Abstract
Introduction
Endomyocardial biopsy (EmBx) is the standard means of establishing cardiac allograft rejection diagnosis. The efficacy of this procedure in xenotransplantation has not been determined. In this study we compare the histology of right ventricular EmBx specimens with the corresponding full cross sections of explanted right ventricle (RV). We also compare RV with the related left ventricle (LV) cross sections.
Methods
Heterotopic CD46 pig to baboon cardiac xenotransplants (n=64) were studied. RVEmBxs were taken at cardiac explant, using either a standard bioptome (RVEmBxBT; n=24) or by sharp dissection (RVEmBxSD; n=40). Hematoxylin-eosin stained sections of RV and LV cross section and RVEmBxs were compared in a blinded fashion. Characteristics of delayed xenograft rejection (DXR) and a global assessment of ischemia, were scored from 0 to 4 based on the percentage of myocardium involved (0 = 0%, 1=1−25%, 2 = 26−50%, 3 = 51−75%, 4 = 76−100%).
Results
Median graft survival was 30 days (range 3–137). Linear regression analysis of histology scores demonstrated that both RVEmBxBT and RVEmBxSD equally represented the histology of RV cross section. Global ischemic injury was strongly correlated between RV and RVEmBx (R2=0.84) and between RV and LV cross sections (R2=0.84). Individual characteristics of DXR showed no significant variation between RV and RVEmBx or between RV and LV (p<0.05).
Conclusions
These results indicate that DXR is a widespread process involving both right and left ventricles similarly. This study shows that histologic assessment of RVEmBx specimens is an effective method for the monitoring of DXR after cardiac xenotransplantation.
INTRODUCTION
The lack of human donors has led investigators to search for alternative sources of organs. The pig is the preferred species for xenotransplantation due to its size plus the existence of established techniques for genetic modifications and designated pathogen free husbandry.1 Pig-to-primate xenotransplantation has been limited by antibody-induced immune rejection. Initially this antibody response was directed towards the Galactose α 1,3 Galactose (α-Gal) antigen, absent in humans and Old World Primates, but produced abundantly in pigs. The presence of preformed recipient anti-Gal antibody results in hyperacute rejection (HAR) of a porcine xenograft within minutes to hours after transplantation. We and others have circumvented this process by using transgenic donor pigs expressing high levels of human complement regulatory proteins combined with α-Gal polymers and specific immunosuppressive regimens that block function and induction of anti-Gal antibody. This approach has resulted in a median survival of 96 days in heterotopic transgenic pig to baboon transplants.2,3 Under conditions where anti-Gal mediated effects are eliminated, including the use of α Gal deficient donor pigs,4,5 xenograft failure remains associated with vascular antibody deposition directed towards non-Gal anti-pig antigens.3,6-8 In this case grafts are lost in a process termed “delayed xenograft rejection” (DXR).9 This rejection is characterized by microvascular thrombosis, and myocardial necrosis without a cellular infiltrate. Antibody (IgM and IgG) is deposited on vascular endothelium, but complement may not be activated.10
Right ventricular endomyocardial biopsy (RVEmBx) specimens has been the gold standard for the early diagnosis of cardiac allograft cellular rejection for 25 years. At later time-points routine biopsy surveillance beyond five years post-transplant is less useful.11
At a lower frequency allograft rejection is associated with induction of allo-antibodies with minimal cellular infiltration. Criteria for the diagnosis of this antibody-mediated cardiac allograft rejection on cardiac biopsies have recently been proposed and include the identification of complement activation12. Whether RVEmBx will prove to have the same utility in the setting of cardiac xenotransplantation, where antibody mediated process dominates, has not been tested. Therefore, we undertook the current study to determine how well endomyocardial biopsy samples represent right ventricular morphology as a whole, and to determine the degree to which pathology occurring in one ventricle compares to pathology in the other in the setting of heterotopic xenotransplantation.
METHODS
Heterotopic Heart Transplantation
Heterotopic abdominal pig-to-baboon cardiac xenotransplantation was performed using standard surgical methods for five prospectively defined transplant protocols. Details of these transplants have been previously described.1-3,7,13,14 All recipients were splenectomized, received CD46 transgenic pig hearts and were treated with maintenance immunosuppression consisting of tacrolimus, sirolimus, tapering steroids and daily infusions of an α-Gal polyethylene glycol polymer (TPC) to block anti-Gal antibody.
The study group is comprised of five transplant groups. Groups 1 – 4 received low maintenance immunosuppression with targeted serum trough levels of tacrolimus and sirolimus of 10 – 15 ng/ml and 5 – 10 ng/ml, respectively. To deplete B cells, these animals also received weekly Rituximab therapy (17 mg/kg i.v.) beginning 1 – 2 weeks prior to transplantation. Group 1 (n=9) received no postoperative anticoagulation. Group 2 (n=13) was treated with Lovenox. Group 3 (n=9) received antiplatelet therapy using aspirin and clopidogrel (Plavix; Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, New York, NY). Group 4 (n=9) was treated with Coumadin (Bristol-Myers Squibb, New York, NY, USA). Group 5 (n = 24) received high maintenance immunosuppression in which the targeted tacrolimus and sirolimus levels were doubled compared to groups 1 – 4. In group 5 fifteen recipients were also anticoagulated with Lovenox. This subgroup received weekly Rituximab therapy as above and was additionally aggressively treated for xenograft rejection using rabbit antithymocyte globulin (SangStat Medical Corporation, Fremont, CA). In group 5 seven recipients were not treated with anticoagulants and were not treated for rejection. ATG was given only as induction therapy on postoperative day 2 – 6 and Rituximab therapy was confined to four weekly treatments beginning 2 weeks prior to transplant.
All animals were housed and received humane care in accordance with the standards established by the Institutional Animal Care and Use Committee of Mayo Clinic and Foundation and as described in the “Guide for the Care and Use of Laboratory Animal” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86−23, revised 1996).
Histologic Analysis
Xenografts were explanted at the time of death or when they were no longer beating as judged by a concomitant decrease in the electrical activity of the graft, a decrease in palpation score and absence of contractility as judged by echocardiography. Under direct visualization, right ventricle endomyocardial biopsies (RVEmBx) and full thickness mid-ventricular sections of right (RV) and left ventricles (LV) of the transplanted heart were collected for light microscopy and frozen for immunohistochemistry (IHC). RVEmBxs were taken either using a standard bioptome (RVEmBxBT; n=24) or by sharp dissection (RVEmBxSD; n=40). In the grafts in which the biopsy was taken with a standard bioptome the mean number of specimens obtained was 2.5 (2 to 4) while the tissue collected with a sharp dissection was represented by a single tissue sample. In addition the size of the standard bioptome biopsies was smaller than the samples obtained by sharp dissection.
Individual features of DXR (coagulative necrosis, myocyte vacuolization, thrombosis, congestion and interstitial hemorrhage) were scored based on amount of involved myocardium (0: 0%, 1: 1−25%, 2: 26−50%, 3: 51−75% and 4: >75%). An overall global assessment of DXR was similarly scored and defined as the sum of areas with reversible ischemia, with myocyte vacuolization and irreversible coagulative necrosis.
For IHC, biopsy samples were embedded in OCT (Miles Laboratories), snap frozen in isopentane and dry ice, and 4 μm thick sections cut on a cryostat. Immunohistochemical staining was assessed estimating the intensity (0, none; 1, minimal; 2, present and easily recognized, characterized by smooth linear decoration of endothelial cells; and 3, intense, with granular staining of endothelial cells) and the extent of reactivity (focal: <25 % of vessels; or diffuse: ≥25 % of vessels).
Sections of RV and LV cross section and RVEmBx were reviewed in a blinded fashion by an experienced cardiac pathologist.
Statistical Analysis
The histology scores (0 – 4) for global ischemic injury and for each characteristic of DXR were compared for all tissue samples. The frequency of histology scores between samples (i.e. comparing the RV and RVEmBx and comparing the RV and LV at explant) that matched perfectly or differed by one histology score were analyzed by linear regression. Similarly a linear regression model was used to compare standard bioptome and sharp dissection biopsies and an F-test was used to determine significance.
RESULTS
Graft Survival and Transplant Groups Histology
A total of 64 transplants were performed. Median implantation time for the group was 30 days (range 3 – 137). Survival was similar among groups except for group 5 in which it was significantly longer.14 Previous analysis found that despite differences in anticoagulation and immunosuppression there was no significant difference in histology between these transplant groups at the time of explant.7,13,14 For this study, we re-examined the histopathology of the RV and LV sections for all samples to detect any disparity in DXR pathology that might be affected by anticoagulation or by the immunosuppression protocol.
The range of the mean histology scores for all the characteristics of DXR in the RV was similar across the transplant groups: global ischemic injury (2.1 – 3.4) coagulative necrosis (1.9 – 3.4), vacuolization (0.7 – 1.6), thrombosis (2.4 – 3.4), congestion (0.8 – 1.5) and hemorrhage (0.4 – 1.3). Group 3 transplants, treated with aspirin and clopidogrel, tended to have a higher level of histological damage compared to the control group 1 but was not significantly different from the other transplant groups (Figure 1). In group 3 global ischemic injury and coagulative necrosis were significantly higher compared to group 1 (p=0.04 and p=0.02 respectively). Vacuolization in Lovenox-treated group 2 was increased compared to group 3 (p=0.04). All other DXR features showed no significant variation between any of the transplant groups (T-test: p>0.05). No transplant-dependent variation was found in histology in LV cross section (data not shown). This analysis is consistent with our previous understanding that the pathology of the DXR is the same regardless of anti-coagulation or immunosuppression. Accordingly all tissue samples were grouped together for further analysis.
Figure 1.
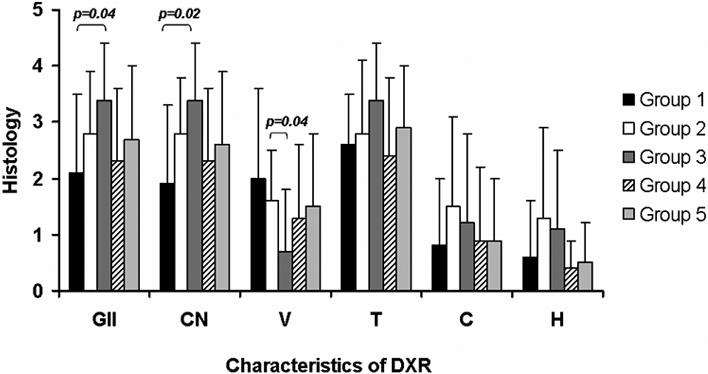
RV cross sections were scored for global ischemic injury (GII), coagulative necrosis (CN), vacuolization (V), thrombosis (T), congestion (C) and hemorrhage (H); mean value for each transplant group, described in material and methods, ± standard deviation is given. Except for global ischemic injury and coagulative necrosis between group 1 and 3 (respectively p=0.04 and p=0.02) and for vacuolization between group 2 vs group 3 (p=0.04) all characteristics of DXR showed no significant difference between the transplant groups (T-test: p>0.05).
Comparing the Histology of RVEmBx (BT or SD) and RV cross section
To determine if RVEmBxBT and RVEmBxSD were equally representative of the histology in the corresponding RV cross section, we produced a matrix of histology scores for each DXR component, as illustrated for coagulative necrosis (Figure 2). Of 24 paired RVEmBxBT and RV tissue samples 18 (75%) were scored identically as depicted by the diagonal of the matrix and 100% of the sample pairs matched within one histology score (Figure 2a). Similarly, 29 of 40 (72%) of RVEmBxSD and RV pairs were identically scored with 97% of the tissues matching within one histology score (Figure 2b). A similar high level of concordance between RVEmBx and RV cross section was observed for each DXR variable regardless of how the biopsy was obtained (Figure 2c). A linear regression analysis of the data found no significant difference (F-test: p>0.05) in concordance with the biopsy type (comparing perfectly matched histology scores) demonstrating that these biopsies are equally representative of the histology in the RV cross section.
Figure 2.
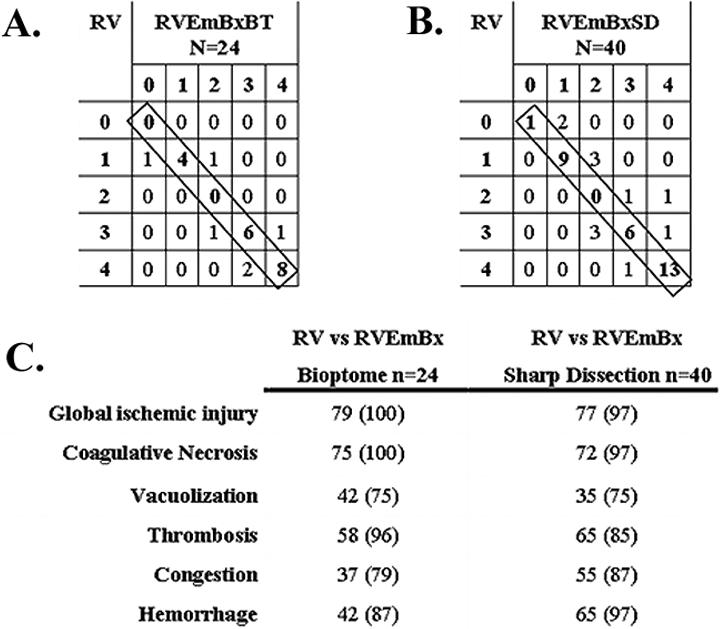
A matrix analysis was used to compare histology scores for each characteristic of DXR. A. Illustrates the matrix comparison between paired samples of RV and RVEmBxBT for the degree of coagulative necrosis. Histology scores ranged form 0 – 4 as described in the Materials and methods. Numbers on the diagonal (boxed) represent samples where the biotome biopsy and RV cross section of a xenograft exhibited the same histology score B. The same analysis as in A for coagulative necrosis, now comparing RV and RVEmBxSD. C. Summarizes the analysis for each characteristic of DXR comparing RV cross sections to biotome and sharp dissection biopsies. Values indicate the percentage of rejected xenografts with identical histology scores. The number in parentheses is the percentage of sample pairs that differed by only one histology score. (p value >0.05 for all the variables examined)
Comparing the Histology between RV and RVEmBx and between RV and LV cross sections
A similar systematic comparison of the histology observed in RV and RVEmBx and in RV and LV was performed for each of the 64 xenografts (Table 1). The mean histology scores for each feature of DXR were consistent across the different tissue samples. In each xenograft there was a high degree of correlation between the histology of the RV and RVEmBx and between the RV and LV samples as illustrated by the percent of tissue pairs with identical histology scores and with scores that differed only by one. Regression analysis of the data found a high degree of correlation between each sample pair. This correlation was strongest for end-stage effects of DXR such as global ischemic injury and coagulative necrosis, and less powerful for transient histologic features such as vacuolization, congestion and hemorrhage, nonetheless all sample pairs showed a significant correlation (p<0.05).
Table 1.
Comparison of DXR histology between RV cross section and RVEmBx and between RV and LV cross section. Histology scores represent mean histology scores for each characteristic of DXR for the RV cross section, RVEmBx and LV cross section. Percent matching is the percent of samples with matched histology scores. Values in parenthesis indicate the percent of sample pairs with histology scores that differ by only one value. There were no significant differences in the assessment of each DXR feature between the tissue samples: *p<0.0001; **p<0.05.
| Global Ischemic Injury | Coagulative Necrosis | Vacuolization | Thrombosis | Congestion | Hemorrhage | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RV | RVEmBx* | LV* | RV | RVEmBx* | LV* | RV | RVEmBx** | LV* | RV | RVEmBx* | LV* | RV | RV EmBx* | LV* | RV | RVEmBx* | LV* | |
| Histology scores | 2.7 | 2.7 | 2.7 | 2.6 | 2.7 | 2.7 | 1.5 | 1.2 | 1.5 | 2.8 | 2.8 | 2.8 | 1.0 | 0.9 | 0.9 | 0.8 | 0.5 | 0.8 |
| % Matching | 78 (98) | 78 (98) | 73 (98) | 76 (95) | 38 (75) | 37 (78) | 63 (89) | 73 (100) | 48 (84) | 59 (87) | 56 (94) | 63 (95) | ||||||
| R2 | 0.84 | 0.84 | 0.83 | 0.80 | 0.14 | 0.26 | 0.60 | 0.81 | 0.28 | 0.38 | 0.40 | 0.55 | ||||||
In 64 transplants 33 xenografts were rejected as evidenced by the loss of contractility. These grafts uniformly exhibited widespread irreversible ischemic myocyte injury involving over 50% of the RV. Among 64 transplants, however, 31 grafts were explanted due to recipient death and these grafts exhibited variable levels of DXR (Figure 3). To determine if RVEmBxs were representative samples in xenografts with on-going immune injury we divided the samples into three grades of rejection (1 − 3) based on the level of global ischemic injury. Grafts with minimal global ischemic injury (Grade 1; n=21) typically exhibited vigorous contractility at the time of recipient death and had histologic changes of DXR involving 0 – 25% of the myocardium (Figure 3 panel 1). Grafts with Grade 2 DXR (n=18) had changes involving 26 – 75% of the myocardium (Figure 3 panel 2) and grafts with severe rejection, Grade 3 (n=25) showed features typical of fulminant DXR, with involvement of >75% of myocardium. (Figure 3 panel 3). No prominent cellular infiltrates were observed in any of the grafts. Congestion and intramyocardial hemorrhage were rarely observed. The mean histology scores for each of the six characteristics of DXR were similar in each tissue samples and independent of the grade of rejection as illustrated in figure 4 for coagulative necrosis, vacuolization, thrombosis and congestion. This suggests that RVEmBx remains a faithful representation of all the specific histology features.
Figure 3.
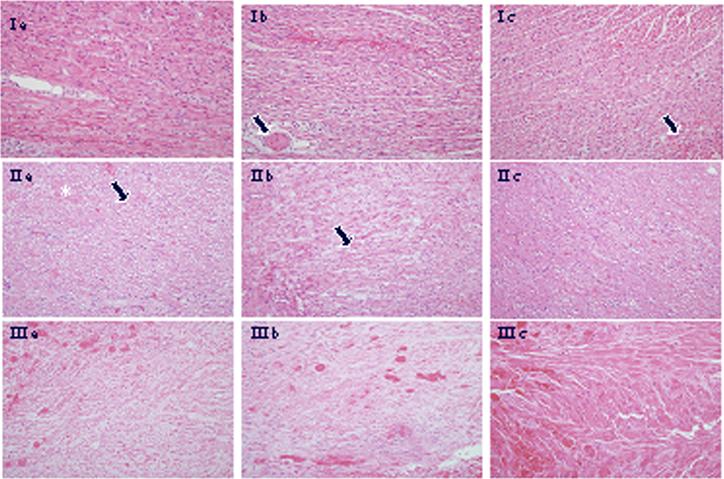
a: RV, b: RVEmBx, c: LV (magnification 200X)
Panel 1: sections from all three sites show well-preserved myocardium, minimal myocyte vacuolization and focal vascular thrombosis (arrows).
Panel 2: intermediate myocardium damage (26 – 75%) in all three sections with more prominent vacuolization (arrows) and coagulative necrosis (asterisk).
Panel 3: in all three sites features typical of coagulative necrosis, characterized by loss of myocyte nuclei, and microvascular thrombosis.
Figure 4.
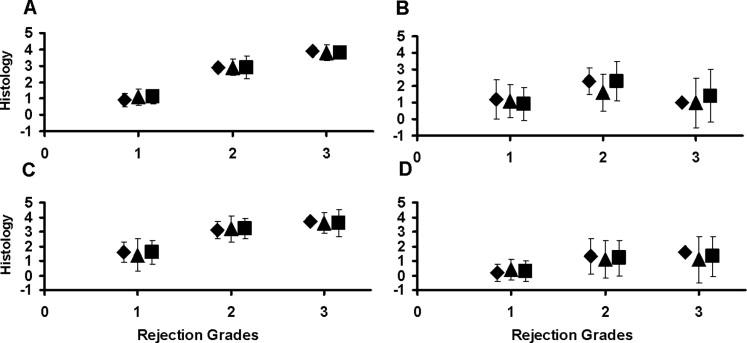
Comparison of histology between RV cross section (◇), RVEmBx (△) and LV cross section (□) samples. A. coagulative necrosis. B. vacuolization. C. thrombosis. D. congestion. Graphs depict the mean histology score for each DXR characteristic (± standard deviation). X-axis values indicate the extent of DXR. Similar results were observed for global ischemic injury and hemorrhage (data not shown).
Immunohistochemistry
Since xenograft rejection is predominantly an antibody mediated process, we examined the intensity and extent of vascular IgM deposition. From 64 transplanted hearts there were 31 pairs of RV and RVEmBx samples that were interpretable. The level of tissue damage in other samples precluded a clear assessment of vascular antibody deposition. The majority of RV and RVEmBx samples (20 of 31 pairs 65%) matched for intensity, the extent of immuno-reactivity or both. In 7 cases (23%) the RVEmBx gave a false negative and in 2 cases (6%) the RVEmBx was a false positive compared to the RV cross section. In the remaining cases (2 of 31) both tissues showed IgM labeling but the intensity or distribution did not match.
There were 35 pairs of IgM stained LV and RV sections that were interpretable. Most samples (26 of 35 pairs 74%) matched for intensity, the extent of immuno-reactivity or both. There were 5 samples (14%) where the LV gave a false negative and 2 samples (6%) where the LV was a false positive compared to the RV cross section. In 2 cases (6%) both the LV and RV were stained but the intensity or extent of staining did not match.
DISCUSSION
In this study we demonstrate that DXR is a global process involving both right and left ventricles to a similar extent. The histology of RV biopsies obtained with a bioptome or by sharp dissection was a reliable indicator of histology in the larger RV and LV cross sections. This correlation was true for xenografts with extensive histologic injury and for grafts with lesser degrees of rejection. Endomyocardial biopsy, therefore, faithfully represents all the specific histologic features of DXR and is an appropriate method to monitor the degree of cardiac xenograft rejection.
Our observation that DXR appears to be a global process is in contrast to those reported by Rose et al15 who found that antibody-mediated rejection occurred with a zonal distribution. For this reason others have suggested that the use of EmBx as a monitoring tool in the setting of xenotransplantation may not be appropriate.15,16 We observed diffuse microvascular thrombosis and coagulative necrosis without any predilection for the specific zones within the myocardium. The disparity between the observations may be explained by differences in procedure since Rose et al used pig-to-baboon heterotransplants in the neck, while ours were all abdominal transplants. In addition, a predominance of the grafts analyzed by Rose showed hyperacute rejection and histologic damage within one hour of reperfusion and in many cases frank hyperacute rejection. In contrast, none of our grafts showed histologic injury in routine 30 minute biopsies and median time from implantation to explant was of 30 days with no hyperacute rejections. It seems possible that the rapidity of HAR might result in zonal injury to the xenograft in contrast to the more chronic vascular injury associated with DXR.
For clinical xenotransplantation to become a reality it is essential that easily obtained and reliable tools for monitoring and treating xenograft rejection be available. Stadler et al.17 have indicated that echocardiography is a superior method for assessing early graft dysfunction compared to palpation score. We have used a combination of changes observed on intramyocardial electrocardiograms (IMEG), manual palpation and biochemical markers like troponin T and aspartate aminotransferase (AST)2 to monitor heterotopic cardiac xenograft rejection. Kuwaki et al have recommended similar criteria.18 In our experience these non-invasive methods are not completely reliable since treating animals with additional immunosuppression for presumed episodes of rejection did not prolong xenograft survival.7 EmBx remains the gold standard in the diagnosis and treatment of cardiac allografts. Although there may be additional non-invasive tools or tests, e.g. echocardiography, serum cytokine levels of INF gamma, TNF alfa, IL 2, that will be helpful in monitoring the immunologic status of the heart after xenotransplantation, histologic analysis is likely to play a key role in diagnosing xenograft rejection, particularly orthotopic cardiac xenografts, as it has in cardiac allografts.
This study was not designed to determine how many pieces of myocardium are optimal for the detection of DXR as has been done for other solid organ transplants.19-21 In cardiac allografts, Zerbe and Arena21 demonstrated that the level of sensitivity improves with an increasing number of endomyocardial biopsy samples but that sensitivity does not improve when more than six pieces are examined. In our study we found a very high degree of correlation between EmBx and RV and LV cross sections, particularly for irreversible characteristics of DXR such as coagulative necrosis and thrombosis. Additional biopsies are not likely to improve this correlation. Additional biopsies may be useful to increase the sensitivity of detecting transient features of DXR (edema, vacuolization) and in improving the correlation for immunostains of vascular antibody and complement deposition. This might help to define earlier stages of DXR. Additional studies are underway to determine the optimal number of endomyocardial biopsy pieces in the setting of xenotransplantation.
Finally, this study was performed using a heterotopic model. While useful, the model has some inherent problems. The heterotopic heart is not life supporting and it is prone to the development of intrachamber clots that can distend the ventricles and interfere with the normal cardiac function and myocyte survival. How these features may affect DXR is unknown. Similar studies will need to be performed in the orthotopic setting to determine if the histopathology of DXR remains global and to estimate the number of pieces of myocardium necessary for adequate surveillance.
In conclusion, this animal study indicates that DXR is a global process involving both the ventricles similarly and without any predilection for specific zones within the myocardium; and that EmBx is an appropriate method to monitor, the immunologic status of the graft.
Acknowledgements
We wish to acknowledge the contributions of Scott Suddendorf, Paul Henke, Michael Timmons, Merilyn Walters and Junice Thompson for technical help and Karen Schumacher for writing assistance.
This research supported by: NIH Grant AI66310 William J von Liebig Foundation Mayo Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Teotia SS, Walker RC, Schirmer JM, et al. Prevention, detection, and management of early bacterial and fungal infections in a preclinical cardiac xenotransplantation model that achieves prolonged survival. Xenotransplantation. 2005;12(2):127–33. doi: 10.1111/j.1399-3089.2005.00205.x. [DOI] [PubMed] [Google Scholar]
- 2.McGregor CG, Teotia SS, Byrne GW, et al. Cardiac xenotransplantation: progress toward the clinic. Transplantation. 2004;78(11):1569–75. doi: 10.1097/01.tp.0000147302.64947.43. [DOI] [PubMed] [Google Scholar]
- 3.McGregor CG, Davies WR, Oi K, et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg. 2005;130(3):844–51. doi: 10.1016/j.jtcvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11(12):1295–8. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng YL, Moran K, Dor FJ, et al. Elicited antibodies in baboons exposed to tissues from alpha1,3-galactosyltransferase gene-knockout pigs. Transplantation. 2006;81(7):1058–62. doi: 10.1097/01.tp.0000197555.16093.98. [DOI] [PubMed] [Google Scholar]
- 6.Lam TT, Paniagua R, Shivaram G, Schuurman HJ, Borie DC, Morris RE. Anti-non-Gal porcine endothelial cell antibodies in acute humoral xenograft rejection of hDAF-transgenic porcine hearts in cynomolgus monkeys. Xenotransplantation. 2004;11(6):531–5. doi: 10.1111/j.1399-3089.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 7.Byrne GW, Schirmer JM, Fass DN, et al. Warfarin or low-molecular-weight heparin therapy does not prolong pig-to-primate cardiac xenograft function. Am J Transplant. 2005;5(5):1011–20. doi: 10.1111/j.1600-6143.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen G, Sun H, Yang H, et al. The role of anti-non-Gal antibodies in the development of acute humoral xenograft rejection of hDAF transgenic porcine kidneys in baboons receiving anti-Gal antibody neutralization therapy. Transplantation. 2006;81(2):273–83. doi: 10.1097/01.tp.0000188138.53502.de. [DOI] [PubMed] [Google Scholar]
- 9.Bach FH, Winkler H, Ferran C, Hancock WW, Robson SC. Delayed xenograft rejection. Immunol Today. 1996;17(8):379–84. doi: 10.1016/0167-5699(96)10024-4. [DOI] [PubMed] [Google Scholar]
- 10.Logan JS. Prospects for xenotransplantation. Curr Opin Immunol. 2000;12(5):563–8. doi: 10.1016/s0952-7915(00)00139-4. [DOI] [PubMed] [Google Scholar]
- 11.Stehlik J, Starling RC, Movsesian MA, et al. Utility of long-term surveillance endomyocardial biopsy: a multi-institutional analysis. J Heart Lung Transplant. 2006;25(12):1402–9. doi: 10.1016/j.healun.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Reed EF, Demetris AJ, Hammond E, et al. Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25(2):153–9. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Schirmer JM, Fass DN, Byrne GW, Tazelaar HD, Logan JS, McGregor CG. Effective antiplatelet therapy does not prolong transgenic pig to baboon cardiac xenograft survival. Xenotransplantation. 2004;11(5):436–43. doi: 10.1111/j.1399-3089.2004.00159.x. [DOI] [PubMed] [Google Scholar]
- 14.Byrne GW, Davies WR, Oi K, et al. Increased immunosuppression, not anticoagulation, extends cardiac xenograft survival. Transplantation. 2006;82(12):1787–91. doi: 10.1097/01.tp.0000251387.40499.0f. [DOI] [PubMed] [Google Scholar]
- 15.Rose AG, Cooper DK. Venular thrombosis is the key event in the pathogenesis of antibody-mediated cardiac rejection. Xenotransplantation. 2000;7(1):31–41. doi: 10.1034/j.1399-3089.2000.00042.x. [DOI] [PubMed] [Google Scholar]
- 16.Goddard MJ, Dunning J, Horsley J, Atkinson C, Pino-Chavez G, Wallwork J. Histopathology of cardiac xenograft rejection in the pig-to-baboon model. J Heart Lung Transplant. 2002;21(4):474–84. doi: 10.1016/s1053-2498(01)00402-8. [DOI] [PubMed] [Google Scholar]
- 17.Stalder M, Tye T, Lam TT, et al. Improved assessment of graft function by echocardiography in cynomolgus monkey recipients of hDAF-transgenic pig cardiac xenografts. J Heart Lung Transplant. 2005;24(2):215–21. doi: 10.1016/j.healun.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 18.Kuwaki K, Knosalla C, Dor FJ, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004;4(3):363–72. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 19.Tazelaar HD, Nilsson FN, Rinaldi M, Murtaugh P, McDougall JC, McGregor CG. The sensitivity of transbronchial biopsy for the diagnosis of acute lung rejection. J Thorac Cardiovasc Surg. 1993;105(4):674–8. [PubMed] [Google Scholar]
- 20.Spiegelhalter DJ, Stovin PG. An analysis of repeated biopsies following cardiac transplantation. Stat Med. 1983;2(1):33–40. doi: 10.1002/sim.4780020105. [DOI] [PubMed] [Google Scholar]
- 21.Zerbe TR, Arena V. Diagnostic reliability of endomyocardial biopsy for assessment of cardiac allograft rejection. Hum Pathol. 1988;19(11):1307–14. doi: 10.1016/s0046-8177(88)80286-7. [DOI] [PubMed] [Google Scholar]


