Abstract
Background
Gene-modified tumor cell vaccines have shown efficacy in animal models of malignancy, including prostate cancer. Class I major histocompatibility complex (MHC) assembly and function in the cellular targets of such therapies is pivotal in determining the efficacy of specific cytokine-secreting tumor vaccines.
Purpose
To help guide development of genetically engineered vaccine therapy for human prostate cancer, potential immune resistance pathways were evaluated by analysis of class I MHC assembly in prostate cancer cells.
Method
Class I MHC assembly in metastasis-derived human prostate cancer cell lines (LNCaP, PPC-1, DU-145, PC-3, and TSU) and a normal prostate-derived cell line (TP-2) were characterized by phenotypic, molecular, and functional assays. Assembled class I MHC and antigen was measured by flow cytometry; mRNA levels of assembly components (class I MHC heavy chain, β2-microglobulin, and the antigen transporter gene product TAP-2) were determined; and antigen processing was measured with a chimeric reconstituted system using vaccinia vectors. Restoration of antigen processing was attempted by interferon gamma stimulation and by transfection with mouse class I MHC heavy-chain cDNA.
Results
Assembled class I MHC was underexpressed in two (LNCaP and PPC-1) of five prostate cancer cell lines compared with normal prostate-derived controls. PPC-1 cells underexpressed TAP-2 mRNA despite abundant class I MHC and β2-microglobulin message. Induction of TAP-2 by interferon gamma indicated that coding sequences for TAP-2 message were present in PPC-1. Resistance to cytotoxic T lymphocytes (CTL) lysis showed a functional defect in antigen transport by PPC-1 cells; reversal of the molecular defect with interferon gamma led to restoration of functional antigen processing. In contrast, LNCaP cells had competent antigen transport but deficient class I MHC heavy-chain function despite abundant class I MHC RNA; though refractory to stimulation by interferon gamma, this defect responded to transfection of class I MHC heavy-chain cDNA.
Conclusions
Metastatic prostate cancer cells can escape T-cell recognition via divergent mechanisms of defective class I MHC assembly. The specific underexpression of TAP-2 gene product in PPC-1 cells contrasts with prior studies of TAP gene underexpression in lung cancer (which concurrently underexpressed class I MHC heavy chain) and provides evidence for a regulatory pathway controlling TAP-2 gene expression in human cancers that may not affect class I MHC heavy-chain expression.
Implications
In clinical application of gene therapy for prostate cancer, these findings provide a rationale for focusing on strategies that can circumvent sole reliance on class I MHC-mediated tumor cell recognition by CTL.
No curative treatment currently exists for human prostate cancer after progression beyond resectable boundaries (1). One therapeutic strategy under current development involves vaccination of patients who have advanced prostate cancer with tumor cell vaccines transduced to secrete immunostimulatory cytokines. Consequent augmentation of T-cell–mediated antitumor immunity can induce regression of pre-established metastases in preclinical models of several malignancies (2–7), including prostate cancer (8). The feasibility of routinely applying such a gene therapy strategy for human prostate cancer clinical trials has also been demonstrated recently (8).
In applying preclinical models of gene-modified autologous cancer vaccine therapy toward clinical use, the choice of which immunoregulatory gene product would confer greatest efficacy depends, in part, on mechanisms of tumor-specific antigen processing and transport by metastatic human prostate cancer cells. Immunotherapy via gene transfer of interferon gamma or tumor necrosis factor-α (TNF-α), for example, relies on interaction of cytotoxic T cells and tumor cell class I major histocompatibility complex (MHC) (3,4). Immunotherapy based on granulocyte–macrophage colony-stimulating factor or interleukin 2 (IL 2) gene transfer, in contrast, has efficacy independent of levels of class I MHC expression and depends rather on class II MHC expression or natural killer (NK)-mediated lysis (9,10). Molecular mechanisms of antigen processing in human prostate carcinoma cells and related potential pathways of immune evasion, however, have not been previously reported. We assayed molecular regulation of class I MHC-mediated antigen processing and presentation in metastatic human prostate cancer to rationally guide clinical translation of gene therapy strategies of immunotherapy.
Materials and Methods
Cell lines
Human metastatic prostate cancer cell lines DU-145, LNCaP, PC-3, PPC-1, and TSU were provided by Dr. William B. Isaacs, Baltimore, Md. These cell lines were passaged in vitro and grown in RPMI-1640 medium containing glutamine (JRH Bioproducts, Lenexa, Kan.) with 10% fetal calf serum (JRH Bioproducts), as was T2, a cell line used as a Northern blot control with somatic-fusion–induced genomic deletion of the TAP-2 gene. Dr. Isaacs also provided TP-2, an immortalized cell line generated from normal prostate epithelium that has been described previously (11). Briefly, immunohistochemical analysis of TP-2 cells previously demonstrated a prostate epithelial luminal phenotype, as evidenced by specific cytokeratin 18 expression and weak prostate-specific antigen staining (11). This cell line was passaged in vitro in RPMI-1640 with glutamine (JRH Bioproducts) and 5% fetal calf serum supplemented with MCDB-105, insulin, transferrin, selenic acid premix, and bovine pituitary extract (all from Sigma Chemical Co., St. Louis, Mo.) prepared according to the manufacturer’s specifications.
Measurement of class I MHC surface expression using fluorescence-activated cell sorter (FACS) analysis
Cultured cells were harvested by trypsinization and washed three times with Hanks’ balanced salt solution (GIBCO-BRL, Grand Island, NY), and resuspended in phosphate-buffered saline with 2.5% fetal calf serum. Cells (105) in 100 μL of staining buffer were then stained for 30 minutes with 5 μg/mL of fluorescein-conjugated w6/32 (Accurate Chemical, Westbury, N.Y.) or fluorescein-conjugated control mouse immunoglobulin G2a (IgG2a) antibody (Becton Dickinson, Mountain View, Calif.). FACScan (Becton Dickinson) standardized with calibrated flow-cytometry beads was then used to measure fluorescence emission and calculate statistics. In some experiments, cells were cultured with interferon gamma (Genzyme Corp., Cambridge, Mass.)—2.5-1000 U/mL for 24 hours—prior to harvesting for FACS analysis.
Northern blot analysis
Total cellular RNA was isolated using a guanidium isothiocyanate-based RNA isolation kit from Promega Corp. (Madison, Wis.). Purified RNA was quantitated using an Orsinol assay (12), and 20 μg of purified RNAs were electrophoresed on 3-(N-morpholino)propanesulfonic (MOPS)–formaldehyde gels (uniform purity was confirmed with ethidium bromide stain) (13), transferred to Zetaprobe nylon membranes (Stratagene Inc., La Jolla, Calif.) and then probed with 32P-labeled (GIBCO-BRL) complementary DNA probes for TAP-2, class I MHC, (β2- microglobulin, and (as control for uniformity of RNA loading and transfer) probes for histone H4 messenger RNA (mRNA) as well as 18S ribosomal RNA (rRNA) (14). Expression of class I MHC assembly components was then quantified by measuring the optical density of assembly components relative to the optical density of H4 mRNA and 18S rRNA control hybridizations of the same blot.
T-cell-mediated lysis of human prostate cancer cells transfected with mouse MHC in a reconstituted system directed against a viral model antigen
Human prostate cancer cells were transfected with 10 plaque forming units (pfu) per cell of recombinant vaccinia virus containing the Kd class I MHC molecule, or wild-type vaccinia virus control, for 1 hour. The vaccinia vectors used and details of the chimeric assay of antigen presentation by human cancer cells have been described previously (14). Briefly, transfected cells were incubated for 3 hours, labeled with Na51CrO4 for 1 hour and then mixed with CD8+ cytolytic T lymphocytes (CTL). After 4 hours at 37 °C, released 51Cr was determined by gamma-counting. Percentage-specific lysis was calculated as follows: 100 × (experimental cpm-spontaneous cpm)/(maximal cpm-spontaneous cpm). Background lysis of wild-type vaccinia-infected controls, lacking mouse Kd, was consistently less than 15% and was subtracted from vac-Kd lysis to derive antigen-specific tumor lysis. To generate the CD8+ CTL, BALB/c mice were immunized by intravenous injection with vaccinia and, after 2 weeks, splenocytes were collected and restimulated in vitro with vaccinia-infected splenocytes at a ratio of 2:1. On day 7 of culture, 2-5 U/mL of IL 2 (Chiron Therapeutics, Emeryville, Calif.) was added, and CD8+ T cells were harvested on day 9 for use as effector CTL (FACS analysis confirmed that this method yielded >90% CD8+ T cells).
Statistical analysis
Pooled-variance t test, separate-variance t test, and paired t test with Bonferroni adjustment for multiple comparisons were applied according to published guidelines. All t tests were two-tailed, and P values <.05 were considered significant (15).
Results
We evaluated class I MHC-mediated antigen processing, transport, and presentation by metastatic human prostate cancer cells. First, five metastatic human prostate cancer cell lines were screened for antigen presentation defects at the cell surface by assaying surface class I MHC expression with FACS, using the conformation-dependent antibody w6/32. (This antibody specifically binds class I MHC associated with β2-microglobulin.) In repeated experiments, two of these cell lines (PPC-1 and LNCaP) underexpressed surface class I MHC-β2-microglobulin heterodimer at the cell surface (Figs. 1 and 2) compared with the level of class I MHC heterodimer expression by cells from normal prostate and the metastasis-derived cell lines TSU and DU-145. In one of these cell lines (PPC-1), class I MHC heterodimer was undetectable on the cell surface by FACS analysis.
Fig. 1.
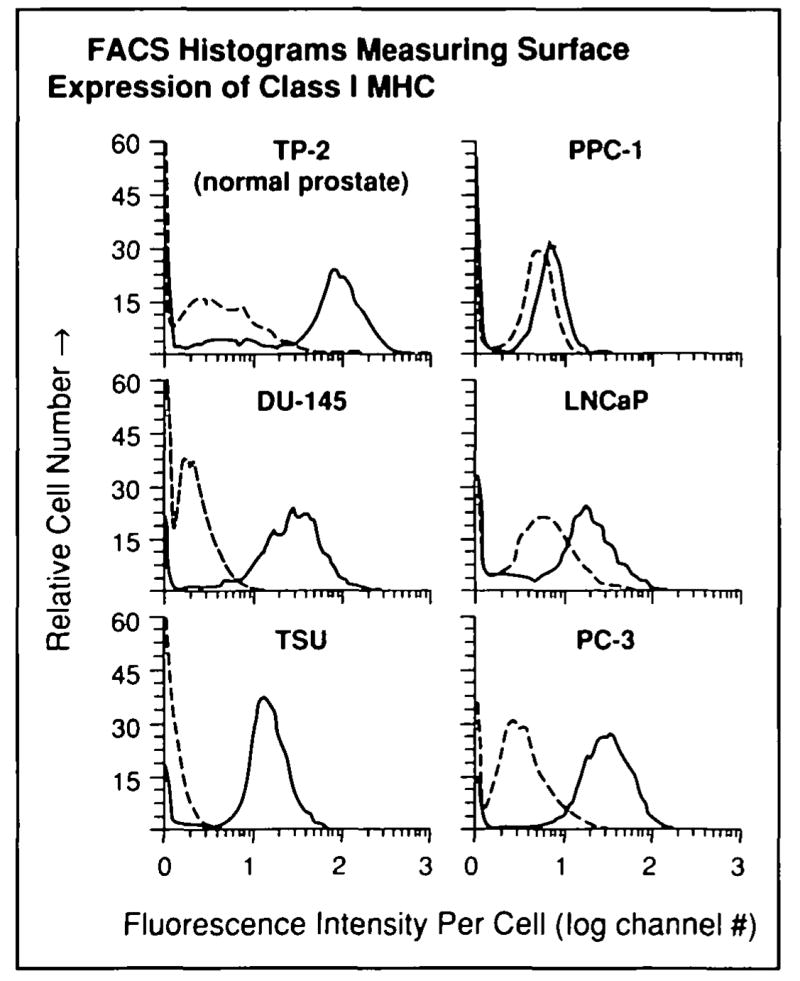
Underexpression of cell-surface class I MHC by human prostate cancer cells. FACS analysis was used to measure class I MHC expression in five cultured human prostate cancer cell lines (DU-145, LNCaP, PC-3, PPC-1, and TSU) and one immortalized cell line derived from normal prostate epithelium (TP-2) as described in the “Materials and Methods” section. Representative FACS histograms: solid lines indicate staining with human class I MHC-specific antibody (w6/32), dashed lines indicate control histograms with fluorescein-conjugated, isotype-matched control antibody determining background fluorescence. Three separate experiments were performed with similar results.
Fig. 2.
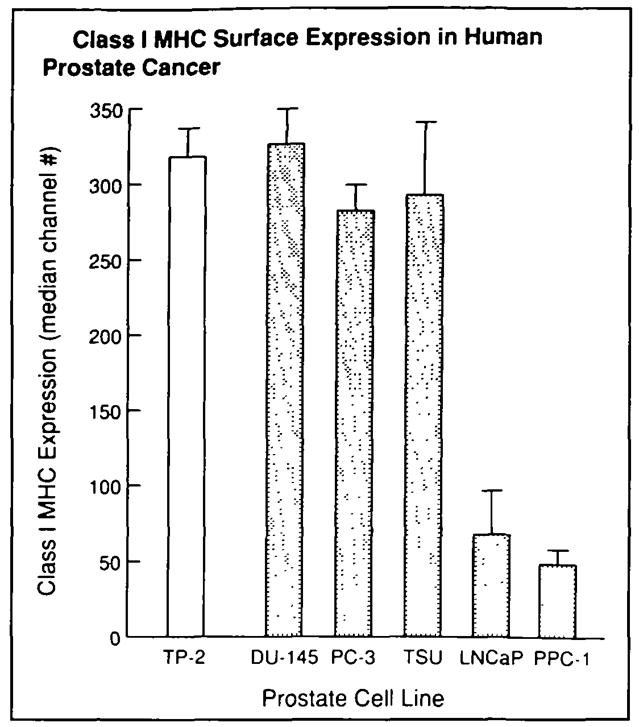
Relative expression of class I MHC was determined by subtracting median fluorescence of isotype-matched controls from median fluorescence of class I MHC antibody-labeled cells. Mean values from three experiments are shown for each cell line; error bars represent SE. LNCaP and PPC-1 were found to have diminished expression of class I MHC relative to the normal prostate control line TP-2. (Paired t test with Bonferroni adjustment for multiple comparisons: LNCaP, P<.025; PPC-1, P<.005).
To dissect molecular mechanisms of class I MHC underexpression by these prostate cancer cells, three principal components in the class I MHC-mediated antigen processing pathway were tested for mRNA expression by Northern blot analysis (Fig. 3): class I MHC heavy chain, β2-microglobulin, and TAP-2 (TAP-2 is a gene product required for competent antigenic peptide transfer and assembly of stable class I MHC) (16–18). Unexpectedly, one cell line lacking cell surface class I MHC expression, PPC-1, had abundant amounts of heavy-chain and light-chain (β2-microglobulin) mRNA expression compared with cell lines with abundant cell surface class I MHC (DU-145, PC-3, and TSU). However, the lack of cell surface class I MHC in PPC-1 was explained by the undetectable expression of TAP-2 mRNA assayed by Northern blot hybridization. Densitometry confirmed absent TAP-2 mRNA in PPC-1 when compared with a control cell line with a deletion of TAP-2 (T2 cells in the control lane in Fig. 3, used as a negative control, which lack TAP-2 message regardless of interferon gamma stimulation), even after adjusting for minor variations of lane loading and transfer by measurement of signal intensity relative to constitutive H4 or 18S rRNA hybridization. The TAP-2 mRNA deficiency in PPC-1 was overcome by induction of TAP-2 message with interferon gamma, indicating a potentially reversible defect of basal transcription in this human prostate cancer cell line.
Fig. 3.
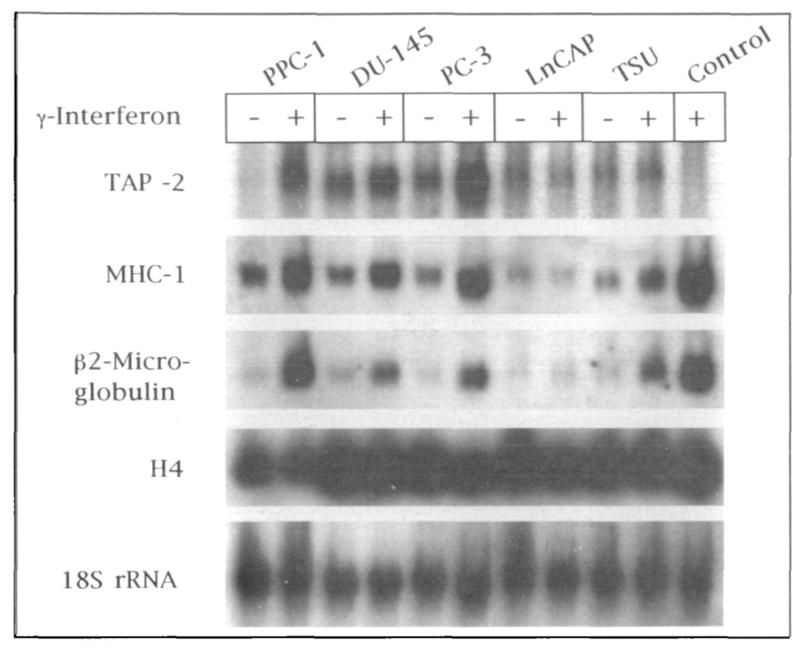
Northern blot hybridization of antigen processing components in human prostate cancer cell lines. RNA from five human prostate cancer cell lines (PPC-1, DU-145, PC-3, LNCaP, and TSU; with and without stimulation by interferon gamma [γ-interferon]) and a control somatic cell-fusion product cell line with deletion of the TAP-2 gene (T-2 control stimulated by interferon gamma) was purified, transferred to nylon membrane, and hybridized with 32P-labeled probes for TAP-2, class I MHC, β2-microglobulin, and, to control for uniformity in RNA loading and transfer, histone H4 mRNA and 18S rRNA. Autoradiographs of each hybridization are shown.
In contrast to PPC-1 cells, mRNA for class I MHC heavy chain, β2-microglobuIin, and TAP-2 was detectable in LNCaP cells (Fig. 3). Although interferon gamma restored TAP-2 mRNA to abundant levels in PPC-1, mRNA levels of all three class I MHC antigen processing components failed to respond to interferon gamma stimulation in LNCaP cells. Densitometry of the Northern blot (Fig. 3) confirmed that, in each of the prostate cancer cell lines except LNCaP, there was an increase in expression of class I MHC assembly components following interferon gamma stimulation (data not shown). Deficient expression of class I MHC by LNCaP cells was not caused by the same reversible basal transcription defect as that seen in PPC-1.
FACS analysis confirmed that restoration of TAP-2 mRNA in PPC-1 cells following interferon gamma stimulation was associated with increased expression of assembled class I MHC complex at the cell surface (Fig. 4), which is consistent with restoration of competent antigenic peptide transport and class I MHC assembly via restoration of TAP-2 expression. In contrast, low levels of class I MHC assembly in LNCaP cells, as measured by FACS (Fig. 4), did not respond to interferon gamma. This inability of interferon gamma to affect class I MHC assembly at the cell surface of LNCaP was consistent with absent interferon gamma induction of mRNA for class I MHC heavy chain, β2-microglobulin, and TAP-2 by Northern blot analysis (Fig. 3).
Fig. 4.
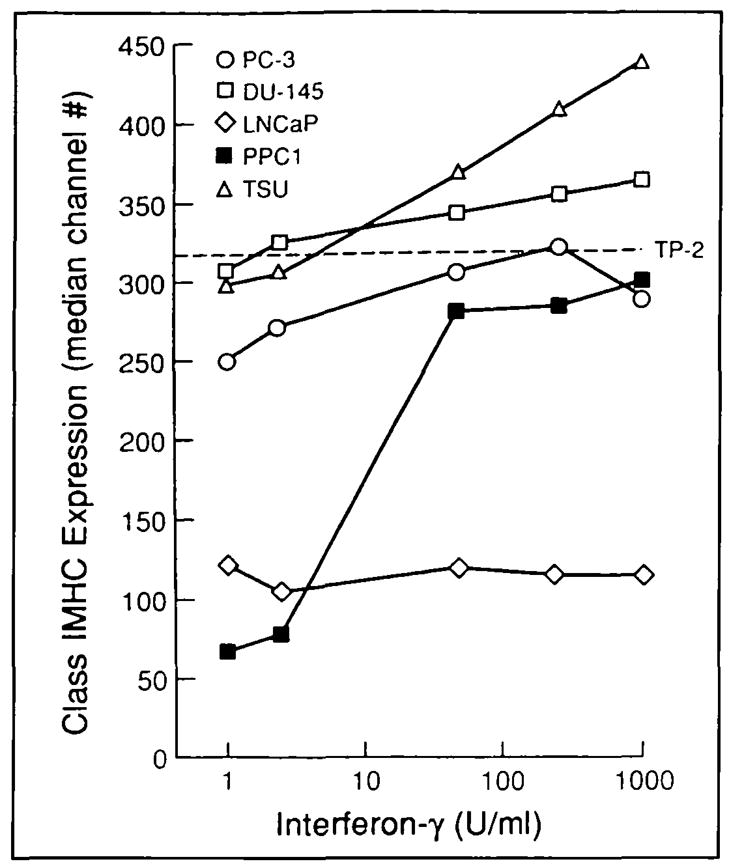
Defective class I MHC expression in human prostate cancer restored using interferon gamma (interferon-γ). Surface expression of class I MHC was measured by FACS 24 hours after culture of human prostate cancer cell lines with interferon gamma. Base-line class I MHC expression by normal prostate-derived TP-2 (dashed line) is shown as a reference standard. PPC-l cells demonstrate a significant, dose-dependent response to interferon gamma (P<.01; t test with pooled variance), whereas LNCaP underexpression of class I MHC is refractory to interferon gamma. Responses to interferon gamma appeared after 8 hours of culture, and stimulation for up to 3 days revealed similar responses (not shown). Three experiments were performed with similar results.
The functional consequences of human prostate cancer-defective antigen processing were also investigated. For this purpose, a chimeric assay of antigen processing and presentation was applied (14). In this assay, vaccinia vector is used to transfect human tumor cells with mouse Kd heavy-chain gene expressed from the vaccinia P7.5 (early/late) promoter. Subsequent constitutive expression of the mouse class I Kd gene by these human cancer cells presenting vaccinia antigen allows them to serve as targets for lysis by vaccinia-specific CTL from in vivo-primed BALB mice. CTL-specific lysis in this assay demonstrates competent function of human genes in antigenic peptide processing, transport, and class I MHC assembly; only class I MHC heavy-chain expression itself remains untested in this assay because heavy chain is constitutively supplied in the form of murine Kd.
Immortalized human normal prostate cells (TP-2) as well as DU-145, PC-3, and TSU human prostate cancer cells were lysed by vaccinia-specific CTL, indicating that a general CTL evasion phenotype is not characteristic of all prostate epithelial cells. In contrast, PPC-1 was not lysed in this assay (Fig. 5). These results show that the transcriptional defect in TAP-2 expression (demonstrated by Northern blot analysis) is clearly associated with a functional defect in cancer cell antigen processing and presentation. In contrast, replacement of human class I MHC with murine class I Kd rendered LNCaP susceptible to CTL-mediated lysis (Fig. 5), indicating that antigen processing components (other than the human class I MHC heavy chain itself) were functional and sufficient for antigen presentation to CTL, despite the relatively low levels of mRNA seen on Northern blots.
Fig. 5.
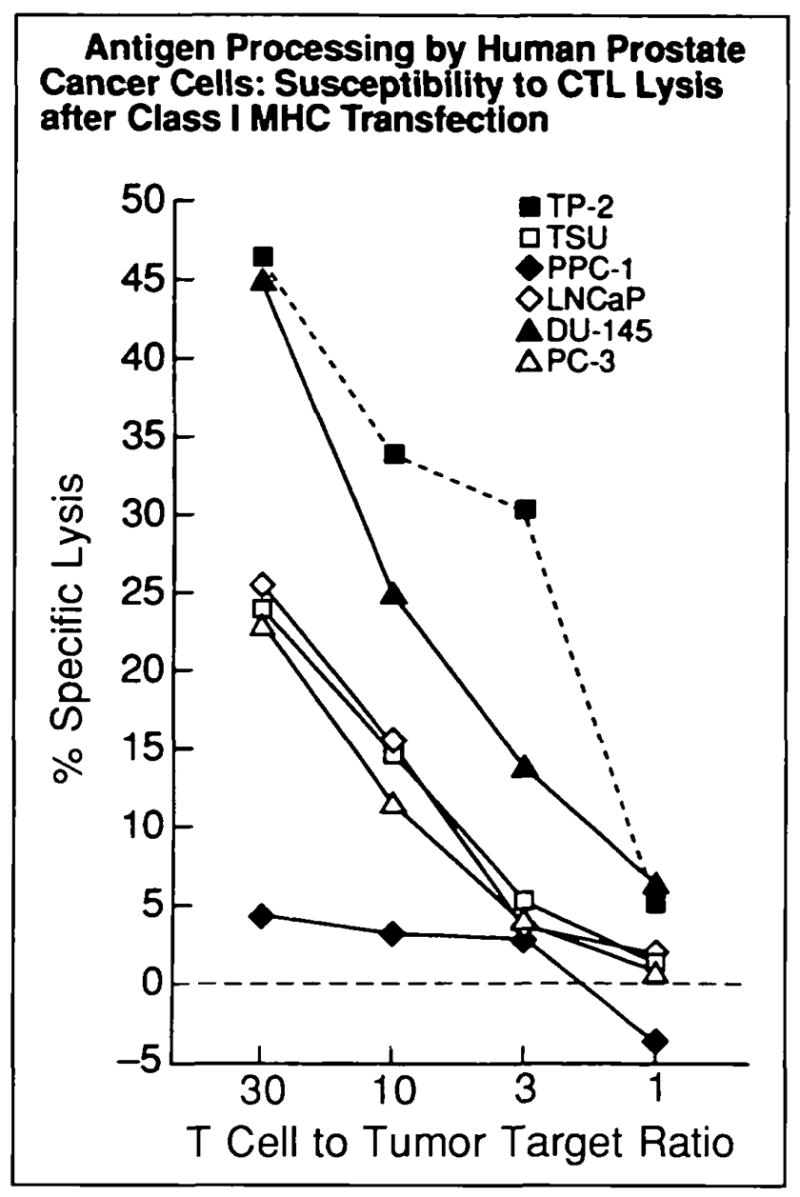
Class I MHC-mediated antigen processing by human prostate cancer. Class I MHC-restncted T-cell killing targeted via a model viral antigen was used t o measure antigen processing and transport by normal and cancerous human prostate. Class I MHC-restricted, vaccinia antigen-specific lysis (y axis vaccinia antigen presented by mouse Kd class I MHC transfected into tumor target cells using vaccinia vector) was measured by chromium release. Dashed line indicates normal prostate control cells; solid lines indicate metastasis-derived human prostate cancer cells. Each data point represents a mean of triplicate measurements (SE consistently <10%); this experiment was repeated twice with similar results. T-cell killing of all lines except PPC-1 was significant (P<.01).
The effect of specifically reversing the TAP-2 transcriptional defect by TAP-2 message induction with interferon gamma (Fig. 3) was also tested using this functional assay (Fig. 6). Transcriptional upregulation of TAP-2 expression was associated with restoration of competent peptide processing and transport; induction of TAP-2 rendered PPC-1 susceptible to specific, Kd-restricted lysis. The absence of lysis of control PPC-1 targets transfected with wild-type vaccinia virtually eliminates the possibility that CTL lysis in this assay occurs from nonspecific, antigen-independent cellular interactions. Susceptibility of LNCaP t o lysis by CTL was expectedly unchanged after interferon gamma stimulation in this assay (Fig. 6), consistent with the unresponsiveness of this cell line to interferon gamma as determined by FACS analysis and Northern blot hybridization.
Fig. 6.
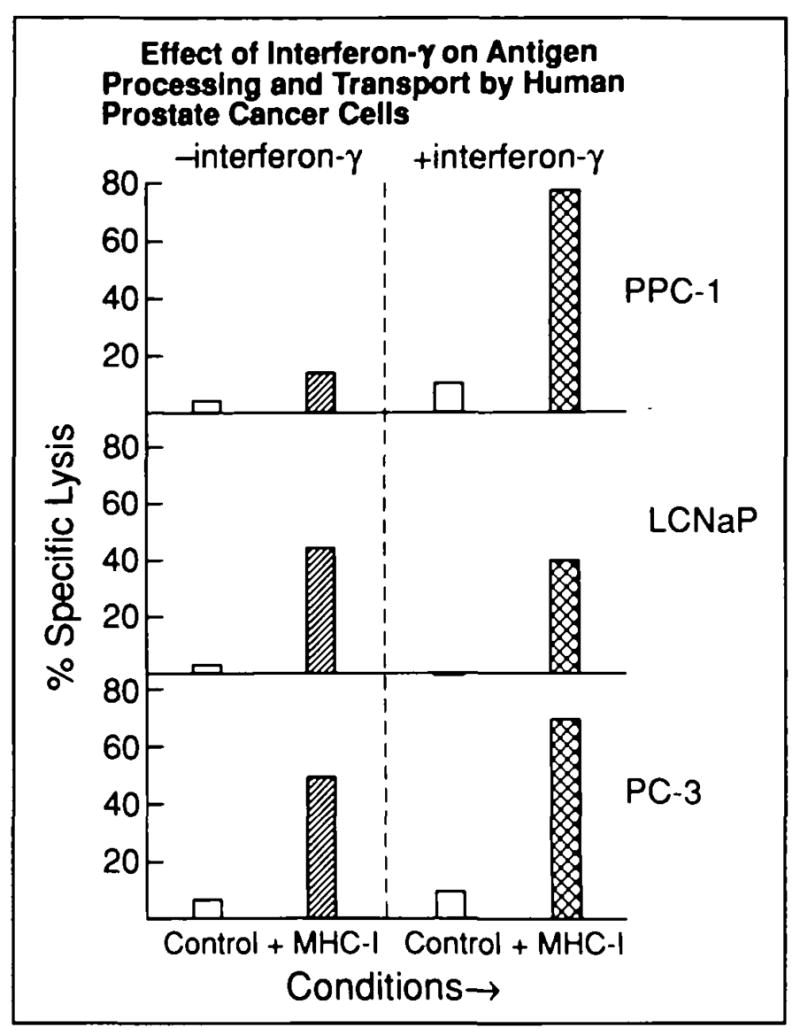
Prostate cancer cell lines PPC-1, LNCaP, and PC-3 were cultured with or without 250 U/mL of interferon gamma (interferon-γ) for 24 hours, and their susceptibility to CTL-mediated killing was then measured. Parallel conditions are identified on the x axis: clear columns (control lysis) = lysis of cells transfected with wild-type vaccinia (lacking Kd) or nonspecific, background lysis (with or without interferon gamma stimulation of targets prior to transfection); single-hatched columns (class I MHC-specific lysis) = lysis of cancer cells transfected with vac-Kd; cross-hatched columns (class I MHC-specific lysis) = lysis of cancer cells transfected with vac-Kd after interferon gamma treatment. Tumor lysis (T cell to tumor ratio = 30.1) is shown on y axis for each condition; each value represents a mean of triplicate measurements (SE consistently <10%). Lysis was T-cell dependent as in Fig. 5 (10:1, 3:1, and 1:1 dilutions not shown here). Increase in PPC-1 killing after interferon gamma treatment was statistically l significant (P<.005; pooled variance t test).
Discussion
The efficacy of gene-modified tumor vaccines in treating animals with hormone-refractory prostate cancer has been described (8,19,20). Translating preclinical studies of molecular immunotherapy to rational clinical strategies of therapy requires a rigorous understanding of immune-resistance mechanisms operative in human prostate cancer. Studies were conducted not only to characterize class I MHC expression on the cell surface of metastatic human prostate cancer cells but also to identify underlying molecular mechanisms responsible for human prostate cancer phenotypes capable of evading recognition by CTL.
Two of five metastasis-derived human prostate cancer cell lines (LNCaP and PPC-1) were defective in cell surface expression of class I MHC when compared with other prostate cancer cell lines and with a control prostate epithelial cell line derived from normal tissue. These findings confirm that deficient class I MHC-mediated antigen presentation, permitting evasion of CTL recognition, can occur in human prostate cancer progression and metastasis, consistent with immunohistochemical studies of class I MHC expression in other urological malignancies (21). Analysis of mRNA demonstrated that PPC-1 cells specifically underexpressed TAP-2, a critical mediator of antigen transport and consequent class I MHC assembly. Class I MHC heavy- and light-chain mRNA levels were unexpectedly abundant. A chimeric assay using vaccinia vectors demonstrated that the escape of PPC-1 cells from CTL-mediated recognition and killing was associated with the defective TAP-2 expression.
Although a prior study has identified deficient TAP expression in human small-cell lung carcinoma cell lines (14), these cell lines also underexpressed class I MHC heavy-chain message (Restifo NP: unpublished data). Functionally, PPC-1 mimics the radiation-induced murine cell line RMA-S and the human somatic fusion hybrid T-2 (16–18); to our knowledge, a metastasis-derived human cell line has not been described with such a specific defect in antigen transport (not associated with concurrent defects in class I MHC or β2-microglobulin message expression).
Previously described mechanisms underlying specific defects in class I MHC-associated antigen transport (as seen in somatic cell fusion or radiation-induced mutants) have involved abnormalities in coding sequences of TAP genes; RMA-S cells exhibit a premature stop in the message of the mouse TAP-2 gene, and T-2 cells demonstrate loss of the entire class II region encoding TAP-2 as well as TAP-1 (16–18). In contrast, we found restoration of TAP-2 message expression (Fig. 3) and functional antigen transport (Fig. 6) after transcriptional stimulation by interferon gamma, indicating the presence of inducible and functional coding sequences for TAP-2 mRNA in PPC-1 cells. These findings provide clear evidence of a constitutive pathway governing TAP gene expression (distinct from the interferon gamma response element), which cancer cells can dysregulate to the potential advantage of immune evasion.
In contrast to the responsiveness of PPC-1 cells to interferon gamma, LNCaP cells, which underexpress class I MHC at the cell surface, did not respond to transcriptional stimulation by interferon gamma (Fig. 4). The possibility of androgen-modulated proteolysis of interferon gamma by LNCaP cells has not been ruled out and is the subject of ongoing studies. The chimeric assay of antigen presentation demonstrated that replacement of class I MHC heavy chain with murine Kd was sufficient to restore competent antigen presentation by LNCaP cells (Fig. 6), indicating that gene products from the TAP-2 and β2-microglobulin genes were functional in these cells and suggesting that deficient class I MHC assembly in this cell line is related specifically to abnormal heavy-chain gene product function. Because class I MHC message is abundant in LNCaP, the possibility of gene mutations or defective post-transcriptional processing of human class I MHC heavy chain by this cell line is raised. Although not entirely elucidated, the underlying mechanism responsible for deficient class I MHC assembly in LNCaP cells clearly differs from that present in PPC-1 cells.
These studies show that class I MHC antigen presentation can be functional or defective in metastatic human prostate cancer cells. Functional class I MHC antigen processing and presentation defects are clearly one mechanism of prostate cancer immune evasion that must be accounted for by future rational immunotherapies for human prostate cancer. The PPC-1 cell line demonstrates a novel regulatory defect among cancers in general, showing underexpression of the antigen transporter subunit TAP-2 independent of class I MHC heavy-chain expression. As evidenced by LNCaP cells, however, mechanisms underlying defective class I MHC assembly in human prostate cancer include alterations that are not consistently reversible with interferon gamma. Fortunately, gene transfer-based strategies of immunotherapy using the class II MHC pathway have recently been identified that circumvent the need for class I MHC-mediated recognition of cancer cells by CTL (9,10). Based on the heterogeneity of mechanisms underlying class I MHC underexpression in human prostate cancer, such strategies are potentially advantageous in clinical protocols of prostate cancer immunotherapy that use cytokine gene-transduced cancer cell vaccines.
Acknowledgments
Supported by Public Health Service (PHS) grant CA58236 from the National Cancer Institute (NCI), National Institutes of Health (NIH), Department of Health and Human Services (DHHS). J. W. Simons was supported by PHS grant CA01495 of NCI, NIH, DHHS.
References
- (1).Walsh PC. Prostate cancer kills: a strategy to reduce deaths. Urology. 1994;44:463–466. doi: 10.1016/s0090-4295(94)80039-1. [DOI] [PubMed] [Google Scholar]
- (2).Golumbek PT, Lazenby AJ, Levitsky HI, et al. Treatment of established renal cell cancer by tumor cells engineered to secrete interleukin-4. Science. 1991;254:713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- (3).Asher AL, Mulé JJ, Kasid A, et al. Murine tumor cells transduced with the gene from tumor necrosis factor-alpha. Evidence for paracrine immune effects of tumor necrosis factor against tumors. J Immunol. 1991;146:3227–3234. [PMC free article] [PubMed] [Google Scholar]
- (4).Restifo NP, Spiess PJ, Karp SE, et al. A nonimmunogenic sarcoma transduced with the cDNA for interferon gamma elicits CD8+ T cells against the wild-type tumor: correlation with antigen presentation capability. J Exp Med. 1992;175:1423–1431. doi: 10.1084/jem.175.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Trojan J, Johnson TR, Rudin SD, et al. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993;259:94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- (6).Porgador A, Tzehoval E, Katz A, et al. Interleukin 6 gene transfection into Lewis lung carcinoma cells suppresses the malignant phenotype and confers immunotherapeutic competence against parental metastatic cells. Cancer Res. 1992;52:3679–3686. [PubMed] [Google Scholar]
- (7).Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Sanda MG, Ayyagari SR, Jaffee EM, et al. Demonstration of a rational strategy for human prostate cancer gene therapy. J Urol. 1994;151:622–628. doi: 10.1016/s0022-5347(17)35032-2. [DOI] [PubMed] [Google Scholar]
- (9).Levitsky HI, Lazenby A, Hayashi R, et al. In vivo priming of two distinct antitumor effector populations: the role of MHC class I expression. J Exp Med. 1994;179:1215–1224. doi: 10.1084/jem.179.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Connor J, Bannerji R, Saito S, et al. Regression of bladder tumors in mice treated with interleukin 2 gene-modified tumor cells [published erratum appears in J Exp Med 177:following 1831, 1993] J Exp Med. 1993;177:1127–1134. doi: 10.1084/jem.177.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Isaacs WB. Immortalization of normal human prostate epithelial cells by a retrovirus expressing the adenovirus E1A gene. J Urol. 1992;147:415a. [Google Scholar]
- (12).Schneider WC. Determination of nucleic acids in tissues by pentose analysis. Methods Enzymol. 1957;3:680. [Google Scholar]
- (13).Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual, Section 7. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. Electrophoresis of RNA through gels containing formaldehyde; pp. 7.43–7.45. [Google Scholar]
- (14).Restifo NP, Esquivel F, Kawakami Y, et al. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Shott S. Hypothesis testing. In: Shott S, editor. Statistics for Health Professionals. Philadelphia: Saunders; 1990. pp. 105–143. [Google Scholar]
- (16).Monaco JJ, Cho S, Attaya M. Transport protein genes in the murine MHC: possible implications for antigen processing. Science. 1990;250:1723–1726. doi: 10.1126/science.2270487. [DOI] [PubMed] [Google Scholar]
- (17).Trowsdale J, Hanson I, Mockridge I, et al. Sequences encoded in the class II region of the MHC related to the ‘ABC’ superfamily of receptors [see comment citation in Medline] Nature. 1990;348:741–744. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- (18).Spies T, Bresnahan M, Bahrman S, et al. A gene in the human major histocompatability complex class II region controlling the class I antigen processing pathway [see comment citation in Medline] Nature. 1990;348:744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- (19).Moody DB, Robinson JC, Ewing CM, et al. Interleukin-2 transfected prostate cancer cells generate a local antitumor effect in vivo. Prostate. 1994;24:244–251. doi: 10.1002/pros.2990240505. [DOI] [PubMed] [Google Scholar]
- (20).Vieweg J, Rosenthal FM, Bannerji R, et al. Immunotherapy for prostate cancer in the Dunning rat model, use of cytokine gene modified tumor vaccines. Cancer Res. 1994;54:1760, 1765. [PubMed] [Google Scholar]
- (21).Cordon-Cardo C, Fuks Z, Drobnjak M, et al. Expression of HLA-A,B,C antigens on primary and metastatic tumor cell populations of human carcinomas. Cancer Res. 1991;51(23 Pt 1):6372–6380. [PubMed] [Google Scholar]


