Abstract
Chemically induced renal neoplasms in male rats, developed coincident with α2u-globulin nephropathy, are not considered predictive of risk to humans by the International Agency for Research on Cancer (IARC) and the U.S. Environmental Protection Agency. Criteria have been defined to establish the role of α2u-globulin nephropathy in renal carcinogenesis, based on a proposed mode of action involving sustained tubular cell proliferation resulting from α2u-induced nephropathy, with consequent development of neoplastic lesions. Recent NTP studies demonstrated inconsistencies with this proposed mechanism, including in some cases, far weaker kidney tumor responses than expected based on the extent of α2u-globulin nephropathy. NTP studies with decalin, propylene glycol mono-t-butyl ether and Stoddard solvent IIC included extended evaluations of α2u-related nephropathy, and were thus used in assessing the linkage between key events in 90-day studies with renal tumors in 2-year studies. This review revealed no or at best weak associations of tumor responses with renal α2u-globulin concentrations, indices of cell turnover, or microscopic evidence of α2u-associated nephropathy in prechronic studies. While tumor responses corresponded somewhat with a measure of cumulative α2u-associated nephropathy (linear mineralization of the papilla) at the end of the 2-year studies, the severity of chronic nephropathy was generally in best agreement with the pattern of tumor response. These results suggest that while α2u-globulin nephropathy may contribute to the renal tumor response, the critical component(s) of the nephropathy most closely associated with the development of tumors could not be clearly identified in this review.
Keywords: α2u-globulin nephropathy, male rats, renal tubular cell tumors, pathogenesis
Introduction
Tumors in experimental animal cancer studies that are associated with species-specific mechanisms or modes of action may not be considered predictive of similar risk to humans. If a mode of action can be clearly demonstrated to not operate in humans, experimental animal responses are not considered relevant for cancer risk assessment (Cohen et al., 2003). A framework for human relevance analysis of information on carcinogenic modes of action has been developed and tested (Meek et al., 2003). Inappropriate use of experimental animal data can have adverse public health implications either leading to restriction of the use of a beneficial substance, or exposure of humans and the environment to a hazardous substance. Thus, it is critically important that modes of cancer action in rodent studies that are accepted by the scientific and regulatory communities as not relevant for humans, be periodically reexamined for consistency and coherence with data from emerging studies.
The development of kidney tumors in male rats in association with chemically induced α2u-globulin nephropathy is one mechanism not considered a predictor of carcinogenic risk to humans by the IARC and the U.S.EPA (EPA, 1991; Swenberg and Lehman-McKeeman, 1999). The lack of relevance of the α2u-globulin mechanism for the evaluation of carcinogenic risk is based on the absence of production of an analogous protein in humans. Strict scientific criteria have been outlined for establishing the role of α2u-globulin-associated nephropathy in male rat renal carcinogenesis (Table 1).
Table 1.
Criteria to establish the role of α2u-globulin nephropathy in renal carcinogenesis.
| US EPA (1991)a |
| Increased number and size of hyaline droplets in renal proximal tubule cells of treated male rats |
| Protein in the hyaline droplets is α2u-globulin Additional pathological sequence of lesions |
| IARC (1999)b |
| Essential Evidence |
| Tumors occur only in male rats |
| Acute exposure exacerbates hyaline droplet formation |
| α2u-Globulin accumulates in hyaline droplets |
| Subchronic lesions include granular casts and linear papillary mineralization |
| Absence of hyaline droplets and other histopathological changes in female rats and mice |
| Negative for genotoxicity |
| Additional Supporting Evidence |
| Reversible binding of chemical to α2u-globulin |
| Increased sustained cell proliferation in proximal tubule (P2 segment) |
| Dose-response relationship between hyaline droplet severity and renal tumor incidence |
These criteria were based on an hypothesized mechanism of action involving renal tubular cell death resulting from accumulation of a chemical-α2u-globulin complex resistant to lysosomal degradation, compensatory sustained cell proliferation, and ultimately, development of neoplasms. In part because only very few chemicals have been shown to fulfill every one of the necessary criteria, an alternative mechanism of action of α2u-related kidney tumor formation has been proposed (Melnick, 1992; Kohn and Melnick, 1999; Melnick and Kohn, 1999), and the subject has been the topic of extensive debate (Borghoff et al., 1993; Melnick, 1993; Ashby, 1996; Huff, 1996; Ashby, 1997; de la Iglesia et al., 1997; Dietrich, 1997; Melnick, 1997; Melnick et al., 1997).
A number of structurally diverse chemicals have been shown to induce α2u-globulin nephropathy in the male rat. In addition to increases in the number, size and altered shape of hyaline droplets in the proximal tubules, α2u-globulin nephropathy has been defined to include additional histological alterations (Hard et al., 1993; Swenberg and Lehman-McKeeman, 1999). Lysosomal dysfunction associated with excessive accumulation of α2u-globulin is thought to initiate cell death, degeneration and necrosis of tubular epithelial cells. Cell loss, in turn, produces accumulation of α2u-globulin and cellular debris as granular casts primarily at the cortico-medullary junction, and stimulates regenerative epithelial cell proliferation. Upon continuing exposure, mineralization within the loops of Henle in linear profiles, exacerbation of age-related chronic progressive nephropathy and atypical renal tubular hyperplasia occur after several months of treatment. Although direct evidence for this is lacking, it is thought that atypical hyperplastic foci, in turn, progress to renal adenomas and carcinomas (Hard, 1990).
Recent studies conducted by the National Toxicology Program (NTP) have comprehensively characterized the renal toxicity and carcinogenicity of several α2u-globulin-inducing chemicals. Male rats exposed for 3 months via whole-body inhalation to decalin, propylene glycol mono-t-butyl ether (PGMBE) and Stoddard solvent IIC (SS IIC) developed alterations suggestive of α2u-globulin nephropathy, including overall dose-related increases in renal α2u-globulin protein and cell labeling indices (Dill et al., 2003; Doi et al., 2004a; Doi et al., 2004b). Interestingly, the development of renal neoplasms in chronic bioassays of PGMBE and SS IIC was unremarkable, although the magnitude of the α2u-globulin nephropathy appeared equivalent to that previously seen with decalin and in other studies. Our analysis was conducted to systematically review key events in α2u-globulin nephropathy in select NTP studies, and to evaluate the association among these key events and renal tumor outcomes. The purpose of this review was to uncover possible dissimilarities in chemical-related α2u-globulin nephropathy in 3-month studies that might explain the variation in tumor responses subsequently observed in the 2-year studies.
Materials and Methods
Source of Data
The NCI/NTP database was searched for studies with a diagnosis of hyaline droplet accumulation in kidney tubules of rats in prechronic studies. Additional chemicals were identified that were associated with α2u-globulin accumulation in studies reported by others (Swenberg and Lehman-McKeeman, 1999; Melnick and Kohn, 1999; Lock and Hard, 2004) and also studied for cancer by the NCI/NTP. Because the purpose of this evaluation was to examine associations among key events in the proposed α2u-globulin nephropathy mode of tumor development in male rats, the datasets selected for evaluation could include only those for which sufficient information was available to conduct such analysis. Although many chemicals in the NTP database have been shown to induce α2u-globulin in renal tubules (Table 2), only those satisfying all of the following criteria were included in this review: a) Diagnosis of hyaline droplet accumulation in kidney tubules of male rats in 3-month studies; b) Additional pathologies consistent with α2u-globulin nephropathy; c) Development of renal tumors in 2-year studies exclusive to male rats; and d) At least one common exposure/dose level utilized in both the 3-month and 2-year studies. Studies of only 3 contemporary chemicals met these criteria and are included in this review (Table 3). Results from these studies are compared with data available for the prototypical α2u-globulin inducing chemical d-limonene.
Table 2.
List of 2-year rat cancer studies with renal tumors from the NCI/NTP database.
| Chemical Name | TRb | Year | Reason for Excluding Study from Analysis |
|---|---|---|---|
| Pentachloroethane | 232 | 1983 | Lack of hyaline droplet diagnosis in 90-day studies |
| Isophorone | 291 | 1986 | Lack of hyaline droplet diagnosis in 90-day studies |
| Tetrachloroethylene | 311 | 1986 | Lack of hyaline droplet diagnosis in 90-day studies |
| 1,4-Dichlorobenzene | 319 | 1987 | Kidney sections were transverse and thus, not comparable to other studies |
| Dimethyl methylphosphonate | 323 | 1987 | Transitional cell neoplasms in the kidney |
| Hexachloroethane | 361 | 1989 | No dose was carried over from 90-day to 2-year studies |
| Hydroquinone | 366 | 1989 | Lack of hyaline droplet diagnosis in 90-day studies |
| α-Methylbenzyl alcohol | 369 | 1990 | Lack of hyaline droplet diagnosis in 90-day studies |
| ADBAQ | 383 | 1996 | Renal tumors occurred in male and female rats |
| t-Butyl alcohol | 436 | 1995 | Low survival in all groups in the study |
| p-Nitrobenzoic acid | 442 | 1994 | Renal tumors not considered chemical related |
| Pyridine | 470 | 1997 | No renal tumors in any dose group carried over from 90-day to 2-year studies |
| Emodin | 493 | 2001 | Lack of additional α2u-related pathologies |
| Anthraquinone | 494 | 1999 | Renal tumors occurred in male and female rats |
| p-Nitrotoluene | 498 | 2002 | Lack of additional α2u-related pathologies |
Technical Report Number.
Studies are with a diagnosis of hyaline droplet accumulation in kidney tubules of males in 90-day or 2-year studies, or that have been associated with α2u-globulin accumulation in other reportsa, but not considered further in this analysis.
Table 3.
List of NTP studies included in the analysis, and evidence of carcinogenicity based on kidney responses in male F344/N rats.
| Doses/Concentrations |
||||||
|---|---|---|---|---|---|---|
| Chemical Name | TRa | Year | Route of Administration | 90-Day | 2-Year | Evidence of Carcinogenicityb |
| d-limonene | 347 | 1990 | Gavage | 0, 150, 300, 600, 1200, 2400 mg/kg | 0, 75, 150 mg/kg | Clear evidence |
| decalin | 513 | 2005 | Inhalation | 0, 25, 50, 100, 200, 400 ppm | 0, 25, 50, 100, 400c ppm | Clear evidence |
| propylene glycol mono-t-butyl ether | 515 | 2004 | Inhalation | 0, 75, 150, 300, 600, 1200 ppm | 0, 75, 300, 1200 ppm | Equivocal evidence |
| Stoddard solvent IIC | 529 | 2004 | Inhalation | 0, 138, 275, 550, 1100, 2200 mg/m3 | 0, 138, 550, 1100 mg/m3 | Equivocal evidenced |
Technical Report Number.
Five categories of evidence of carcinogenic activity are used to summarize the strength of the evidence observed in bioassays conducted by the NTP: “Clear Evidence” and “Some Evidence” are used for chemical-related positive studies, “Equivocal Evidence” is used for uncertain findings that may be interpreted as chemical-related; “No Evidence” refers to no observable effects, and “Inadequate Study” is used for experiments that cannot be evaluated because of major flaws.
The 400 ppm exposure concentration in the decalin study was an additional group of 20 animals included because of uncertainty over the possibility that this exposure concentration would be too toxic for animals to survive throughout a two-year study. This was not the case, and data from 15 randomly selected animals from this exposure group are included in Figures 5, 6, and 7.
There was some evidence of carcinogenic activity of Stoddard solvent IIC in male rats based on increased incidences of adrenal medulla neoplasms; the slight increases of renal tubule adenoma may have been related to Stoddard solvent IIC exposure. The Equivocal Evidence call reflects only the kidney response.
Selected Studies and Quantitative Endpoints
This retrospective evaluation included only male F344/N rats. Decalin, PGMBE and SS IIC-exposed animals (whole-body inhalation studies, 6 hours per day, 5 days per week, for 14 or 104/105 weeks) were 6 weeks of age at the beginning of the studies. Note for the studies on decalin and PGMBE the top exposure concentration used in the 3-month study was the same as that used in the 2-year study and retrospective histopathology evaluations on rats exposed for 3 months used materials from the original prechronic studies. For SS IIC, the 2-year study top concentration was half that used in the 3-month study, and the retrospective prechronic histopathology analyses included this higher dose. However, measures of cell proliferation and renal α2u-globulin content were performed on a satellite group examined 3-months into the 2-year study at the exposure concentrations employed in that study (0, 138, 550, and 1,100 mg/m3). Doses or exposure concentrations are shown in Table 3, and more information on experimental design and materials and methods for these studies can be found elsewhere (NTP, 1990; 2004; 2004a; 2005; Dill et al., 2003; Doi et al., 2004a, 2004b).
For the studies with d-limonene, animals were dosed starting at 7 weeks of age with the chemical in corn oil by gavage once per day, 5 times per week for 3 months or 2 years. Because limonene was the index α2u-globulin nephropathy chemical, characterization of the nephropathy was carried out in a subsequent 14 exposure, 21-day study performed following completion of the 2-year cancer study. Animals in this subsequent study were 18 weeks of age at study start. Animals in all prechronic studies were 19-21 weeks old at the time that measurements were performed. Analyses of renal concentrations of α2u-globulin were performed in kidney homogenates using a competitive indirect ELISA technique (Borghoff et al., 1992). Estimates of renal tubular cell proliferation rates were conducted using Proliferating Cell Nuclear Antigen (PCNA) labeling for decalin and PGMBE (Dill et al., 2003; Doi et al., 2004b), and bromodeoxyuridine (BrdU) labeling for SS IIC (Doi et al., 2004a). This endpoint was not evaluated in the limonene study.
Histopathology Reviews
The diagnoses of pathologies in NTP studies are uniformly consistent in the identification of lesions within a particular study. However, some diagnostic variation can exist among different studies, particularly in the scoring of severity of lesions because this is in part dependent on the total spectrum of lesions present in a given study. Thus, renal lesions of interest from prechronic studies were retrospectively evaluated for incidence and/or severity by a single pathologist (GH) to allow for direct comparisons across studies. Severity grades ranged from 1-4, with 1 = minimal, 2 = mild, 3 = moderate and 4 = marked.
Entire longitudinal, H&E-stained kidney sections from male rats from prechronic studies (n = 10 unless otherwise specified) were evaluated for the presence of intracytoplasmic hyaline droplets in the proximal tubules, regenerative cortical tubules, and granular casts in the corticomedullary junction. Hyaline droplets were graded as 0 = no protein droplets observed, 1 = occasional small protein droplets observed, 2 = frequent small protein droplets observed, 3 = frequent small to moderately sized protein droplets observed, 4 = frequent large protein droplets observed. Regenerative tubules defined as clusters of basophilic tubules in which epithelial cells had increased nuclear density and occasional mitotic figures were counted throughout the cortex. All granular casts of cellular debris within tubules in the outer medulla/corticomedullary junction were counted. Counts of clusters of tubular regeneration and granular casts are not standard practice, but were performed in the current study as a more quantitative, and hence, less subjective approach to estimate the severity of these changes.
Histopathology slides from 2-year studies were also reevaluated by a single pathologist (JS) for incidence and severity of chronic nephropathy, linear papilla mineralization, and atypical renal tubular hyperplasia, as well as incidence of tubular adenomas, or adenomas and carcinomas combined. A fraction (30/50) of randomly selected single longitudinal kidney sections from 2-year studies was included in this review. Evaluation of only 60% of the animals in each dose group was done because using more than 30 animals per group achieved relatively smaller increases in power to detect endpoint associations. Because slides from only a portion of the animals from the studies were evaluated, the lesion counts do not match those in the original technical reports of these studies.
Nephropathy consisted of a spectrum of lesions including multifocal tubular regeneration, tubular protein casts, thickening of the tubular and glomerular basement membrane, interstitial fibrosis, and chronic inflammatory cell infiltration. The criteria used for grading the severity of nephropathy have been previously published (Rao et al., 2001). Linear mineralization designated regularly arranged basophilic mineralized linear deposits distributed mainly in the lower half of the renal papillae (minimal = involvement of papillae up to 25%; mild = 26-50%; moderate = 51-75%; marked = 76% and above).
Atypical renal tubular hyperplasia consisted of discrete, focal to multifocal proliferative lesions of renal tubules characterized by expansion of a single or several profiles of the same tubule by a multilayered epithelium, containing cells somewhat larger than normal with prominent nucleoli. The cytoplasm of affected cells is faintly basophilic with a homogenous-tinctorial sheen. When present, the tubular basement membrane is often variable in thickness. Although capillaries may be noted in association with hyperplastic lesions, invasion into the lesion is not present, a point of differentiation along with loss of tubular integrity when comparing hyperplasia to small adenomas. Grading of hyperplasia is generally subjective based primarily on the size of the lesion, number of affected tubular profiles present and complexity of the hyperplastic epithelium.
Renal tubular adenomas were larger discrete lesions that ranged from greater than 5 tubule diameters to 1 mm or more in size. They consisted of a solid mass of large, closely packed tubular epithelial cells. Cells were moderately pleomorphic. Renal tubular carcinomas were differentiated from adenomas in that they were usually larger, less discrete, had a prominent vascular supply, and more anaplasia and cellular atypia.
Statistical Methods
Tests of significance included pairwise comparisons of each exposed group with controls. Continuity corrected Poly-3 tests (Bailer and Portier, 1988) were used in the analysis of lesion incidence and reported P values are one sided. Average severity values were analyzed for significance with the Mann-Whitney U-test (Hollander and Wolfe, 1973).
Results
Quantitative Endpoints
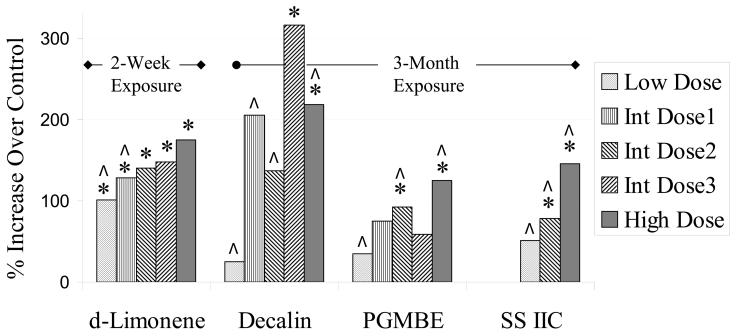
Increases in renal concentrations of α2u-globulin, relative to controls, are depicted in Figure 1 and numerical data presented in Table 4. Data for d-limonene were expressed as ng/μg total protein and are from the study where the chemical was dosed 14 times over 21 days, while data for decalin, PGMBE, and SS IIC were expressed as ng/μg soluble protein, and were collected at the end of 3-month exposures. α2u-Globulin concentrations were significantly increased following exposure to all doses of d-limonene (∼100-180%), and generally, to the highest concentrations of decalin (∼ 220-320%), PGMBE (∼ 90-120%), and SS IIC (∼ 80-150%). Relative increases in α2u-globulin concentrations were higher for decalin than for PGMBE or SS IIC. Data are presented in Figure 1 as percent increase over controls because the assays were done at different times using different reagents.
Figure 1.
Relative increases of mean renal α2u-globulin concentrations, following 3-month exposures to decalin, propylene glycol mono-t-butyl ether (PGMBE), and Stoddard solvent IIC (SS IIC) to male rats; d-limonene-treated animals were exposed for 2 weeks. All rats (n = 10) were approximately 18 weeks old at the time of measurements. Analysis of renal concentrations of a2u-globulin was performed in kidney homogenates using a competitive indirect ELISA technique. Doses used in the 2-week limonene studies were 75, 150, 300, 600, and 1,200 mg/kg. Doses or exposures in prechronic studies that were common to those in 2-year studies are indicated by ^. Significantly greater than controls, p ≤ 0.05.
Table 4.
Concentrations of α2u-globulin in the kidney of male F344/N rats exposed to d-limonene, decalin, propylene glycol mono-t-butyl ether and Stoddard solvent IIC.
| Chemical | d-limonene | decalin | propylene glycol mono-t-butyl ether | Stoddard solvent IIC |
|---|---|---|---|---|
| Exposure duration | 21 Days | 3 Months | 3 Months | 3 Months |
| Number of animals | 5 ng/ μg Total Protein | 5 ng/ μg Soluble Protein | 5 ng/μg Soluble Protein | 10 ng/μg Soluble Protein |
| Control | 204 ± 14 | 60 ± 17 | 113 ± 37 | 198 ± 46 |
| Low Dose | 409 ± 19** | 75 ± 18 | 153 ± 22 | 300 ± 34 |
| Intermediate Dose 1 | 465 ± 22** | 184 ± 98 | 198 ± 44 | |
| Intermediate Dose 2 | 489 ± 14** | 142 ± 38 | 218 ± 25 * | 353 ± 30* |
| Intermediate Dose 3 | 505 ± 27** | 251 ± 69** | 179 ± 25 | 488 ± 72** |
| High Dose | 561 ± 18** | 192 ± 93* | 254 ± 45* | |
Notes: Data are presented as mean ± S.E.
Significantly different (p ≤ 0.05) from controls by Dunn’s or Shirley’s test.
Significantly different (p ≤ 0.05) from controls by the Kruskal-Wallis multiple comparison test (d-limonene) or Dunn’s or Shirley’s test (decalin, propylene glycol mono-t-butyl ether and Stoddard solvent IIC).
Labeling indices of tubular epithelial cells were calculated by dividing the number of labeled nuclei by the number of total nuclei. Increases in labeling indices relative to controls are shown in Table 5 for decalin and PGMBE. Cell labeling in these studies was measured with PCNA immunostaining at 3 time points during the course of the 13-week studies. For the SS IIC study, cell labeling was accomplished using a 24-hour infusion of BrdU with osmotic minipumps. Thus, the data are not directly comparable with the measures taken from the decalin and PGMBE studies. An analysis of tubular cell labeling index was not performed for the d-limonene study. Labeling indices were significantly increased following exposure to all concentrations of decalin, and the top 2 concentrations of PGMBE at all time points and at the 550 and 1,100 mg/m3 concentrations of SS IIC (∼ 60-135%). The highest relative increases in cell labeling with PCNA (approximately 2-fold) were seen with the highest concentrations of PGMBE at 13 weeks and decalin at 6 weeks.
Table 5.
Cell labeling indices determined with PCNA immunostaining during the 13-week prechronic inhalation studies with decalin and propylene glycol mono-t-butyl ether.
| Propylene glycol mono-t-butyl ether | Chamber Control | 75 ppm | 150 ppm | 300 ppm | 600 ppm | 1,200 ppm |
|---|---|---|---|---|---|---|
| Labeling index (%) n = 5 | ||||||
| Week 2 | 3.6 ± 0.1 | 3.6 ± 0.2 | 3.9 ± 0.2 | 3.4 ± 0.1 | 4.4 ± 0.2* | 5.5 ± 0.4** |
| Week 6 | 3.6 ± 0.1 | 3.2 ± 0.1 | 3.4 ± 0.2 | 3.9 ± 0.1 | 4.3 ± 0.0* | 4.9 ± 0.3* |
| Week 13 | 3.2 ± 0.2 | 3.8 ± 0.2 | 4.3 ± 0.2** | 4.1 ± 0.2* | 5.0 ± 0.4** | 6.6 ± 0.4** |
| Decalin | Chamber Control | 25 ppm | 50 ppm | 100 ppm | 200 ppm | 400 ppm |
| Labeling index (%) n = 5 | ||||||
| Week 2 | 3.3 ± 0.2 | 4.2 ± 0.1* | 3.9 ± 0.3 | 4.3 ± 0.2** | 4.2 ± 0.3* | 4.9 ± 0.3** |
| Week 6 | 3.2 ± 0.1 | 4.2 ± 0.1** | 4.8 ± 0.1** | 4.6 ± 0.3** | 5.0 ± 0.2** | 5.9 ± 0.3** |
| Week 13 | 3.1 ± 0.2 | 4.5 ± 0.1** | 3.7 ± 0.3* | 4.1 ± 0.2* | 4.5 ± 0.2** | 4.5 ± 0.2** |
Significantly different (p < 0.05) from the chamber control group.
p < 0.01, data are presented as mean + S.E.
Retrospective Histopathology Evaluation of Prechronic Studies
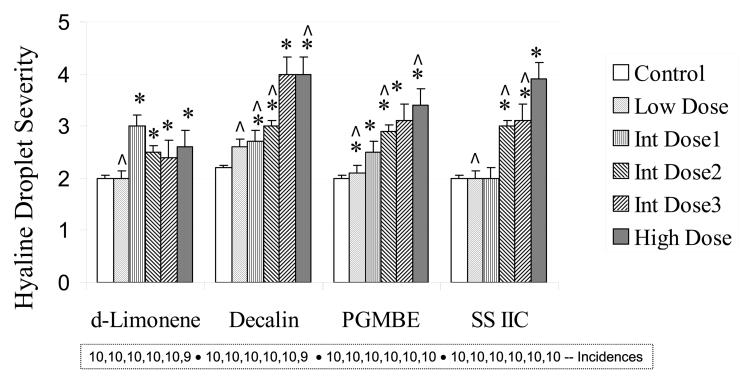
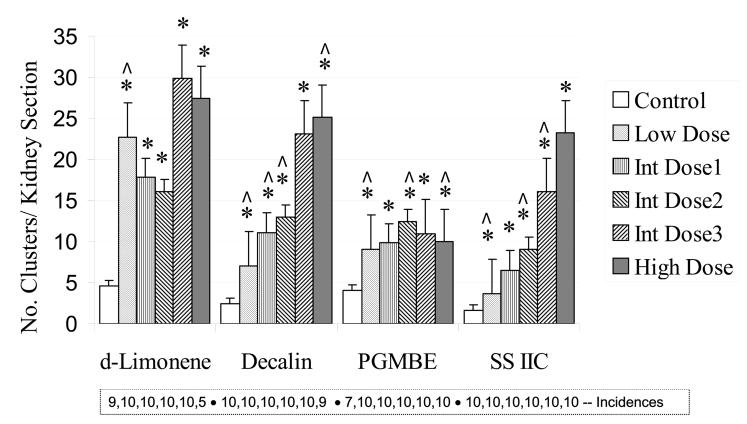
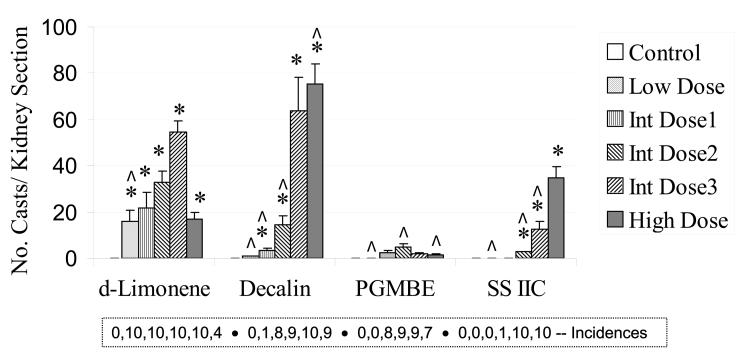
Hyaline droplet accumulation in renal tubular cells was diagnosed in essentially all animals in all groups of control and treated male rats, however, the severity of the lesion was higher in some dose groups (Figure 2). Hyaline droplet severity was generally dose related for decalin, PGMBE and SS IIC. Regenerating clusters of injured renal tubules were also observed in most animals in the prechronic studies. Incidences and cluster counts are shown in Figure 3. The data for decalin and SS IIC showed quite similar, dose-related increases in cluster counts at concentrations ultimately used in the 2-year studies, whereas the changes in counts for PGMBE were minimal across the exposure concentrations. Figure 4 shows the numbers of granular casts present in the cortical tubules. d-Limonene, decalin and SSIIC showed generally dose or concentration-related increases, while this lesion was rare in the PGMBE study.
Figure 2.
Severity of hyaline droplet accumulation in renal proximal tubules of male rats (n = 10), following 3-month exposures to d-limonene, decalin, propylene glycol mono-t-butyl ether (PGMBE), and Stoddard solvent IIC (SS IIC). Data are presented as mean ± S.E.; *significantly greater than controls, p ≤ 0.05. Incidences of the lesion are presented in the box insert. Doses or exposures in prechronic studies that were common to those in 2-year studies are indicated by ^.
Figure 3.
Tubular regeneration cluster count in a single longitudinal section of the renal cortex of male rats (n = 10), following 3-month exposures to d-limonene, decalin, propylene glycol mono-t-butyl ether (PGMBE), and Stoddard solvent IIC (SS IIC). Data are presented as mean ± S.E.; *significantly greater than controls, p ≤ 0.05. Numbers of animals with the lesion are presented in the box insert. Doses or exposures in prechronic studies that were common to those in 2-year studies are indicated by ^.
Figure 4.
Granular cast count in a single longitudinal section of the renal outer medulla of male rats (n = 10), following 3-month exposures to d-limonene, decalin, propylene glycol mono-t-butyl ether (PGMBE), and Stoddard solvent IIC (SS IIC). Data are presented as mean ± S.E.; *significantly greater than controls, p ≤ 0.05. Numbers of animals with the lesion are presented in the box insert. Doses or exposures in prechronic studies that were common to those in 2-year studies are indicated by ^.
Retrospective Histopathology Evaluation of 2-Year Studies
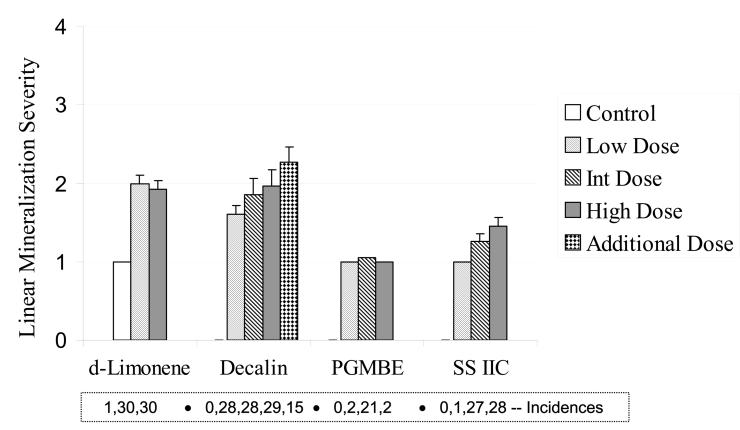
The severity of linear mineralization in the kidneys of male rats at the end of the 2-year studies was increased in exposed animals in all the studies (Figure 5). Although the increases were modest, of the measures taken at 2 years, this endpoint showed the clearest dose response, particularly for decalin and SS IIC when also considering the incidence of the lesion in the groups. The incidence of this lesion in the top exposure group of the PGMBE study was surprisingly low when compared to the middle exposure concentration. This lesion was noted in only one control rat across the 4 studies.
Figure 5.
Severity of linear mineralization in the renal papilla of male rats (n = 30), following 2-year exposures to d-limonene, decalin, propylene glycol mono-t-butyl ether (PGMBE), and Stoddard solvent IIC (SS IIC). Additional dose in decalin treatment consisted of 15 male rats. Data are presented as mean ± S.E.; statistical analyses were not performed because of the low or zero incidences in controls. Numbers of animals with the lesion are presented in the box insert.
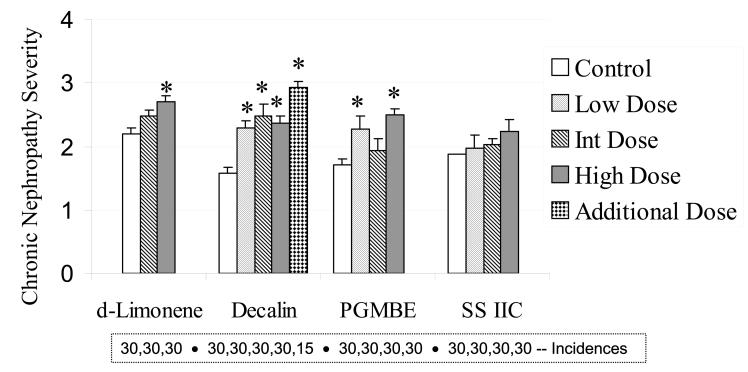
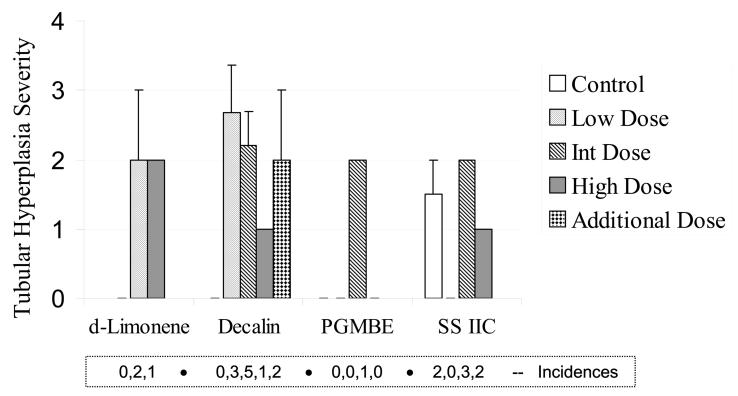
Chronic nephropathy is a common age-related lesion in male F344 rats, and exposure to 3 chemicals resulted in a moderate increase in the severity of this lesion, with decalin again showing the strongest evidence for a chemically related response when compared to the controls (Figure 6). For SS IIC, the severity of chronic nephropathy was not increased, and as outlined next, this chemical had the fewest renal tumors of the 4 at the conclusion of the 2-year studies. The assessment of tubular hyperplasia is shown in Figure 7. These lesions, which are considered preneoplastic, occurred at a low frequency in the 4 separate 2-year studies and with no clear dose response in either incidence or severity.
Figure 6.
Severity of chronic nephropathy in male rats (n = 30), following 2-year exposures to d-limonene, decalin, propylene glycol mono-t-butyl ether (PGMBE), and Stoddard solvent IIC (SS IIC). Additional dose in decalin treatment consisted of 15 male rats. Data are presented as mean ± S.E.; *significantly greater than controls, p ≤ 0.05. Numbers of animals with the lesion are presented in the box insert.
Figure 7.
Severity of renal tubular hyperplasia in male rats (n = 30), following 2-year exposures to d-limonene, decalin, propylene glycol mono-t-butyl ether (PGMBE), and Stoddard solvent IIC (SS IIC). Additional dose in decalin treatment consisted of n = 15 male rats. Data are presented as mean ± S.E.; *significantly greater than controls, p ≤ 0.05. Numbers of animals with the lesion are presented in the box insert.
Tumor Response
The tumors seen in the animals evaluated in these studies are shown in Table 6. The majority of the tumors were tubular cell adenomas. The incidences in the decalin and PGMBE studies exhibited a dose response, while the top exposure group in the SS IIC study had only 1 tumor in the 30 animals examined (standard sections). Renal tubular cell tumors typically occur in NTP studies with an average incidence of about 0.4%, thus a single tumor in an exposed group may or may not have resulted from the chemical under study. In fact, the tumor responses in the PGMBE and SS IIC studies (based on the entire set of 50 animals) were determined insufficient to be considered related with certainty to the chemical exposure.
Table 6.
Neoplastic lesions in the kidney of male rats (n = 30) following 2-year exposures to d-limonene, decalin, propylene glycol mono-t-butyl ether (PGMBE), and Stoddard solvent IIC (SS IIC). Additional dose in decalin treatment consisted of 15 male rats. Neoplastic incidences herein reported represent a review of 30 out of 50 animals from the original NTP studies, and thus, do not reflect NTP 2-year bioassay results for these chemicals.
| d-limonene |
Decalin |
PGMBE |
SS IIC |
|||||
|---|---|---|---|---|---|---|---|---|
| Adenomas | Adenomas or Carcinomas | Adenomas | Adenomas or Carcinomas | Adenomas | Adenomas or Carcinomas | Adenomas | Adenomas or Carcinomas | |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Low Dose | 5* (17%) | 7** (23%) | 1 (3%) | 1 (3%) | 1 (3%) | 1 (3%) | 0 | 0 |
| Intermediate Dose | 2 (7%) | 2 (7%) | 2 (7%) | 2 (7%) | 0 | 0 | ||
| High Dose | 3 (10%) | 4 (13%) | 6* (20%) | 6* (20%) | 3 (10%) | 3 (10%) | 1 (3%) | 1 (3%) |
| Additional Dosef | 3* (20%) | 4** (27%) | ||||||
n = 15.
Significantly greater than controls, p ≤ 0.05.
p ≤ 0.01.
Based on a qualitative assessment, none of the nonneoplastic endpoints considered part of the α2u-globulin nephropathy syndrome evaluated in these prechronic or 2-year studies was consistently predictive of the ultimate tumor outcome in the 2-year studies. However, the pattern of increased severity of chronic nephropathy at the end of the 2-year studies was somewhat consistent with the renal tumor response.
Discussion
Renal tubular cell tumors occurring in chemical carcinogenesis studies are often considered not predictive of human hazard when they appear coincident with nonneoplastic changes suggestive of α2u-globulin related nephropathy. In fact, the unique susceptibility of the male rat for tumors arising through this postulated sequence of events has been used to showcase how mode of action can be used in the determination of the potential human relevance of tumors arising in animal cancer studies (Cohen et al., 2003).
The key events in α2u-globulin nephropathy associated tumorigenesis and associated histopathological features have been described in great detail (Swenberg and Lehman-McKeeman, 1999; Meek et al., 2003). Yet it has been our experience in reviewing the results of the studies shown in Table 3 that the syndrome actually presents in a wide variety of ways. The studies of t-butyl alcohol are particularly illustrative in that the involvement of α2u-globulin in the renal pathology caused by this chemical in the male rat has been interpreted differently among pathologists even within the same institution reviewing the same set of slides (Lindamood et al., 1992; NTP, 1995).
The findings from these current studies provide a more systematic basis on which to evaluate quantitative relationships between certain key events in the α2u-globulin hypothesis and renal tubular cell tumors in rats. The results support our earlier impressions of the lack of a strong association of any particular manifestation of the syndrome with the ultimate tumor outcome. It must be pointed out that our evaluations of these events are largely limited to 2 times during the studies, 3 months and 2 years, and only during the prechronic studies is α2u-globulin present in measurable amounts.
For this reason, the retrospective pathology review focused on renal changes that reflected persistent damage from earlier insult, i.e., linear mineralization of the papillae, and chronic nephropathy. To the extent that these measures represent cumulative damage, it could be argued that they might better represent the contribution of the totality of the α2u-globulin related pathology occurring during the 2-year study and that may not be apparent at the 3-month time point.
The initial indication that a chemical may induce α2u-globulin-related nephropathy is often an observation in 14- or 90-day studies of an increase in hyaline droplets in the tubular epithelium. Droplets are typically seen in control male rats, and increases have resulted from exposures to a variety of hydrocarbons and some pharmaceuticals (Gopinath et al., 1987). These droplets are thought to be largely comprised of α2u-globulin, and immuno-assays have been developed to provide a quantitative measure of renal accumulation. Using these methods we found that the largest increases in accumulation of α2u-globulin in relation to controls occurred in the decalin study, which had the most robust renal tumor response. However, we also noted a significant increase in renal α2u-globulin accumulation at the top exposure concentration in the SS IIC studies, which was associated with a much more modest tumor response.
We examined 3 measures of renal tubular damage in prechronic studies, counts of granular casts, clusters of regenerating tubules and cell labeling indices. The accumulation of casts was again most marked in the decalin studies, although quite similar counts were seen at the top exposure concentration with SS IIC as were noted at the tumorigenic 150 mg/kg dose in the limonene study and the 100 ppm exposure concentration in the decalin study. Cluster counts also showed close similarities in the SS IIC studies and counts with limonene and decalin at doses/concentrations that caused tumors in the 2-year studies. The limited direct comparisons that could be made with the cell labeling studies were mentioned earlier, but it is interesting to note the overall pattern of response for PGMBE where an elevation in cell labeling equivalent to that seen with decalin was associated with a moderate number of regenerating tubules, few granular casts, and a much more modest tumor response.
Of the measures evaluated at 2 years, the incidence and severity of linear papilla mineralization and the severity of chronic nephropathy appeared at least somewhat predictive of the tumor outcome. The severity of linear mineralization was greatest with limonene and decalin, but the incidence and severity were also markedly increased with SS IIC in the absence of a significant tumor response. Increases in the severity of chronic nephropathy were noted with limonene, decalin and PGMBE, but not with SS IIC. Chronic nephropathy is a common condition in aging male rats and the severity of this lesion is increased by a wide variety of chemical exposures, including many chemicals that do not induce or bind to α2u-globulin. It is possible that α2u-globulin associated nephropathy may simply contribute to a weak background tumorigenic stimulus provided by age-related chronic progressive nephropathy.
Alternatively, other as yet undetermined factors may better account for the renal tubular cell tumor responses in male rats in chemical carcinogenesis studies. These results suggest that while α2u-globulin nephropathy may contribute to the renal tumor response, the critical component(s) of the nephropathy most closely associated with the development of tumors cannot clearly be identified. Thus reliance on evidence of α2u-globulin associated nephropathy in determining the potential human hazard from chemicals that cause renal tubular cell tumors in rats may need to be reconsidered.
Acknowledgment
The authors thank Drs. Ron Melnick, James Huff, Tom Goldsworthy, Susan Elmore and John Boyce for their kind comments and helpful suggestions on the manuscript. This research was supported by the Intramural Research Program of the NIH and NIEHS.
Abbreviations
- NTP
National Toxicology Program
- IARC
International Agency for Research on Cancer
- US EPA
United States Environmental Protection Agency
- NCI
National Cancer Institute
- PCNA
Proliferating cell nuclear antigen
- BrdU
Bromodeoxyuridine
- SS IIC
Stoddard solvent IIC
- PGMBE
Propylene glycol monobutyl ether
References
- Ashby J. Alpha 2-mu-globulin nephropathy in white ravens. Environ Health Perspect. 1996;104:1264. doi: 10.1289/ehp.104-1469542. (Correspondence) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby J. The relevance of mechanistic data to the interpretation and extrapolation to humans of rodent carcinogenicity data. Environ Health Perspect. 1997;105:902–3. doi: 10.1289/ehp.105-1470344. (Correspondence) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailer AJ, Portier CJ. Effects of treatment-induced mortality and tumor-induce mortality on tests for carcinogenicity in small samples. Biometrics. 1988;44:417–31. [PubMed] [Google Scholar]
- Borghoff SJ, Lehman-McKeeman LD, Short BG, Hard GC, Swenberg JA. Critique of R. Melnick’s “An alternative hypothesis on the role of chemically induced protein droplet (α2u-globulin) nephropathy in renal carcinogenesis.”. Regul Toxicol Pharmacol. 1993;18:357–64. doi: 10.1006/rtph.1993.1061. [DOI] [PubMed] [Google Scholar]
- Borghoff SJ, Youtsey NL, Swenberg JA. A comparison of European High Test gasoline and PS-6 unleaded gasoline in their abilities to induce α2u-globulin nephropathy and renal cell proliferation. Toxicol Lett. 1992;63:21–33. doi: 10.1016/0378-4274(92)90104-r. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Meek ME, Klaunig JE, Patton DE, Fenner-Crisp PA. The human relevance of information on carcinogenic modes of action: overview. CRC Crit Rev Toxicol. 2003;33:581–9. doi: 10.1080/713608371. [DOI] [PubMed] [Google Scholar]
- de la Iglesia FA, Gough AW, Sigler RE. α2u-globulin nephropathy and ravens: do ravens of a different feather flock together? Environ Health Perspect. 1997;105:903–4. doi: 10.1289/ehp.105-1470350. (Correspondence) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich DR. Doubting nongenotoxic mechanisms of renal cancer: comparing apples and oranges in the α2u-Globulin hypothesis. Environ Health Perspect. 1997;105:898–902. doi: 10.1289/ehp.105-1470358. (Correspondence) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill JA, Lee KM, Renne RA, Miller RA, Fuciarelli AF, Gideon KM, Chan PC, Burka LT, Roycroft JH. α2u-Globulin nephropathy and carcinogenicity following exposure to decalin (decahydronaphthalene) in F344/N Rats. Toxicol Sci. 2003;72:223–34. doi: 10.1093/toxsci/kfg028. [DOI] [PubMed] [Google Scholar]
- Doi AM, Peckham JC, Chou BJ, Dill JA, Renne RA, Grumbein SL, Chhabra RS. Development of α2u-globulin nephropathy and adrenal medullary pheochromocytomas in male rats following exposure to Stoddard Solvent IIC. Inhal Toxicol. 2004a;16:247–57. doi: 10.1080/08958370490427842. [DOI] [PubMed] [Google Scholar]
- Doi AM, Roycroft JH, Herbert RA, Haseman JK, Hailey JR, Chou BJ, Dill JA, Grumbein SL, Miller RA, Renne RA, Bucher JR. Inhalation toxicology and carcinogenesis studies of propylene glycol mono-t-butyl ether in rats and mice. Toxicology. 2004b;199:1–22. doi: 10.1016/j.tox.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Gopinath C, Prentice DE, Lewis J. Atlas of Experimental Toxicological Pathology. MTP Press Limited; Boston: 1987. pp. 77–90. [Google Scholar]
- Hard GC. Tumors of the kidney, renal pelvis and ureter. In: Turusov V, Mohr U, editors. Pathology of Tumors in Laboratory Animals. 1—Tumours of the Rat. IARC Scientific Publications; Lyon, France: 1990. p. 304. No. 99. [PubMed] [Google Scholar]
- Hard GC, Rodgers IS, Baetcke KP, Richards WL, McGaughy RE, Valcovic LR. Hazard evaluation of chemicals that cause accumulation of α2u-globulin, hyaline droplet nephropathy, and tubule neoplasia in the kidneys of male rats. Environ Health Perspect. 1993;99:313–49. doi: 10.1289/ehp.9399313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander M, Wolfe DA. Nonparametric Statistical Methods. John Wiley and Sons; New York: 1973. pp. 120–3. [Google Scholar]
- Huff J. Response: alpha-2-mu-globulin nephropathy, posed mechanisms, and white ravens. Environ Health Perspect. 1996;104:1264–7. doi: 10.1289/ehp.104-1469546. (Correspondence) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn MC, Melnick RL. A physiological model for ligand-induced accumulation of α2uglobulin in male rat kidney—Roles of protein synthesis and lysosomal degradation in the renal dosimetry of 2,4,4-trimethyl-2-pentanol. Toxicology. 1999;136:89–105. doi: 10.1016/s0300-483x(99)00063-3. [DOI] [PubMed] [Google Scholar]
- Lindamood C, III, Farnell DR, Giles HD, Prejean JD, Collins JJ, Takahashi K, Maronpot RR. Subchronic toxicity studies of t-butyl alcohol in rats and mice. Fundam Appl Toxicol. 1992;19:91–100. doi: 10.1016/0272-0590(92)90032-d. [DOI] [PubMed] [Google Scholar]
- Lock EA, Hard GC. Chemically induced renal tubule tumors in the laboratory rat and mouse: review of the NCI/NTP database and categorization of renal carcinogens based on mechanistic information. CRC Crit Rev Toxicol. 2004;34:211–99. doi: 10.1080/10408440490265210. [DOI] [PubMed] [Google Scholar]
- Meek ME, Bucher JR, Cohen SM, Dellarco V, Hill RN, Lehman-McKeeman LD, Longfellow DG, Pastoor T, Seed J, Patton DE. A framework for human relevance analysis of information on carcinogenic modes of action. CRC Crit Rev Toxicol. 2003;33:591–653. doi: 10.1080/713608373. [DOI] [PubMed] [Google Scholar]
- Melnick RL. An alternative hypothesis on the role of chemically induced protein droplet (α2u-globulin) nephropathy in renal carcinogenesis. Regul Toxicol Pharmacol. 1992;16:111–25. doi: 10.1016/0273-2300(92)90052-b. (Comment) [DOI] [PubMed] [Google Scholar]
- Melnick RL. Critique does not validate assumptions in the model on α2u-globulin and renal carcinogenesis. Regul Toxicol Pharmacol. 1993;18:365–8. doi: 10.1006/rtph.1993.1062. (Comment) [DOI] [PubMed] [Google Scholar]
- Melnick RL, Kohn MC. Possible mechanisms of induction of renal tubule cell neoplasms in rats associated with α2u-globulin: role of protein accumulation versus ligand delivery to the kidney. In: Capen CC, Dybing E, Rice JM, Wilbourn JD, editors. Species Differences in Thyroid, Kidney and Urinary Bladder Carcinogenesis. IARC Scientific Publications; Lyon, France: 1999. pp. 119–37. No. 147. [PubMed] [Google Scholar]
- Melnick RL, Kohn MC, Huff J. Weight of evidence versus weight of speculation to evaluate the α2u-globulin hypothesis. Environ Health Perspect. 1997;105:904–6. doi: 10.1289/ehp.105-1470356. (Correspondence) [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program . Toxicology and Carcinogenesis Studies of d-Limonene (CAS No. 5989-27-5) in F344/N Rats and B6C3F1 Mice (Gavage Studies) NTP, Research Triangle Park; NC: 1990. NTP TR 347, NIH Publication No. 90-2802. [PubMed] [Google Scholar]
- National Toxicology Program . Toxicology and Carcinogenesis Studies of t-Butyl Alcohol (CAS No. 75-65-0) in F344/N Rats and B6C3F1 Mice (Drinking Water Studies) NTP, Research Triangle Park; NC: 1995. NTP TR 436, NIH Publication No. 95-3167. [PubMed] [Google Scholar]
- National Toxicology Program . Toxicology and Carcinogenesis Studies of Stoddard Solvent IIC (CAS No. 64742-88-7) in F344/N Rats and B6C3F1 Mice (Inhalation Studies) NTP, Research Triangle Park; NC: 2004. NTP TR 519, NIH Publication No. 04-4453. [PubMed] [Google Scholar]
- National Toxicology Program . Toxicology and Carcinogenesis Studies of Propylene Glycol Mono-t-Butyl Ether (CAS No. 57018-52-7) in F344/N Rats and B6C3F1 Mice and a Toxicology Study of Propylene Glycol Mono-t-Butyl Ether in Male NBR Rats (Inhalation Studies) NTP, Research Triangle Park; NC: 2004a. NTP TR 515, NIH Publication No. 04-4449. [PubMed] [Google Scholar]
- National Toxicology Program . Toxicology and Carcinogenesis Studies of Decalin (CAS No. 91-17-8) in F344/N Rats and B6C3F1 Mice and a Toxicology Study of Decalin in Male NBR Rats (Inhalation Studies) NTP, Research Triangle Park; NC: 2005. NTP TR 513, NIH Publication No. 05-4447. [PubMed] [Google Scholar]
- Rao GN, Morris RW, Seely JC. Beneficial effects of NTP-2000 diet on growth, survival, and kidney and heart diseases of Fischer 344 rats in chronic studies. Toxicol Sci. 2001;63:245–55. doi: 10.1093/toxsci/63.2.245. [DOI] [PubMed] [Google Scholar]
- Swenberg JA, Lehman-McKeeman LD. α2-Urinary globulin-associated nephropathy as a mechanism of renal tubule cell carcinogenesis in male rats. In: Capen CC, Dybing E, Rice JM, Wilbourn JD, editors. Species Differences in Thyroid, Kidney and Urinary Bladder Carcinogenesis. IARC Scientific Publications; Lyon, France: 1999. pp. 95–118. No. 147. [PubMed] [Google Scholar]
- US Environmental Protection Agency . Prepared for the Risk Assessment Forum. EPA/625/3-91/019F; Washington, DC: Sep, 1991. Alpha2u-Globulin: Association with Chemically Induced Renal Toxicity and Neoplasia in the Male Rat. [Google Scholar]