Abstract
The hippocampus is thought to contribute to episodic memory in part by binding stimuli to their spatiotemporal context. The present study examined how hippocampal neuronal populations encode spatial and temporal context as rats performed a task in which they were required to remember the order of trial-unique sequences of odors. The results suggest that a gradual change in the pattern of hippocampal activity served as a temporal context for odor sampling events and was important for successful subsequent memory for the order of those odors.
Episodic memory involves our capacity to bring to mind events in the context in which they were experienced, including the order and the location in which they occurred (Tulving and Madigan, 1970; Tulving, 2002). The hippocampus is essential for episodic memory and is thought to be involved in binding stimuli to their spatiotemporal context, connecting the “what” with the “where” and “when” of the memory (Morris, 2001; Eichenbaum, 2004). An abundance of data indicates that hippocampal activity is strongly modulated by spatial context (Burgess et al., 2001; Jeffrey et al., 2004), but little is known regarding how events are encoded in their temporal context, that is, when they occur within an episode.
The present study assessed the influence of temporal as well as spatial cues on hippocampal neuronal activity in rats performing a task in which they were required to remember the order of trial-unique sequences of odors. Our hypothesis was that temporal cues—cues derived from background information that gradually changes over time, including a sense of satiety or fatigue as well as the accumulated residue of memories for recently experienced odor stimuli—could lead to a representation of temporal context that would be particularly important to memory for the order of ongoing events. According to this view, odor sampling events that occurred close together in time would have been accompanied by similar temporal cues, whereas events occurring farther apart in time would likely have been accompanied by dissimilar temporal cues. To the extent that these temporal cues influence hippocampal neural populations as a contextual signal, the similarities and differences in temporal context signals could organize memories for the order in which the odors were encountered. Our results indicate that the hippocampus had access to both a representation of the presented odors as well as a gradually-changing representation that could function as temporal context. These findings suggest that successful memory for order occurs when the odor stimuli are bound in a sequence by association with their temporal context.
Results and Discussion
On each trial, rats alternated between two sides of a testing enclosure as they sampled a unique sequence of 5 odors (Figure 1). At subsequent test, rats were presented with a randomly selected pair of non-adjacent sample odors and were rewarded for choosing the odor that had appeared earlier in the sample phase. Across 19 recording sessions, each of 5 rats completed at least 19 trials and performed on average 78.6 percent correct (SEM = 1.7%). For odors separated at study by 1, 2 and 3 intervening odors (adjacent study items were never presented together at test), rats performed at 72.9 (±2.9), 82.4 (±2.9), and 76.8 (±3.5) percent correct, respectively (mean ± SEM, all pairwise comparisons, p >.1), indicating that rats generally remembered the order of all items in the sequence. Unless specified otherwise, the following analyses considered data from only trials that were performed correctly.
Figure 1.
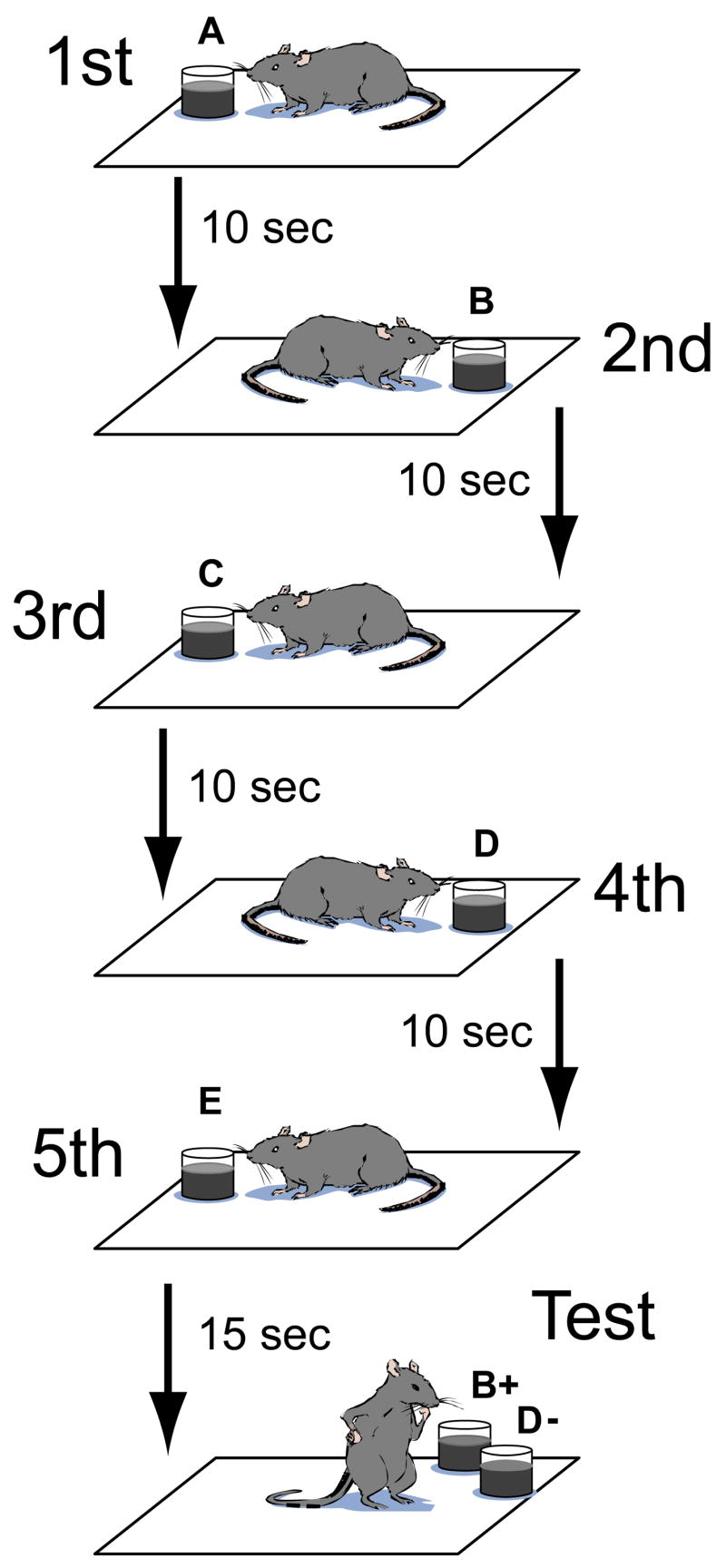
Schematic of the task. On each trial, rats alternated between left and right sides of the enclosure as they encountered a trial-unique sequence of 5 odors. Rats were then probed with a non-adjacent pair of the odors and were rewarded for selecting the odor that had appeared earlier in the sample sequence.
We first sought to verify our previous finding that hippocampal CA1 activity carries information about the identity of the individual odors encountered during performance of a memory task (Wood et al., 1999). We conducted an ensemble analysis on population vectors constructed for each odor sampling event during the study phase of the experiment (see Supplementary Material). To quantify the fidelity of odor representation in the hippocampal ensembles, we conducted an analysis in which we sampled cells randomly across sessions and attempted to reconstruct the identity of the odor presented. We measured the mutual information between the actual odor sequence and the predicted odor sequence and found an approximately linear relationship between the information transmitted about odor and the number of cells (see Supplementary Figure 1). A linear extrapolation of this relationship suggests that an ensemble of approximately 1000 cells could identify the odors as well as the animals identified their order in the present task (i.e., at 78.6% correct). This estimate is comparable in magnitude to previous estimates for odor coding in the orbital-frontal cortex (Schoenbaum and Eichenbaum, 1995), a structure that receives prominent olfactory input. These findings indicate that the hippocampus sparsely coded the odor cues used in each sequence.
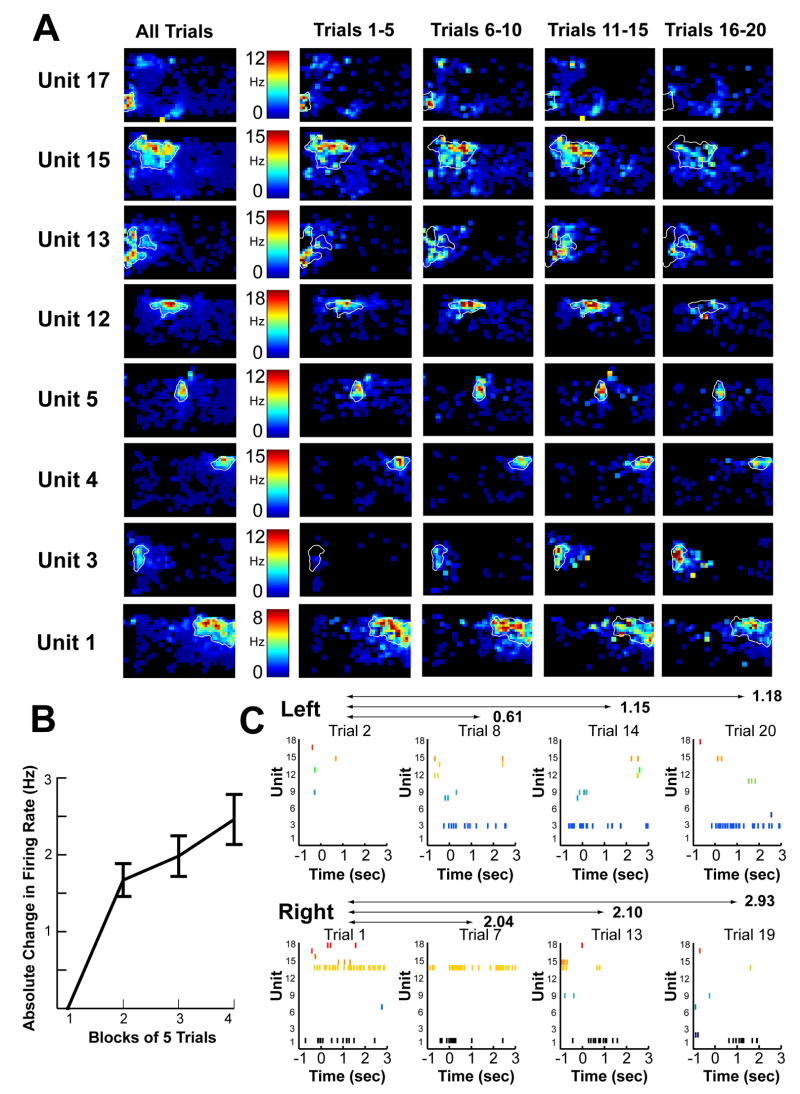
Next we addressed our primary hypotheses, that activity in the hippocampus during the sampling of trial-unique sequences of odors might have been influenced by both spatial and temporal cues and that the influence of temporal cues would be particularly important for performance on the task. There were diverse potential sources for both types of cues. Available spatial cues included prevalent visual stimuli surrounding the testing enclosure as well as internal self motion cues and path integration cues. Potential temporal cues included increasing fatigue and satiety, an internal sense of time passing, and lasting representations of recently presented odors. We characterized the influence of both types of cues indirectly by asking whether the activity of hippocampal cells changed as a function of the spatial location of the animal and over elapsed time. Figure 2 shows for an example session the tendency of some cells to exhibit a high degree of spatial specificity (“place fields”) as well as the tendency of some cells to increase or decrease their firing rates over the course of the testing session. Moreover, a substantial number of cells exhibited a combination of these properties as a change (increase or decrease) in the infield firing rate over the course of the session (see Figure 2B).
Figure 2.
Examples of gradual changes in hippocampal firing across a testing session. A. Example of hippocampal firing rates as a function of the rat’s location in a rectangular testing enclosure for the 8 (out of 18) pyramidal cells that met the criterion for exhibiting at least one place field in this example session. The results are shown for the entire test session (leftmost column) and separately for blocks of five trials (rightmost four columns). B. Average absolute change in in-field firing rate for all pyramidal units with place fields from all sessions used in the study. Some cells showed increases in in-field firing rates, whereas others showed decreases or no changes, and thus the graph shows absolute differences relative to the first block of trials (as a consequence, block 1 is shown as 0; effect of block for blocks 2–4: F=1.76, p=.01). C. Example rasters for trials 2, 8, 14, and 20 (all of which occurred on the left side of the testing enclosure) and for trials 1, 7, 13, and 19 (all of which occurred on the right side of the testing enclosure) for the same example session shown in A. The numbers above double arrows indicate the Distance Index between the events (all events were from the 4th ordinal position on each trial).
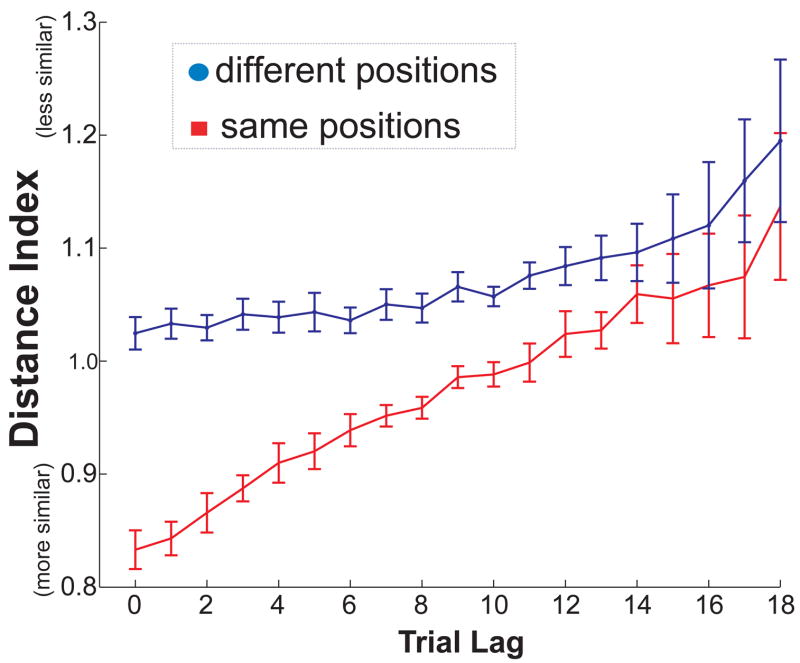
To examine the influence of temporal cues, we asked whether odor sampling events that occurred close together in time were represented more similarly to one another by ensemble patterns of hippocampal activity as compared to sampling events that occurred farther apart in time. Similarly, to quantify the influence of spatial cues during the period surrounding odor sampling, we asked whether odor sampling events that occurred in the same location were represented more similarly to one another by ensemble patterns of hippocampal activity as compared to sampling events that occurred in different locations. We operationally defined contextual representations as the activity of simultaneously recorded groups of neurons from 1 sec before to 3 sec after onset of each sniffing event. We constructed N-dimensional population vectors for each study event. The distance between two population vectors can be thought of as a measure of the similarity (or dissimilarity) in the pattern of firing across two odor sniffing events. We measured the distance between population vectors using Mahalanobis distance, a standard multivariate measurement of distance. The Mahalanobis distance has several properties that make it particularly useful for the present analyses. First, it scales the components of the vectors such that cells with a high firing rate do not dominate the measurement. Second, the Mahalanobis distance is sensitive to the correlational structure of the data, such that it controls for redundancies between cells. To allow for fair comparisons across sessions with different numbers of cells, we calculated a Distance Index by dividing the Mahalanobis distance by two times the number of units recorded in that session. Thus, a lower Distance Index represented more similar events, and a higher Distance Index represented less similar events. We expected greater similarity of contextual population vectors between sniffing events occurring in the same location as compared to different locations and between sniffing events occurring close together in time as compared to events occurring farther apart in time. Figure 2 shows several examples of the activity of recorded neurons taken from events spread across one example session and includes the Distance Indices of ensemble contextual representations between these events.
We first compared the similarities of contextual representations between odor sampling events from across the entire session as a function of the number of trials separating the events. The influence of spatial contextual cues was assessed by separately plotting comparisons involving the same spatial position and comparisons involving different spatial positions. In this analysis, both correct and incorrect trials were included due to the fact that incorrect responses occurred on different trials for different sessions. The results are shown in Figure 3 and indicate the influence on hippocampal ensemble representations for both spatial and temporal context. First, the contextual representation was more similar for events that occurred in the same position as compared to events that occurred in different positions (two way repeated-measures ANOVA, effect of position: F(1,18) = 45.61, p < .001), reflecting the tendency of many hippocampal neurons to show a high degree of spatial specificity. Second, the contextual representation was more similar for events that occurred closer together in a session and was more dissimilar for events that occurred farther apart (two-way repeated measure ANOVA, effect of trial lag: F(1,18) = 6.49, p. <.001). The two way ANOVA revealed a significant position by lag interaction (F(1,18)=7.72, p. < .001), suggesting that the effect of trial lag might have been more robust for comparisons of events occurring in the same location. Nevertheless, the effect of trial lag was still apparent when the analysis was repeated separately for comparisons involving same locations (one way repeated-measures ANOVA, main effect of lag: F(18,18) = 10.60, p < .001) and for comparisons involving different locations (F(18,18) = 2.91, p < .001). Thus, the results suggested that hippocampal activity reflected a contextual representation that changed across the entire session.
Figure 3.
Similarity of ensemble responses according to temporal lag between trials. Results from odors encountered in the same position (red) are plotted separately from odors encountered in different positions (blue). A lower Distance Index corresponds to greater similarity. Error bars show SEM for the variability across the 19 sessions analyzed.
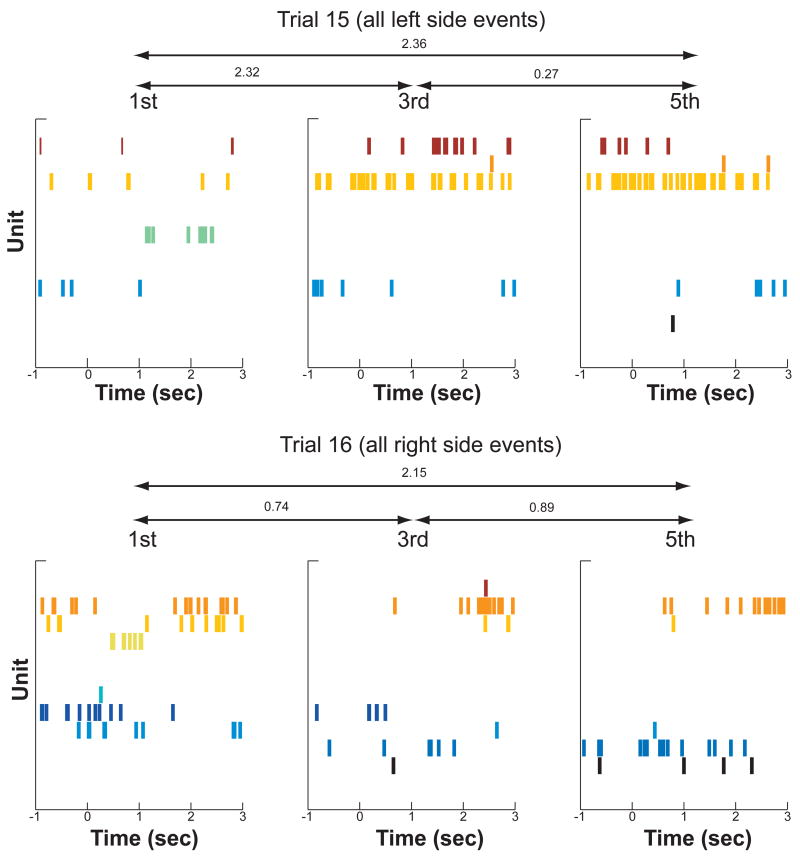
We next assessed whether the change in contextual representation observed over the entire session would also be apparent within individual trials. The task protocol allowed us to examine the influence of spatial as well as temporal context on each trial (see Figure 1). In particular, rats alternated between two spatial positions while sampling the five odors on each trial, and thus temporally adjacent odors (lag = 1; e.g., A and B) as well as odors separated by two intervening odors (lag = 3; e.g., A and D) appeared on opposite sides of the testing enclosure. Odors separated by 1 (lag = 2) or 3 (lag = 4) intervening odors appeared on the same side of the enclosure. Figure 4 shows examples of hippocampal activity from two trials and illustrates the influence of temporal lag between events and the influence of spatial position. Figure 5 shows examples of hippocampal activity that changed within a trial for events occurring in the same location. The question of interest was whether temporal cues would have an influence on hippocampal activity in addition to the expected influence of spatial cues. Therefore, we separately compared the Distance Index of hippocampal ensemble representations at a lag of 1 (different positions) to that at a lag of 3 (different positions) and ensemble representations at a lag of 2 (same position) to that a lag of 4 (same position).
Figure 4.
Example from one session of changes in the pattern of hippocampal activity within a trial. The top row of graphs show the 1st, 3rd and 5th events on trial 15 of this session. Because the rat alternated sides of the testing enclosure, these events all occurred on the left side of the enclosure. The bottom row shows the 1st, 3rd, and 5th events on trial 16 of the same session. These events all occurred on the right side of the enclosure, and the difference in pattern of activity between the top row and bottom row illustrates the influence of location on the hippocampal ensemble response. The difference between events within a trial illustrates the effect of temporal context on the hippocampal ensemble, and the numbers above the double arrows show the Distance Indices for the indicated comparison.
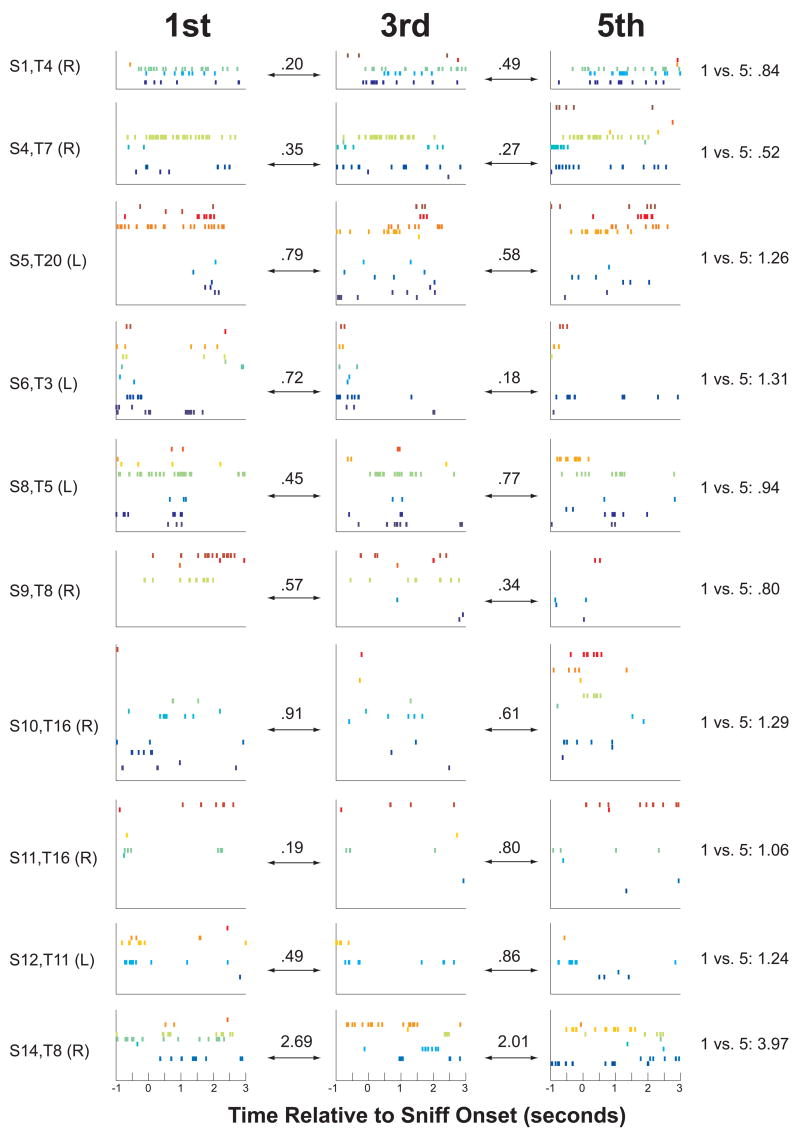
Figure 5.
Examples of changes in the pattern of hippocampal activity within a trial from 10 different sessions. Each row of graphs is taken from a different session and shows activity from the 1st, 3rd, and 5th odor from one trial. The numbers above the double arrows (and in the rightmost column) show the Distance Indices for the indicated comparison. The session number, trial number, and right/left side of the enclosure are indicated by text to the left of each row (in the format “S[session number], T[trial number] (L[eft]/R[ight])”).
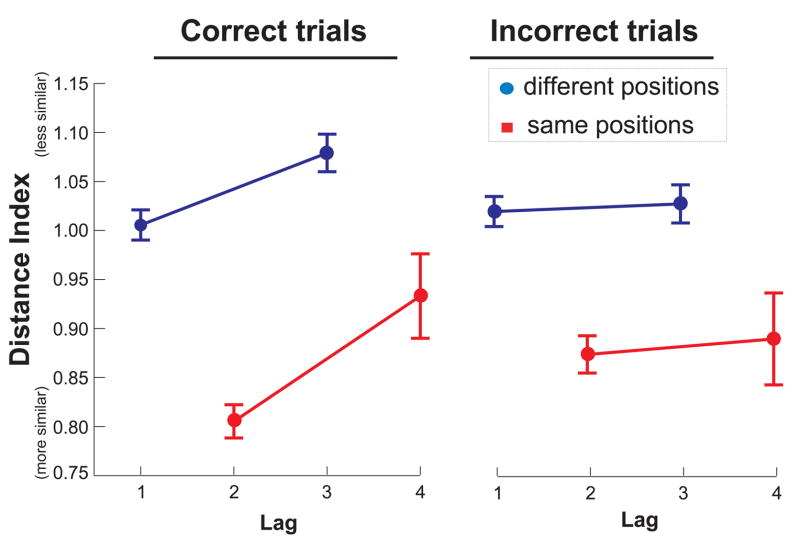
Figure 6 shows the average Distance Indices of contextual representations associated with pairs of odor sampling events associated with temporal lag and with location. The results were plotted separately for trials on which the animal subsequently correctly identified the order of odors and for those on which the animal subsequently incorrectly judged order. For correct trials, sampling events that occurred closer together in the sequence were represented more similarly than those farther apart, both for events that occurred in the same locations (lag 1 vs. lag 3) and for events that occurred in different locations (lag 2 vs. lag 4). A 2X2 repeated measures ANOVA revealed a main effect of location (same vs. different; F(1, 18) = 25.38, p < .001) and temporal lag (lag 1 and lag 2 vs. lag 3 and lag 4; F(1, 18) = 15.57, p = .001). Thus, both spatial and temporal cues had a significant influence on hippocampal activity when the rats were encoding trial-unique sequences of odors. In contrast, for trials in which the animal subsequently failed to remember the order of sample odors, a similar ANOVA revealed only a main effect of location (F(1,18) = 19.53, p < .001) but no effect of temporal lag (F(1,18) = 0.18, p > .1). An additional post-hoc test directly comparing scores on correct versus incorrect trials indicated that Distance Indices between short lags and long lags averaged across same and different positions was greater for correct trials than for incorrect trials (t(18) = 2.25, p < .05), suggesting that the temporal cues were especially important for subsequent memory for temporal order. In contrast, the influence of spatial cues on hippocampal activity was present on both correct and error trials.
Figure 6.
Similarity of ensemble responses according to temporal lag between odors encountered during the sample phase. Results from odors encountered in the same position (red) are plotted separately from odors encountered in different positions (blue). In addition, results from trials that were subsequently performed correctly are plotted separately from incorrect trials. A lower Distance Index corresponds to greater similarity. Error bars show SEM for the variability across the 19 sessions analyzed.
The findings of a hippocampal ensemble representation of spatial and temporal context were robust across analysis parameters and statistical measures. The same pattern of results were obtained when measures other than the Mahalanobis distance were used to estimate similarities of contextual representations. For instance, in another analysis, a measurement of similarity between contextual representations for two sampling events was obtained by averaging the differences in firing rates (calculated in terms of standard deviation) between the two events for each pyramidal neuron in the session (although this analysis was based on a univariate statistic, all 302 cells were included to perform an analysis that was parallel to the ensemble analyses). Using this statistic, there was still a significant effect of trial lag (F(1,18) = 7.70, p.<.001) and position (F(1,18) = 52.10, p < .001), as well as an interaction (F(1,18) = 19.17, p. <.001) for the analysis considering data from across the entire session. For the within trial analysis, there was still a main effect of lag (F(1,18) = 15.71, p=.001) and position (F(1,18) = 61.53, p<.001) for correct trials. For incorrect trials, there was an effect of position (F(1,18) = 57.40, p<.001) but not lag (F(1,18) = 0.09, p>.1). Further, the results obtained using Mahalanobis distance for both the across session analysis and the within trial analysis were not dependent on a particular time window. Indeed, similar, statistically significant results were obtained using a variety of time widows around the sniffing event, including windows that were both shorter and longer than the one reported here. One example is the result provided below for the time window when the rat was sniffing the odor and retrieving and consuming the food reward
We inspected the activity during the inter-stimulus intervals (3 seconds after onset of sniffing to 13 seconds after onset of sniffing, during which behavior was uncontrolled). The pattern of activity during this period showed the same kind of across-session changes in the pattern of activity as we observed during the odor sampling period. Specifically, a two way repeated measures ANOVA similar to that performed on the data shown in Figure 3 showed an effect for the preceding stimulus of lag (F(1,18) = 15.48, p<.001), of spatial position (F(1,18) = 28.23, p<.001) and an interaction of position and lag (F(1,18)=5.83, p<.001). These changes in activity over the entire course of the session during the inter-trial interval are consistent with the idea that hippocampal activity is typically under the influence of a variety of general temporal cues. In comparison to the across-trial effect, we did not observe a significant influence of temporal context during the inter-trial interval that was apparent within a trial (F(1,18)=1.35, p =.26), although the effect of location was still apparent (F(1,18) = 42.41, p<.001). Presumably this time window was sufficiently removed from the actual odor sampling period for the odors and other general features of the odor sampling event to exert an effect on activity that would have been apparent on the data from within a trial.
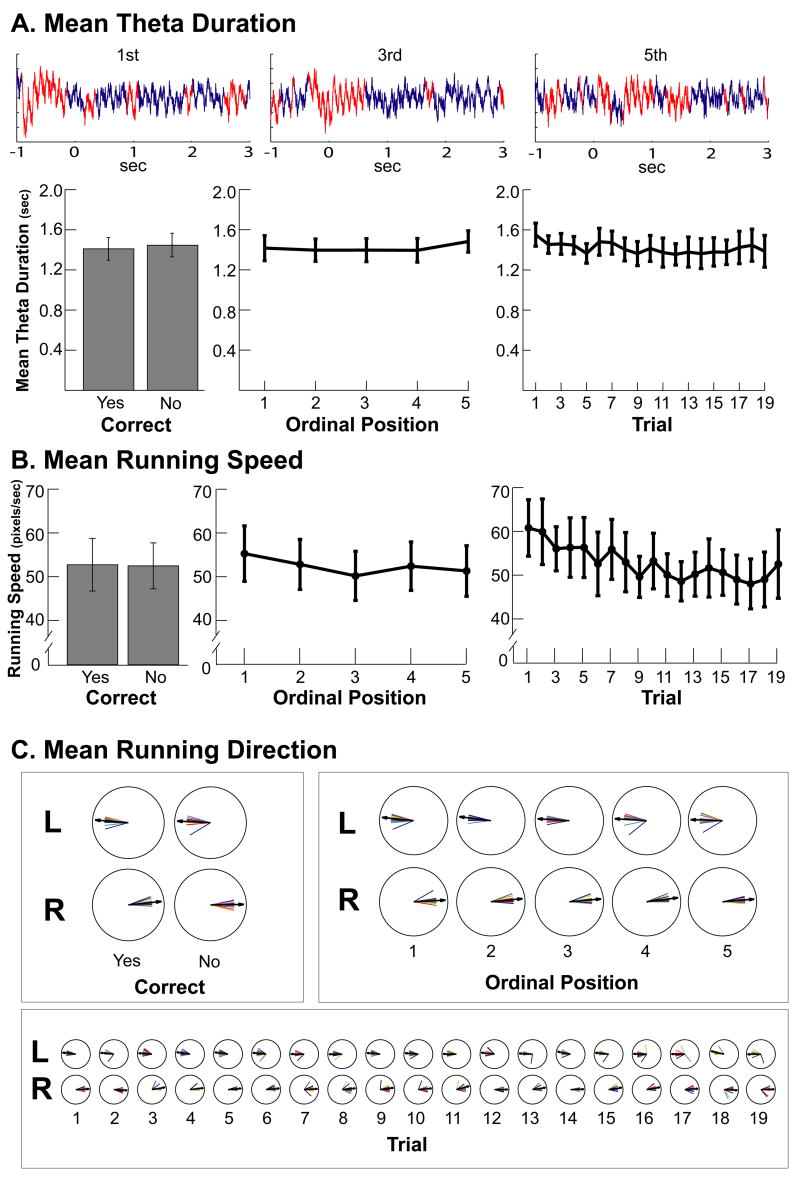
The findings on temporal context representation do not seem to be explained by nonspecific factors. We considered whether differences in running speed, running direction, and duration of time spent in theta activity for each event could be a source of the temporal changes we observed in the hippocampal activity during odor sampling. Figure 7 shows for sampling events the mean theta duration (Fig. 7A), the mean running speed (Fig. 7B), and the mean running direction (Fig. 7C). None of these factors accounted for our observations in the changing ensemble patterns of firing rates. Theta duration and running direction did not differ between correct and incorrect sampling events, across ordinal positions, or across trials (All ps > .1; see caption for Figure 7). Although running speed for the −1 to + 3 sampling period decreased slightly across ordinal positions (F(4,14)=4.97, p<.01) and across trials F(18,14)=2.61, p <.01), the influence of temporal context was still readily apparent when we reanalyzed our ensemble data for a 0 (onset of sniffing) to + 3 sampling period, a period when the rat was not running (during this period, the rat retrieved and consumed the food reward). Using this time window, there was still a significant effect of trial lag (F(1,18) = 5.86, p.<.001) and position (F(1,18) = 56.27, p < .001), as well as an interaction (F(1,18) = 7.21, p. <.001) for the analysis considering data from across the entire session. For the within trial analysis, there was still a main effect of lag (F(1,18) = 10.35, p<.01) and position (F(1,18) = 37.94, p<.001) for correct trials. For incorrect trials, there was an effect of position (F(1,18) = 20.28, p<.001) but not lag (F(1,18) = 0.82, p>.1). Also, running speed did not differ between correct and incorrect trials. Finally, although running speed differed slightly across ordinal positions, there was not an ordinal position by accuracy interaction (F(1,14) = 1.17, p = .34; a similar analysis could not be performed across trials because rats performed incorrectly on different trials). The similar slight decline of running speed across incorrect and correct trials indicated that running speed was unlikely to have propelled the different patterns of Distance Indices between correct and incorrect trials. Furthermore, all of the statistical results were the same even when the analysis included only the time periods when the rat was not running. Thus, the slight changes in running speed observed did not seem to account for the robust changes in hippocampal representations.
Figure 7.
A. Mean theta duration. Example traces of the local field potential (recorded in the pyramidal layer; 1–400 Hz) are shown that represent the first, third, and fifth sampling events from one trial. The local field potential was divided into theta-present (red) and theta-absent sections (blue; see text). The amount of time spent in hippocampal theta did not differ between correct and incorrect events (t(18)=1.00, p > .1), across ordinal positions (F(4,18)=1.37, p>.1), or across trials (F(18,18)=0.62, p >.1). B. Mean running speed. The mean running speed did not differ between correct and incorrect events (event = 1 sec prior to onset of sniffing to 4 seconds later; t(14)=0.18, p > .1), but showed a slight decrease across ordinal positions (F(4,14)=4.97, p<.01; this difference was equivalent on correct and incorrect trials, see text) and across trials (F(18,14)=2.61, p <.01). Nevertheless, the main results regarding temporal context were unchanged when the spiking data was considered only when the rat was stationary (see text). 50 pixels/sec corresponded to 13.9 cm/sec. C. Running Direction. The polar plots show the mean running direction for each session (colored lines) and the average for all sessions (black arrows). The mean running direction was similar for correct and incorrect events, for all ordinal positions, and for all trials. Position data (and therefore running speed and running direction) was unavailable for 4 sessions (see text).
In addition, the data for each tetrode for each session was carefully inspected for evidence of tetrode movement, and data was excluded for any tetrode that showed evidence of movement, which was infrequently the case. In particular, we carefully inspected data from every tetrode by viewing unit clusters in numerous 3-dimensional projections in which timestamp was one of the projections, allowing any changes in the clusters over time to be observed (Offline Sorter; Plexon inc.). Figure 8 shows data from an example tetrode and shows similar unit cluster projections across quartiles of a session. In those rare instances in which drift over the session was observed, we excluded all data from that tetrode.
Figure 8.
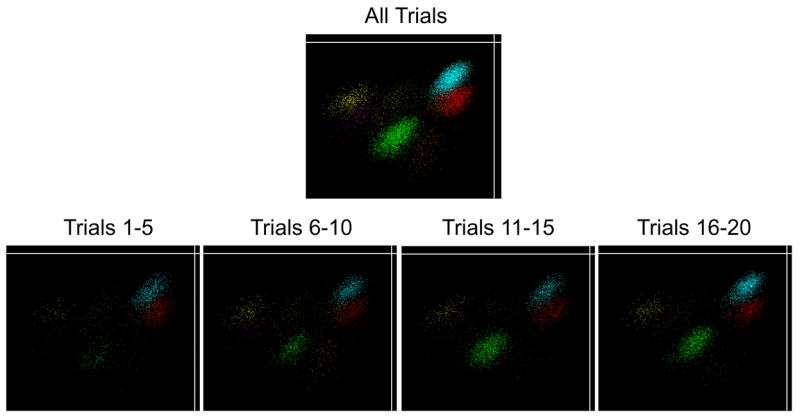
Example data from one tetrode showing similar cluster projections across quartiles of the session. Individual points correspond to neuronal spikes and are colored to represent the different units recorded on this tetrode. The Y axis shows the amplitude of each spike as it was recorded on one wire of the tetrode, and the X axis shows the amplitude of another wire from the same tetrode. The clusters are similar in each of the quartiles, indicating that no tetrode movement occurred. The green cluster corresponds to Unit 2 in Figure 2, a neuron that showed changes in firing rate over the session without showing any evidence of tetrode movement (although the change in firing rate for that unit is apparent as an increase in the number of spikes in the cluster across the quartiles).
The findings that the pattern of hippocampal ensemble activity grew more dissimilar over the course of a testing session and even over the span of a single, correctly performed trial strongly suggested that a gradually changing temporal context played an important part in the rats’ memory for the order of odors. Nevertheless, we sought to address other possible ways in which activity in the hippocampus might have contributed to performance on the task in addition to this representation of temporal context. We considered the possibility that hippocampal neurons might have provided a stronger memory representation for more recent odors and that this signal could be used to judge differences in the recency of test odors without a specific memory for order per se. We also explored the possibility that the hippocampal neural representations enabled rats to associate an ordinal tag (e.g., “third” or “fifth”, by analogy) with each odor it encountered during the study period and that the activity of individual CA1 neurons might have reflected this association. However, thorough analysis of both single-unit and ensemble data found no evidence that activity in the hippocampus contributed to performance in either of these possible ways (see Supplementary Material). The results regarding recency are consistent with the results of a previous lesion study that used the same task (Fortin et al., 2002), which found that information about the recency of items did not enable rats to perform above chance in remembering the order of items. Thus, the results suggest that hippocampal activity contributed to performance of the task not by supporting judgments of recency or by associating ordinal tags with each odor but instead by reflecting temporal context by a gradual change in the pattern of activity over time.
General Discussion
The present results supported our hypothesis that hippocampal activity during learning episodes would be influenced by both spatial and temporal contextual cues and that temporal context would be especially important for remembering the order in which events occurred within an episode. In particular, the finding that the hippocampal representation of temporal context became more dissimilar over the entire session strongly suggested that individual odors were not encoded in isolation but were prominently influenced by a gradually changing representation of the contextual cues that surrounded each odor sampling event. The finding that temporal context representation was important for successful performance on individual trials indicated that temporal contextual cues were a key part of the resulting memory for temporal order. Taken together with the confirmation that hippocampal ensembles also encode the sampled odors, the present results suggest that memory for individual odors might have been bound in a sequence by the overlapping contextual representations of temporally adjacent odors (Eichenbaum, et al., 2007; Howard et al., 2005).
A remaining uncertainty concerns the factors that caused the pattern of activity in the hippocampus to change gradually over time. One possibility is that the gradual changes in the pattern of hippocampal activity were simply the result of random drift in network states. A second possibility is that the gradual changes in hippocampal activity reflected gradual changes in external stimuli, such as the visual cues that unfold as one navigates. A third possibility is that the presentation of the odors themselves propelled the gradual changes in the hippocampus. By this view, the general network state of the hippocampus changed gradually because the recent history of odor sampling underwent continual update and decay (Howard et al., 2005). The present results indicate that, regardless of why activity in the hippocampus changed gradually over time, these gradual changes were important for remembering the order in which the odors were encountered. Although the link between behavior and hippocampal activity is correlational, the observation that changes in activity during the sampling events predicted correct subsequent responses suggests a close relationship between gradual changing temporal context signals and the ability to remember sequences of events.
More research will be needed to identify the temporal cues that are used to differentiate points in time in a way that resembles the many previous efforts to identify the spatial cues that are used to define particular locations. One theme in the effort to characterize the relevant spatial cues has been the observation that the hippocampal memory system participates in the integration of information about external landmarks with internal information such as ideothetic cues and attentional set (McNaughton et al., 2006). Thus, it is possible that future studies will find that the hippocampal memory system also participates in combining information about the external environment that changes over time (for example, visual cues that unfold as one navigates) with gradually changing internal cues (for example, one’s continually updating memory for prior events).
It has been proposed that the brain encodes temporal information in at least two general ways, via oscillations (Buzsaki and Draguhn, 2004) and via gradual changes in network states (Karmarkar and Buonomano, 2007) like those observed in the present study. Oscillations may provide a means for precise spike timing and for sequencing the order of spiking activity. Indeed, there is accumulating data regarding the importance of oscillations in the hippocampus for timing successive behavioral events (Buzsaki, 2002). For example, a recent study of rats running on a track found that the dynamics between hippocampal spiking activity and theta oscillations in the local field potential resulted in a sequencing of spiking activity from different cells on a millisecond time scale that corresponded to a the rat traversing a sequence of locations across several seconds (Dragoi et al., 2006). That is, hippocampal theta oscillations provided a means for compressing the sequential representation of the rat’s path to a time scale amenable to cellular processes related to plasticity without disrupting the sequence of the representation.
Gradual changes in network states, like those observed in the hippocampus in the present study, have long been thought to be important for memory (Estes, 1955). More recently, this proposal has gained renewed interest in computational modeling and cognitive psychology (Burgess and Hitch, 2005; Howard et al., 2005; Kahana et al., in press). For example, recency and contiguity effects in episodic recall, which have been hypothesized to depend on a gradually-changing representation of temporal context, have been observed across many tens of seconds and even across lists of items (Howard, et al., in press). What has been missing has been physiological evidence that processes such as these actually occur in the brain and relate to memory performance in an important way. Thus, the present results provide the first evidence that hippocampal ensembles exhibit a gradually changing pattern of neural activity and that this activity contributes to episodic memory for temporal order.
The idea that the hippocampus is particularly important for encoding spatiotemporal context is a classic formulation of memory systems in the brain (Hirsh, 1974). One difficulty with this idea has been the challenge to define what is meant by context. Nevertheless, many spatial and nonspatial features of the physical environment as well as factors related to internal motivation or attentional set have been found to influence hippocampal activity (for a review, see Jeffery et al., 2004). The present results suggest that the set of contextual cues that influence hippocampal activity should be expanded to include cues that change gradually over time and thereby distinguish the temporal context of sequential events.
Remembering the order in which items are encountered is likely a complex process subserved by multiple neural mechanisms and multiple brain areas (Brown and McCormack, 2006; Marshuetz, 2005). For example, it remains possible that a hippocampal process related to recency could support judgments of temporal order if the amount of time separating items in a sequence was large relative to the study-test interval. Also, results from brain damaged patients (Kesner et al., 1994; Shimamura et al., 1990), functional imaging studies in humans (Henson et al., 2000; Marshuetz et al., 2000) and unit recording studies in monkeys (Funahashi et al., 1997) have indicated that the prefrontal cortex plays an important role in memory for ordinal position. Thus, the results of the present study should be considered as an addition to the list of neural mechanisms supporting episodic memory for the order of events.
Experimental Procedures
Five male Long-Evans rats were trained on a task that was similar to that used in two previous studies (Fortin et al., 2002; Kesner et al., 2002), which both found that damage to the hippocampus resulted in severe deficits in memory for order and not the ability to discriminate or remember the occurrence of particular odors. On each trial, rats sampled 5 odors one at a time (e.g., A, B, C, D, then E) at approximately 10 sec intervals and then at test were presented again with an arbitrarily selected pair of non-adjacent odors (e.g., B and D). The rat was rewarded during study for sampling each odor and during the test phase for choosing the odor that had appeared earlier in the sample phase. On each trial the sample odors were selected randomly from a set of 10 and the sequence of odors was randomly determined. The rat remained in the recording chamber (0.76 m by 0.38 m wooden enclosure with 0.43 m high walls) throughout testing, including a 2.5-min inter-trial interval. The placement of the pot containing the scented sand on each side of the enclosure was guided by marks on the floor of the testing enclosure. Rats were given 8 trials a day (5 days a week) until they reached a criterion of at least 75% correct for three successive testing days. The number of trials needed to reach criterion ranged approximately from 600 to 1200.
After a rat reached criterion performance, it was implanted with a chronic recording headstage above the left dorsal hippocampus (3.6 mm posterior and 2.6 mm lateral to bregma). The recording headstage contained from 6 to 12 independently movable tetrodes aimed at CA1. Each tetrode was composed of 4 12.5 μm nichrome wires whose tips were plated with gold to bring the impedance to 200 kΩ at 1 kHz. Animals were allowed to recover for 4 to 6 days, and the tetrodes were then moved down slowly, over the course of 2 to 3 weeks, until the tips reached the pyramidal cell layer of CA1 and the animal’s performance had again met criterion levels. Tetrodes were never turned on the day of the recording, and tetrode drift during the approximately 1-hour recording sessions was rarely observed. Nevertheless, since the possibility of tetrode drift was an important concern, we carefully inspected data from every tetrode by viewing unit clusters in numerous 3-dimensional projections in which timestamp was one of the projections (Offline Sorter; Plexon inc.). In those rare instances in which tetrode movement was observed, we excluded all data from that tetrode (also see General Discussion for more details).
Following recovery, animals performed 20 trials per day. The placement of the tetrode tips was verified by several CA1 pyramidal electrophysiological hallmarks (complex spikes, theta-modulated spiking, multi-unit bursts accompanied by 200 Hz “ripples” in the field potential) and by histology. During testing, spike activity was amplified (10,000X), filtered (600–6000 Hz), and saved for offline analysis (DataWave Technologies). For single-unit analyses, data were analyzed for each tetrode from only one recording session to avoid analyzing a single unit more than once. This process resulted in 126 cells being analyzed. For ensemble analyses, data from all tetrodes were included such that each session represented sampling with replacement from the total population of neurons. This process resulted in 302 cells being analyzed. For both types of analyses, all pyramidal units that emitted at least a minimum of activity (>3 spikes during any event window) were included.
Local field potentials were also recorded from tetrodes in the CA1 pyramidal layer for each session (1,500X; 4–400Hz). The sampling frequency for field potentials was 1,000 Hz for some sessions and 250 Hz for other session. The field potential from the entire session was divided into theta-present and theta-absent sections by first filtering the recorded wide-band field potential (1–400 Hz) to a more restricted theta band (4–12 Hz). Peaks and troughs of the oscillation in the filtered signal that departed more than one standard deviation from the baseline (determined by the grand mean of the filtered signal) were marked, and sections of the field potential lasting at least one full cycle of marked peaks and troughs was considered to be a theta-present section. Thus, the minimum length of time marked as a theta-present section was 1 theta cycle (typically around 1/7th of a second).
Behavior was recorded with high-resolution digital video (30 frames per sec) that was synchronized with the acquisition of neural data. Onset of sniffing was defined as the video frame on which the rat’s nose was first within 1 cm of the pot. During post-session video analysis, the position of the rat’s head was marked on every frame using a mouse pointer. Although labor intensive, this process yielded a highly accurate frame-by-frame record of the rat’s position that did not suffer from false detections or unmarked frames, an occasional occurrence with automated LED position tracking. Running speed and running direction were calculated based on these head positions. Specifically, running speed for a given time period (e.g., the 1-sec approach period that ended with the onset of sniffing) was calculated by summing the number of pixels traversed and dividing by the number of seconds. The running trajectory during the 1-sec approach period was nearly always a direct path for all rats, and thus a meaningful running direction could be obtained. For this period, a running direction was calculated by measuring the angle between the start point and the end point. Rat position data was available for only 15 of 19 sessions due to a corruption of 4 video files after those files had been scored for sniffing behavior.
Supplementary Material
Acknowledgments
Supported by NIH grant MH051570 (HBE) and NIH NRSA Fellowship MH068982 (JRM). We thank Norbert Fortin, Daniel Hashimoto, Robert Komorowski, Kimberly Ong, Lisa Pytka, and Alex Vasserman for their help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brown GDA, McCormack T. The role of time in human memory and binding: a review of the evidence. In: Zimmer HD, Mecklinger A, Lindenberger U, editors. Handbook of binding and memory: perspectives from cognitive neuroscience. Oxford: Oxford University Press; 2006. pp. 251–290. [Google Scholar]
- Burgess N, Becker S, King JA, O’Keefe J. Memory for events and their spatial context: models and experiments. Philos Trans R Soc Lond B Biol Sci. 2001;356:1493–503. doi: 10.1098/rstb.2001.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Hitch G. Computational models of working memory: putting long-term memory into context. Trends Cog Sci. 2005;9:535–41. doi: 10.1016/j.tics.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Buzsaki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50:145–57. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Declarative memory: insights from cognitive neurobiology. Annu Rev Psychol. 1997;48:547–72. doi: 10.1146/annurev.psych.48.1.547. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–20. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–52. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes WK. Statistical theory of spontaneous recovery and regression. Psychol Rev. 1955;62:145–54. doi: 10.1037/h0048509. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5:458–62. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Inoue M, Kubota K. Delay-period activity in the primate prefrontal cortex encoding multiple spatial positions and their order of presentation. Behav Brain Res. 1997;84:203–23. doi: 10.1016/s0166-4328(96)00151-9. [DOI] [PubMed] [Google Scholar]
- Henson RN, Burgess N, Frith CD. Recoding, storage, rehearsal and grouping in verbal short-term memory: an fMRI study. Neuropsychologia. 2000;4:426–40. doi: 10.1016/s0028-3932(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Hirsh R. The hippocampus and contextual retrieval of information from memory: A theory. Behavioral Biology. 1974;12:421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Howard MW, Fotedar MS, Datey AV, Hasselmo ME. The temporal context model in spatial navigation and relational learning: toward a common explanation of medial temporal lobe function across domains. Psychol Rev. 2005;112:75–116. doi: 10.1037/0033-295X.112.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Youker TE, Venkatadass V. The persistence of memory: Contiguity effects across several minutes. Psychonomic Bulletin & Review. doi: 10.3758/pbr.15.1.58. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery KJ, Anderson MI, Hayman R, Chakraborty S. A proposed architecture for the neural representation of spatial context. Neurosci Biobehav Rev. 2004;28:201–18. doi: 10.1016/j.neubiorev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Howard MW, Polyn SM, Roediger HL, editors. Learning and memory: A comprehensive reference. Elsevier; Associative retrieval processes in episodic memory. in press. [Google Scholar]
- Karmarkar UR, Buonomano DV. Timing in the absence of clocks: encoding time in neural network states. Neruon. 2007;53:427–438. doi: 10.1016/j.neuron.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Lee I, Hargreaves EL. Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory. Hippocampus. 2006;16:755–64. doi: 10.1002/hipo.20203. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav Neurosci. 2002;116:286–90. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hopkins RO, Fineman B. Item and order dissociation in humans with prefrontal cortex damage. Neuropsychologia. 1994;32:881–91. doi: 10.1016/0028-3932(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- Marshuetz C. Order information in working memory: an integrative review of evidence from brain and behavior. Psychol Bull. 2005;131:323–39. doi: 10.1037/0033-2909.131.3.323. [DOI] [PubMed] [Google Scholar]
- Marshuetz C, Smith EE, Jonides J, DeGutis J, Chenevert TL. Order information in working memory: fMRI evidence for parietal and prefrontal mechanisms. J Cogn Neurosci. 2000;12(Suppl 2):130–44. doi: 10.1162/08989290051137459. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7:663–78. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Morris RG. Episodic-like memory in animals: psychological criteria, neural mechanisms and the value of episodic-like tasks to investigate animal models of neurodegenerative disease. Philos Trans R Soc Lond B Biol Sci. 2001;356:1453–65. doi: 10.1098/rstb.2001.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. II. Ensemble activity in orbitofrontal cortex. J Neurophysiol. 1995;74:751–62. doi: 10.1152/jn.1995.74.2.751. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;8:803–13. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Tulving E, Madigan SA. Memory and Verbal Learning. Annu Rev Psychol. 1970;21:437–84. [Google Scholar]
- Wood ER, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–6. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.