induced depression. This may reflect several possibilities: difficulties with accurately diagnosing past MDE retrospectively using the SCID, different “types” of MDE have different vulnerabilities, this could have been the first time for some subjects to encounter a truly depressogenic stimulus, we excluded patients with active MDD, and/or vulnerability may change over the life-span.
Important caveats include: (i) Many HCV patients already have co-morbid psychiatric disorders [50-52]. This study was designed to assess IFN-α's influence on euthymic subjects, limiting clinical generalizability to this specific group. (ii) Depression symptoms would likely worsen in the absence of intervention. This was effectively censored by psychiatric treatment, necessary for ethical reasons and necessitating our focus on one month for quantitative data. (iii) A placebo control was not used. However, we estimate that less than 1% developed MDD unrelated to the IFN-α treatment -- given that the incidence of MDD in medical illness is about 2% per year [53-55]. (iv) We did not control for the dose and brand of ribavirin or pegylated IFN-α2. Whether different brands (e.g. PEG-IFN-α2b vs PEG-IFN-α2a vs. non-pegylated) and/or doses can differentially influence these psychiatric side effects will require future controlled trials.
In summary, this study supports the conclusion that even pegylated IFN-α can induce major depression, and it can do so in patients without a history of major depression. Moreover, IFN-α can trigger a distinct condition of worsening hostility. Prophylactic therapy has been considered for euthymic patients to prevent IFN-α-induced depression [20, 35, 56], but this may not be risk free [20, 25, 26]. The results from this study suggest that vulnerability may be clinically predictable, and therefore prophylaxis could be appropriately targeted. This may have implications both for understanding vulnerability to MDD as well as for appropriately targeting prophylactic antidepressant therapy.
Acknowledgements.
This work was supported by grants MH74012 and MH16804 from the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Chou R, Clark EC, Helfand M. Screening for Hepatitis C Virus Infection: A Review of the Evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2004;140:465–79. doi: 10.7326/0003-4819-140-6-200403160-00014. [DOI] [PubMed] [Google Scholar]
- 2.Donnelly S. Patient management strategies for interferon alfa-2b as adjuvant therapy of high-risk melanoma. Oncological Nursing Forum. 1998;25:921–7. [PubMed] [Google Scholar]
- 3.Malaguarnera M, Di Fazio I, Restuccia S, Pistone G, Ferlito L, Rampello L. Interferon alpha-induced depression in chronic hepatitis C patients: Comparison between different types of interferon alpha. Neuropsychobiology. 1998;37:93–7. doi: 10.1159/000026485. [DOI] [PubMed] [Google Scholar]
- 4.Trask PC, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. Journal of Clinical Oncology. 2000;18:2316–26. doi: 10.1200/JCO.2000.18.11.2316. [DOI] [PubMed] [Google Scholar]
- 5.Ho SB, Nguyen H, Tetrick LL, Opitz GA, Basara ML, Dieperink E. Influence of psychiatric diagnoses on interferon-alpha treatment for chronic hepatitis C in a veteran population. American Journal of Gastroenterology. 2001;96:157–64. doi: 10.1111/j.1572-0241.2001.03468.x. [DOI] [PubMed] [Google Scholar]
- 6.Bonaccorso S, Meltzer HY, Maes M. Psychological and behavioural effects of interferons. Current Opinions in Psychiatry. 2000;13:673–7. [Google Scholar]
- 7.Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: A review. American Journal of Psychiatry. 2000;157:867–76. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- 8.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-a in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–52. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 9.Maes M. Psychological stress, cytokines, and the inflammatory response system. Current Opinions in Psychiatry. 1999;12:695–700. [Google Scholar]
- 10.Schwarz MJ, Chiang S, Muller N, Ackenheil M. T-Helper-1 and T-helper-2 responses in psychiatric disorders. Brain Behavior and Immunology. 2001;15:340–70. doi: 10.1006/brbi.2001.0647. [DOI] [PubMed] [Google Scholar]
- 11.Vollmer-Conna U. Acute sickness behavior: an immune system-to-brain communication? Psychological Medicine. 2001;31:761–7. doi: 10.1017/s0033291701003841. [DOI] [PubMed] [Google Scholar]
- 12.Capuron L, Dantzer R. Cytokines and depression: The need for a new paradigm. Brain Behavior and Immunology. 2003;17:S119–S24. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 13.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behavior and Immunology. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 14.Dantzer R, Wollman L, Vitkovic L, Yirmiya R. Cytokines and depression: fortuitous or causative association? Molecular Psychiatry. 1999;4:328–32. doi: 10.1038/sj.mp.4000572. [DOI] [PubMed] [Google Scholar]
- 15.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends in Neurosciences. 2002;25:154–9. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 16.Leonard BE, Song C. Stress, depression, and the role of cytokines. Advances in Experimental Medicine and Biology. 1999;461:251–65. doi: 10.1007/978-0-585-37970-8_14. [DOI] [PubMed] [Google Scholar]
- 17.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Progress in Neuropsychopharmacology and Biologic Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- 18.Hauser P, Soler R, Reed S, Kane R, Gulati M, Khosla J, Kling MA, Valentine AD, Meyers CA. Prophylactic treatment of depression induced by interferon-a. Psychosomatics. 2000;41:439–42. doi: 10.1176/appi.psy.41.5.439. [DOI] [PubMed] [Google Scholar]
- 19.Loftis JM, Hauser P. Comanagement of depression and HCV treatment. Psychiatric Annals. 2003;33:385–91. [Google Scholar]
- 20.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. New England Journal of Medicine. 2001;344:961–6. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 21.Goldsmith J, Hauser P. Psychiatric issues in patients with hepatitis C. Psychiatric Annals. 2003;33:357–60. [Google Scholar]
- 22.Goldsmith RJ, Mendenhall C, Harrer J. Co-morbid behavioral emotional disturbances (BED) associated with hepatitis C virus (HCV): prevalence, compliance and treatment responses using a multidiscipline approach. Hepatology (abstract) 2002;36:292A. [Google Scholar]
- 23.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric Adverse Effects of Interferon-a: Recognition and Management. CNS Drugs. 2005;19:105–23. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mistler LA, Brunette MF, Marsh BJ, Vidaver RM, Luckoor R, Rosenberg SD. Hepatitis C treatment for people with severe mental illness. Psychosomatics. 2006;47:93–107. doi: 10.1176/appi.psy.47.2.93. [DOI] [PubMed] [Google Scholar]
- 25.Hayasaka S, Nagaki Y, Matsumoto M, Sato S. Interferon associated retinopathy. British Journal of Ophthalmology. 1998;82:323–5. doi: 10.1136/bjo.82.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hejny C, Sternberg P, Lawson DH, Greiner K, Aaberg TMJ. Retinopathy associated with high-dose interferon alfa-2b therapy. American Journal of Ophthalmology. 2001;131:782–7. doi: 10.1016/s0002-9394(01)00836-4. [DOI] [PubMed] [Google Scholar]
- 27.Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, Bauer J, Lieb K. Prefrontal cortical hypometabolism during low-dose inteferon alpha treatment. Psychopharmacology. 2001;152:383–9. doi: 10.1007/s002130000549. [DOI] [PubMed] [Google Scholar]
- 28.Mapou RL, Law WA, Wagner K, Malone JL, Skillman DR. Neuropsychological effects of Interferon Alfa-n3 treatment in asymptomatic human immunodeficiency virus-1-infected individuals. Journal of Neuropsychiatry & Clinical Neurosciences. 1996;8:74–81. doi: 10.1176/jnp.8.1.74. [DOI] [PubMed] [Google Scholar]
- 29.Adams F, Quesada JR, Gutterman JU. Neuropsychiatric manifestations of human leukocyte interferon therapy in patients with cancer. JAMA. 1984;252:938–41. [PubMed] [Google Scholar]
- 30.Malaguarnera M, Laurino A, Di Fazio I, Pistone G, Castorina M, Guccione N, Rampello L. Neuropsychiatric effects and type of IFN-a in chronic hepatitis C. Journal of Interferon and Cytokine Research. 2001;21:273–8. doi: 10.1089/107999001300177457. [DOI] [PubMed] [Google Scholar]
- 31.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer HY, Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-[alpha]-based immunotherapy are related to interferon-[alpha]-induced changes in the serotonergic system. Journal of Clinical Psychopharmacology. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Hunt CM, Dominitz JA, Bute BP, Waters B, Blasi U, Williams DM. Effect of interferon-a treatment of chronic hepatitis C on health-related quality of life. Digestive Diseases and Sciences. 1997;42:2482–6. doi: 10.1023/a:1018852309885. [DOI] [PubMed] [Google Scholar]
- 33.Pariante CM, Orru GM, Baita A, Farci MG, Carpiniello B. Treatment with interferon-a in patients with chronic hepatitis and mood or anxiety disorders. Lancet. 1999;354:131–2. doi: 10.1016/S0140-6736(98)04793-X. [DOI] [PubMed] [Google Scholar]
- 34.Renault PF, Hoofnagle JH, Park Y, Mullen KD, Peters M, Jones DB, Rustgi V, Jones EA. Psychiatric complications of long-term interferon alfa therapy. Archives of Internal Medicine. 1987;147:1577–80. [PubMed] [Google Scholar]
- 35.Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-a-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44:104–12. doi: 10.1176/appi.psy.44.2.104. [DOI] [PubMed] [Google Scholar]
- 36.Kraus MR, Schafer A, Faller H, Csef H, Scheurlen M. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2b therapy. Journal of Clinical Psychiatry. 2003;64:708–14. doi: 10.4088/jcp.v64n0614. [DOI] [PubMed] [Google Scholar]
- 37.Rockstroh JK, Mudar M, Lichterfeld M, Nischalke HD, Klausen G, Golz J, Dupke S, Notheis G, Stein L, Mauss S. Pilot study of interferon alpha high-dose induction therapy in combination with ribavirin for chronic hepatitis C in HIV-co-infected patients. AIDS. 2002;16:2083–5. doi: 10.1097/00002030-200210180-00016. [DOI] [PubMed] [Google Scholar]
- 38.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FLJ, Haussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon afla-2a plus ribavirin for chronic hepatitis C virus infection. New England Journal of Medicine. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 39.Caraceni A, Gangeri L, Martini C, Belli F, Brunelli C, Baldini M, Mascheroni L, Lenisa L, Cascinelli N. Neurotoxicity of interferon-a in melanoma therapy: results from a randomized controlled trial. Cancer. 1998;83:482–9. doi: 10.1002/(sici)1097-0142(19980801)83:3<482::aid-cncr17>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 40.Pavol MA, Meyers CA, Rexer JL, Valentine AD, Mattis PJ, Talpaz M. Pattern of neurobehavioral deficits associated with interferon alfa therapy for leukemia. Neurology. 1995;45:947–50. doi: 10.1212/wnl.45.5.947. [DOI] [PubMed] [Google Scholar]
- 41.Cotler SJ, Wartelle CF, Larson AM, Gretch DR, Jensen DM, Carithers RLJ. Pretreatment symptoms and dosing regimen predict side-effects of interferon therapy for hepatitis C. Journal of Viral Hepatitis. 2000;7:211–7. doi: 10.1046/j.1365-2893.2000.00215.x. [DOI] [PubMed] [Google Scholar]
- 42.Koskinas J, Merkouraki P, Manesis E, Hadziyannis S. Assessment of depression in patients with chronic hepatitis: effect of interferon treatment. Digestive Diseases & Sciences. 2002;20:284–8. doi: 10.1159/000067682. [DOI] [PubMed] [Google Scholar]
- 43.Gohier B, Goeb JL, Rannou-Dubas K, Fouchard I, Cales P, Garre JB. Hepatitis C, alpha interferon, anxiety and depression disorders: a prospective study of 71 patients. World Journal of Biological Psychiatry. 2003;4:115–8. doi: 10.1080/15622970310029904. [DOI] [PubMed] [Google Scholar]
- 44.Bonaccorso S, Marino V, Biondi M, Grimaldi F, Ippoliti F, Maes M. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. Journal of Affective Disorders. 2002;72:237–41. doi: 10.1016/s0165-0327(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 45.Pomova NI, Ivanikov IO, Siutkin VE. Use of peg-intron in combined treatment of chronic liver disease caused by HIV infection. Eksperimental'Naia i Klinicheskaia Gastroenterologiia. 2003;1:42–5. [PubMed] [Google Scholar]
- 46.Horikawa N, Yamazaki T, Izumi N, Uchihara M. Incidence and clinical course of major depression in patients with chronic hepatitis type C undergoing interferon-alpha therapy: a prospective study. General Hospital Psychiatry. 2003;25:34–8. doi: 10.1016/s0163-8343(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 47.Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, Gulati M, Thornton AJ, Schultz RL, Valentine AD, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Molecular Psychiatry. 2002;7:942–7. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 48.Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patients initial affective state. New England Journal of Medicine. 1999;340:1370. doi: 10.1056/NEJM199904293401716. [DOI] [PubMed] [Google Scholar]
- 49.Boyce P, Mason C. An overview of depression-prone personality traits and the role of interpersonal sensitivity. Australian & New Zealand Journal of Psychiatry. 1996;30:90–103. doi: 10.3109/00048679609076076. [DOI] [PubMed] [Google Scholar]
- 50.Yates WR, Gleason O. Hepatitis C and Depression. Depression and Anxiety. 1998;7:188–93. doi: 10.1002/(sici)1520-6394(1998)7:4<188::aid-da7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 51.Straits-Troster KA, Sloan KL, Dominitz JA. Psychiatric and substance use disorder comorbidity with hepatitis C. Psychiatric Annals. 2003;33:362–8. [Google Scholar]
- 52.Dwight MM, Kowdley KV, Russo JE, Ciechanowski PS, Larson AM, Katon WJ. Depression, fatigue, and functional disability in patients wich chronic hepatitis C. Journal of Psychosomatic Research. 2000;49:311–7. doi: 10.1016/s0022-3999(00)00155-0. [DOI] [PubMed] [Google Scholar]
- 53.Patten SB. Long-term medical conditions and major depression in a Canadian population study at waves 1 and 2. Journal of Affective Disorders. 2001;63:35–41. doi: 10.1016/s0165-0327(00)00186-5. [DOI] [PubMed] [Google Scholar]
- 54.Rymaszewska J, Kiejna A, Hadrys T. Depression and anxiety in coronary artery bypass grafting patients. European Psychiatry: the Journal of the Association of European Psychiatrists. 2003;18:155–60. doi: 10.1016/s0924-9338(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 55.Gerstman BB, Jolson HM, Bauer M, Cho P, Livingston JM, Platt R. The incidence of depression in new users of beta-blockers and selected antihypertensives. Journal of Clinical Epidemiology. 1996;49:809–15. doi: 10.1016/0895-4356(96)00017-0. [DOI] [PubMed] [Google Scholar]
- 56.Miller AH, Musselman DL, Nemeroff CB. Paroxetine for the prevention of depression induced by interferon alfa. New England Journal of Medicine. 2001;345:375–6. [PubMed] [Google Scholar]