Abstract
Some bifidobacteria or lactobacilli exhibit a variety of immunomodulatory effects, such as being anti-inflammatory, increasing IgA secretion, and moderating allergy. We prepared three types of Bifidobacterium components from B. pseudocatenulatum JCM 7041 (Bp) using preparation methods such as sonication, heat treatment, and non-treatment (live Bp). Furthermore, we compared their immunomodulatory effects using in vivo and in vitro immunological bio-assays. We determined immune responses such as cell proliferation and the production of cytokines and IgA in Peyer’s patch cells in vitro following co-culture with bacterial components, and investigated the effects of oral administration of each of them on cytokine and IgA production by Peyer’s patch cells. Live-, ultrasonic treated- and heat-treated Bp exhibited cytokine-inducing and cell proliferation activities. Sonicated Bp in particular showed the greatest immunomodulatory activity in the short term as measured by in vitro and in vivo assays, while heat-treated Bp induced cytokines (e.g. IL-6 and IFN-γ) and IgA production following oral administration for 7 consecutive days. These data showed that Bifidobacterium components prepared by different methods might induce different immune responses. Using scanning electron microscopy we demonstrated that the surface structure of sonicated Bp, which contained more soluble saccharides, was different from other components. These data suggest that the immunomodulatory effect of Bp is dependent upon the bacterial conformation and condition.
Keywords: Bifidobacterium, Probiotics, Immunomodulator, Preparation, IgA, IL-12p40
Introduction
Lactic acid bacteria (LAB) such as some Lactobacillus and Bifidobacterium exhibit various immunomodulatory effects. Ohno et al. (2005) reported that oral administration of Bifidobacterium bifidum G9-1 to mice selectively suppressed total and antigen-specific IgE production by regulation of IL-4, IL-5 and IFN-γ production from splenocytes. Also, oral administration of Lactobacillus casei strain Shirota suppressed the increased IgE titer in OVA-specific receptor transgenic mice, thereby suppressing the allergic reaction (Shida et al. 2002).
Mohamadzadeh et al. (2005) reported that three species of Lactobacillus (L. gasseri; ATCC no. 19992, L. johnsonii; ATCC no. 33200 and L. reuteri; ATCC no. 23272) clearly skewed CD4+ and CD8+ T cells towards T helper 1 cells through activation of human dendritic cells (DCs). Another species, Lactobacillus GG, was associated with increased IgA production (He et al. 2005). Lactobacillus johnsonii (NCC 533) was associated with increased IgA production from Peyer’s patch whole-organ culture supernatants and IgG1 production in the serum. Lactobacillus paracasei (NCC 2461) was associated with increased IgG2a in the serum (Ibnou-Zekri et al. 2003). We previously reported that when Bifidobacterium immunomodulator (BIM), prepared by ultrasonic disintegration, and lyophilized Bifidobacterium pseudocatenulatum JCM 7041 (Bp) was administered orally to female BALB/c mice for 7 consecutive days, IgA production and some cytokine production in Peyer’s patches of the small intestine increased (Nakanishi et al. 2005).
Taken together, these studies indicated that the variety of immunomodulatory effects by LAB was dependent on the bacterial species. In addition, in these studies, bacterial components were prepared by different methods such as sonication, heat treatment and non-treatment as in live bacteria. This indicated that immune responses are induced by dead cells or bacterial components, as well as by live bacteria. Furthermore, the reported findings suggest that the variety of immunomodulatory effects induced by LAB might be related to the condition of the bacteria. Indeed, Kishi et al. (1996) reported that IFN-α production significantly increased following oral administration of live Lactobacillus brevis subsp. coagulans, but not after oral administration of heat-treated bacteria.
The gut has a highly developed and unique immune system, capable of distinguishing various species of bacteria. However, it has not been clarified how specific bacterial components are related to the recognition of the bacteria and to the immunomodulation by the mucosal immune system in the gut. Furthermore, it is currently unknown how the immunomodulatory function of probiotic bacteria prepared by different method affects mucosal immune responses in the intestine. Here we examined the relationship between the chemical condition of Bifidobacterium and immunomodulatory effects by using in vivo and in vitro immunological bio-assays. The results show that immunomodulatory characteristics are dependent on the differences of bacterial condition.
Materials and methods
Mice
Seven-week-old female BALB/c mice (CLEA Japan, Inc., Tokyo, Japan) were maintained in plastic cages at 23 ± 2 °C, at a relative humidity of 50 ± 10%, and under 12 h light cycles (illuminated from 8:00 to 20:00). During the first 3 days of the acclimatization period, mice were allowed free access to solid chow MF (Oriental Yeast Co., Ltd., Tokyo, Japan) and ion-exchanged water. The animal experiments in this study were carried out in accordance with the ‘Guidelines for the Care and Use of Laboratory Animals’ by the College of Bioresource Sciences, Nihon University.
Bacteria
Bifidobacteriumpseudocatenulatum JCM 7041 (Bp), isolated through screening by Lee et al. (1993) was obtained from RIKEN (Bioresource, Japan collection of microorganisms, Tokyo, Japan). Bp was cultured in GAM broth at 37 °C for 18 h.
Preparation of Bp components
Cultured Bp were washed three times in PBS, and suspended to a final cell density of 1 × 107 (or 1 × 108) CFU mL−1 (live Bp). Sonicated Bp (SB) were prepared by ultrasonicating live Bp for 15 min. Heat-treated Bp (HT) were prepared by heating at 85–95 °C for 30 min. Bp prepared by this method were was used for in vitro assay, and for oral administration.
Proliferation assay
Peyer’s patch (PP) cells (2.5 × 105 cells mL−1) were obtained from 7-week-old female BALB/c mice, and cultured with each prepared Bp component (1 × 107 CFU) in RPMI 1640 medium containing 5% FCS in 96-well plates for 36 h. The proliferation activity of PP cells was measured by the [3H]-thymidine uptake test according to a previously reported method (Hosono et al. 1997). [3H]-thymidine uptake was measured with a liquid scintillation counter, LSC-6100 (ALOKA, Tokyo, Japan).
Measurement of IL-12p40 from PP cells after 12 h incubation following single-shot oral administration of each prepared Bp component
Live Bp cells, or each prepared Bp component, were suspended in PBS to a final concentration of 1 × 108 CFU 200 mL−1 per mouse and orally administered by gavage using a feeding tube. Mice in the control group were administered the same amount of PBS only. To obtain PP cells, experimental group mice were sacrificed 24 h after a single-shot administration. PP cells were co-cultured with BIM (10 μg mL−1) for 24 h. After co-cultivation, PP culture supernatant was obtained and IL-12p40 was measured by ELISA according to the previously reported methods (Hosono et al. 1998; Nakanishi et al. 2005).
Immunological assay of PP cells after oral administration of Bifidobacterium for 7 consecutive days
Live Bp cells or each prepared Bp component was suspended in PBS to a final concentration of 1 × 108 CFU 200 mL−1 per mouse and orally administered by gavage using a feeding tube. Mice in the control group were administered the same amount of PBS only. After oral administration for single-shot or 7 consecutive days, cells were prepared from PP, and the amount of IgA, IL-6, IL12p40 and IFN-γ produced by PP cells was measured by ELISA according to the previously reported methods (Hosono et al. 1998; Nakanishi et al. 2005).
Scanning electron microscopy
Scanning electron microscopy observations were performed to assess the effects of sonic treatment and heat treatment on the conformation of Bp. Each Bp sample (e.g. live, sonicated and heat-treated Bp) was washed with 0.1 M phosphate-buffer (PB) three times and suspended to a final concentration of 1 × 106 to 108 CFU after each treatment. For fixation, cold 2% osmium tetroxide solution was added to centrifuged Bp pellets and incubated for 3 h in the dark at 4 °C. After incubation, samples were washed with 0.1 M PB three times. Samples were dehydrated with graded ethanol (30, 50, 70, 80 and 90%) and ethanol/acetone (ethanol:acetone, 90%:90%, 90%:95% and 95%:100%), and suspended in isoamyl acetate. Samples were dried at the critical point of liquid CO2, and coated with carbon. Prepared Bp was observed with the aid of a 3D Real Surface View Microscope VE-8800 (KEYENCE, Tokyo, Japan) at an acceleration voltage of 25 kV.
Measurement of natural carbohydrate content from Bp components
Natural carbohydrate content of Bp was measured by the phenol-sulfuric acid method. Bp samples were suspended in PBS to a final concentration of 2.5 × 107 CFU 500 μl−1. The ultrasonic treatment condition was for 15 min. Heat-treated Bp (HT) was prepared by heating at 85–95 °C for 30 min. Additionally, we separated the supernatants and pellets by centrifugation of each Bp component at 5,500 × g for 3 min. d-mannose was used as a standard (on 0–10 scale; 0–100 mg mL−1). First of all, 0.5 mL of 5% phenol solution was added, followed by 2.5 mL of conc. sulfuric acid to each sample with vigorous stirring for 10 s. Optical density was then read at 490 nm within 20 min.
Statistical analysis
Data are expressed as means ± SD. Differences were examined by one-way analysis of variance (ANOVA), and significant differences found between groups further evaluated by Tukey’s test (SPSS Ver. 13.0, Chicago, IL, USA). Differences were considered significant at p < 0.05.
Results
SB has the strongest immunomodulatory effect on immediate immune responses of prepared Bp components
To determine whether the bacterial components prepared from Bp had immunomodulatory effects, we conducted the [3H]-thymidine uptake test and measured IL-12p40 production. All Bp components induced IL-12p40 production and the proliferation of PP cells in vitro. We then investigated the immunomodulatory effects of Bp components following a single-shot oral administration of each Bp component by measuring IL-12p40 production by PP cells. IL-12p40 production in both in vitro (Fig. 1A) and in vivo (Fig. 1C) experiments as well as the proliferation of PP cells (Fig. 1B) were significantly increased following administration of each prepared Bp component. SB was shown to be the highest inducer of immune responses. HT and live-Bp also significantly increased IL-12p40 production and cell proliferation in PP cells.
Fig. 1.
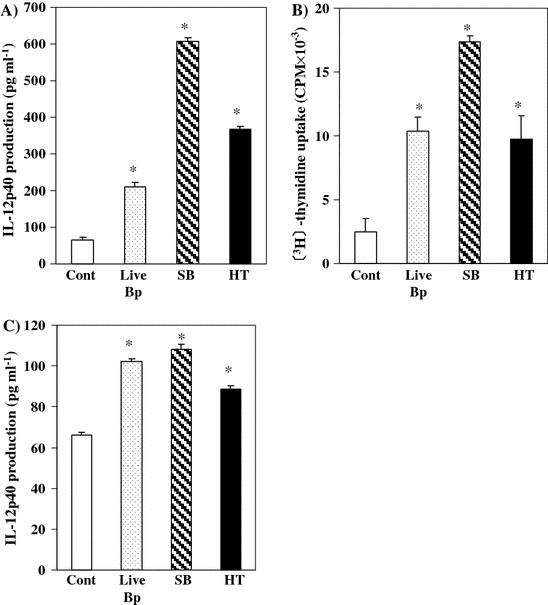
Immune responses of PP cells after stimulation by each prepared Bp component in the short term. Culture supernatant IL-12p40 (A) and PP cell proliferation activity (B) after addition and incubation with each prepared Bp component in vitro. PP cells were obtained from BALB/c mice and pooled. The cells were co-cultured with live-Bp (shadow bar) (1 × 107 CFU), sonicated Bp (hatched line bar) and heat-treated Bp (black bar) for 24 hours or 36 hours. The culture supernatant IL-12p40 was measured by ELISA. PP cell proliferation activity was evaluated by [3H]-thymidine uptake test. Culture supernatant IL-12p40 (C) from PP after single oral administration of live-Bp (1 × 108 CFU), sonicated Bp and heat-treated Bp (black bar). PP cells were obtained and pooled for each experimental group respectively. The cells were co-cultured with BIM 10 μg mL−1 for 24 hours. The culture supernatant IL-12p40 was measured by ELISA. *Significantly different (p < 0.05; Tukey’s test) from control group. The results shown are representative of three independent experiments
We also investigated the effect of repeated oral administration of each prepared Bp component for 7 consecutive days on the amount of IL-12p40 production by PP cells. Although IL-12p40 production was increased by single-shot administration of each of the Bp component, it was not induced by the oral administration for 7 consecutive days (data not shown). Furthermore, no differences in the level of IL-12p40 production were observed following oral administration of PBS (control), live-Bp, SB, or HT for 7 consecutive days (data not shown). Thus, each of the Bp components prepared by different methods demonstrated immunomodulatory effects, but immunomodulatory activity differed for each preparation. In particular, SB demonstrated a strong and immediate immune response as compared to other Bp components prepared.
Of the prepared Bp components, repeated oral administration of HT induced the production of the highest levels of cytokines and IgA
The effect of repeated oral administration of each Bp component for 7 consecutive days on the amount of IgA and various cytokines produced by PP cells was investigated. IL-6 production by PP cells was significantly increased by oral administration of SB and HT groups. However, IL-6 production by PP cells was not changed by oral administration of live-Bp for 7 consecutive days (Fig. 2A). IFN-γ production by PP cells was significantly increased by oral administration of each prepared Bp component group for 7 consecutive days compared with the PBS-control group (Fig. 2B).
Fig. 2.
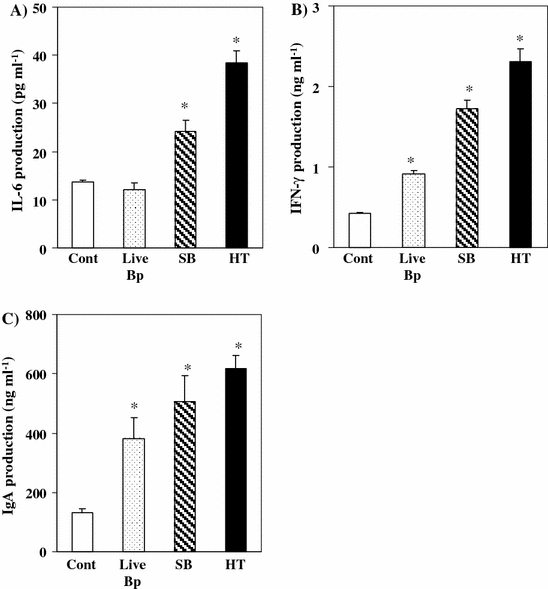
Immunomodulatory effects of oral administration for 7 consecutive days of each prepared Bp component. Culture supernatant IL-6 (A), IFN-γ (B), and IgA (C) from PP cells after oral administration of live-Bp (shadow bar) (1 × 108 CFU), sonicated Bp (hatched line bar) and heat-treated Bp (black bar) for 7 consecutive days. PP cells were obtained and pooled for each experimental group respectively. The cells were co-cultured with BIM 10 μg mL−1 for 24 h, 72 h or 7 days. Culture supernatant cytokines and IgA were measured by ELISA. *Significantly different (p < 0.05; Tukey’s test) from control group. The results shown are representative of three independent experiments
IgA production by PP cells was significantly increased by oral administration of each prepared Bp component group. HT was shown to be a more effective immunomodulator of the production of cytokines and IgA than SB and live-Bp following repeated oral administration.
The conformation of Bp was changed by ultrasonic treatment
Ultrasonic treatment is a physical disruption method, while heat treatment induces heat denaturation of bacterial proteins. For these reasons, it was predicted that the conformation of Bp might be affected differently by each treatment method. On this basis, we studied the conformation of Bp using scanning electron microscopy.
Scanning electron microscopy was performed to observe the conformation of Bp following the different preparation methods (Fig. 3). Live Bp was shown to be clavate in form (Fig. 3A), while the cell walls of Bp were seen to be ruptured by ultrasonic treatment (Fig. 3B, C). The conformation of Bp was not affected by heat treatment (Fig. 3D).
Fig. 3.
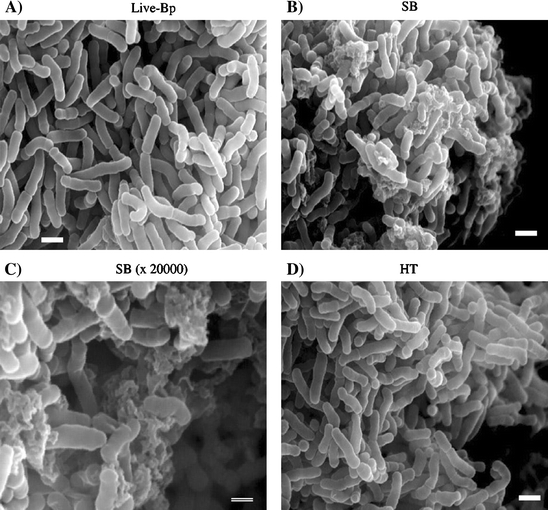
Scanning electron microscopy image of each treated Bp. Scanning electron microscopy images of live-Bp (A), sonicated Bp (B and C) and heat-treated Bp (D) were obtained by 3D Real Surface View Microscope VE-8800 at an acceleration voltage of 25 kV. The bar indicates 1 μm (A, B, and D), and the double line indicates 500 μm (C)
Natural carbohydrate content in supernatants from Bp was increased by ultrasonic treatment
The conformation of Bp was changed by ultrasonic treatment, indicating that polysaccharides, as chief cell wall components, might diffuse into the soluble fraction from the insoluble cell wall. Therefore we measured the levels in supernatants of natural carbohydrates derived from Bp.
Detectable amounts of natural carbohydrates in supernatants were significantly increased by ultrasonic treatment (Fig. 4A), while those detected in pellets were significantly decreased by this treatment (Fig. 4B). Moreover, the detectable amounts of natural carbohydrates in supernatants were dependent on the degree of ultrasonic treatment (data not shown). We observed no differences between live-Bp and heat-treated Bp on the detectable natural carbohydrates in each supernatant fraction (Fig. 4A). The levels of natural carbohydrates in the pellet fraction of SB were significantly lower than in other Bp components (Fig. 4B).
Fig. 4.
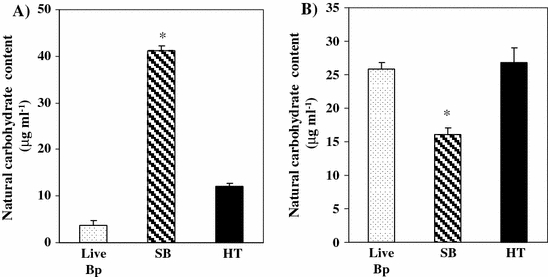
Effect of ultrasonic and heat treatment of Bp on natural carbohydrate content. Natural carbohydrate content from supernatant (A) or pellet (B) was measured by the phenol-sulfuric acid method. Live Bp as control (shadow bar) was suspended in PBS to a final concentration of 2.5 × 107 CFU 500 μl−1. Ultrasound conditions were set for 15 min. HT was prepared by heating at 85–95 °C for 5 min or 30 min (black bar). Optical density was read at 490 nm. *Significant difference of SB from both control and HT groups at p < 0.05 (Tukey’s test). The results shown are representative of two independent experiments
Discussion
In this study, we demonstrated that Bp components prepared by different methods have immunomodulatory effects, but that the immunomodulatory activity was dependent on the method of preparation of Bp components. In particular, the amount of IL-12p40 production was significantly increased by the administration of SB both in the vitro assay and in the single-shot administration in vivo (Fig. 1).
Lee et al. (1993) reported that Bifidobacterium adolescentis M101-4, which was the origin of Bp and derived from human intestinal microflora, had the strongest mitogenic activity on the lymphocytes of mice. In addition, this activity was increased by destruction of the organism. Furthermore, Hosono et al. (1997) characterized the soluble high molecular weight fraction as being immunoactive polysaccharides derived from this strain.
In the present study, scanning electron microscopy confirmed that the Bp cell wall was ruptured by ultrasonic treatment (Fig. 3). In addition, we demonstrated that natural carbohydrates in the supernatant fraction were increased by ultrasonic treatment (Fig. 4). These results suggested that Bp contained immunomodulatory carbohydrates from either the cell wall or cytoplasm, and that the ultrasonic treatment of Bp might facilitate the sensitization of immunocytes or might facilitate the uptake of Bp into the immune tissues in the gut. We demonstrated that sonicated Bp components increased IL-12p40 production by PP cells markedly in in vitro assay, and that the conformation of the cells, including carbohydrate content, was changed dramatically by the ultrasonic treatment. These results strongly suggested that the immunomodulatory effects caused by Bp stimulation might be related to bacterial polysaccharides.
Many reports have indicated that IL-12p40 production by DCs and macrophages (Mϕ) was induced by stimulation with bacterial components through pattern-recognition receptors such as the toll-like receptors (TLRs) and the nucleotide-binding oligomerization domain (NOD) (Uehara et al. 2002; Newton et al. 2007). In particular, it has previously been reported that CpG-DNA is the main promoter of IL-12p40 (Albrecht et al. 2004).
Li et al. (2005) demonstrated that IL-1β, IL-6, IL-12p40, and TNF-α production by the murine macrophage cell line, J774A.1, was increased by CpG-DNA derived from bifidobacteria. We have previously demonstrated that orally administered Bp directly induced innate immune cell responses through phagocytosis by cells such as DCs and macrophages (unpublished data).
In this study, we demonstrated that Bp cell walls were disrupted by ultrasonic treatment. It was possible that exposure of pathogen-associated molecular patterns (PAMPs) such as peptidoglycans, lipoteichoic acids, and unmethylated CpG-DNA might increase with disruption of Bp cell walls by ultrasonic treatment, while the conformation of live-Bp cell walls and heat-treated Bp cell walls were not changed. The results here suggested that increased IL-12p40 production by DC and Mϕ might correlate with exposure of PAMPs following disruption of Bp cell walls by ultrasonic treatment and that bacterial conformation may correlate with the induction of an innate immune reaction.
Although Bp components increased IL-12p40 production by PP cells 24 h after a single-shot oral administration (Fig. 1C), IL-12p40 level was not shown to be different by the oral administration for 7 consecutive days among the experimental groups (data not shown). It was reported that bacterial stimulation induces IL-12 and IL-10 by phagocytes such as Mϕ and DC (Christensen et al. 2002; Ratajczak et al. 2007). We previously demonstrated that oral administration of Bifidobacterium components for 7 consecutive days induced IL-10 production by Thy1.2− PP cells (unpublished data). Furthermore, we confirmed that IL-10 production was increased by oral administration of Bp components for 7 consecutive days (data not shown). It is suggested that IL-10 is a typical anti-inflammatory cytokine which can regulate the IL-12 production by inhibition of pro-inflammatory cytokines (Dennis et al. 2006). Then we can suggest that IL-12p40 production might be induced in the early phase of the oral administration of Bp, and might be down-regulated by IL-10 during 7 days Bp feeding.
Heat treatment might induce the thermal denaturation of proteins on the cell surface of Bp. Although we could not detect the chemical changes in proteins caused by heat treatment of Bp, remarkably, heat treatment of bacterial components can induce alterations in bacterial immunomodulatory effects, e.g. acquired immune responses such as IgA production.
Whitaker and Batt (1991) previously reported that Lactococcus lactis subsp. lactic synthesized heat shock protein under thermal stress. Furthermore, immunological characterizations of this strain changed with the amount of synthesized protein. In another report, the TNF-α inducing activity of Lactobacillus brevis subsp. coagulans was eliminated following heat treatment (Kishi et al. 1996). In this study, we demonstrated that oral administration of heat-treated Bp for 7 consecutive days enhanced cytokine (e.g. IL-6 and IFN-γ) secretion and IgA production (Fig. 2). These results mean that innate immune responses such as IL-12p40 production occurred in the early phase, and then acquired immune responses such as secretion of IL-6 and IFN-γ and IgA production were induced in the late phase. We previously reported that oral administration of BIM induced IgA production by PP cells and cytokine production related to IgA production (e.g. IL-6 and IFN-γ) by CD4+ cells derived from PP (Nakanishi et al. 2005). It suggested that PP CD4 T cell might be activated to induce IgA production by oral administration of Bp components. However, we have no evidence to clarify the relation between acquired immune responses and bacterial protein structure in detail.
In conclusion, in this study we characterized immunomodulation responses induced by Bifidobacterium components prepared using different methods. We demonstrated that a live condition of Bp was not always necessary for the induction of immunomodulation. It indicates that sonication of Bp might be better for induction of innate immunity, and sonication or heat treatment might be effective for the induction of acquired immunity. This knowledge may prove useful in the future in the preparation by different methods of probiotic immunomodulators for use as anti-infectious supplements. Further studies about the applicable treatment and the specific chemical structure of immunoactive components are needed for application of probiotic immunomodulators as an anti-infection or an anti-allergy.
Acknowledgment
We are grateful to Dr. Taketo Kawarai for his help with the scanning electron microscopy analysis.
References
- Albrecht I, Tapmeier T, Zimmermann S, Frey M, Heeg K, Dalpke A (2004) Toll-like receptors differentially induce nucleosome remodelling at the IL-12p40 promoter. EMBO Rep 5:172–177 Epub 2004 Jan 2023 [DOI] [PMC free article] [PubMed]
- Christensen HR, Frokiaer H, Pestka JJ (2002) Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol 168:171–178 [DOI] [PubMed]
- Dennis VA, Jefferson A, Singh SR, Ganapamo F, Philipp MT (2006) Interleukin-10 anti-inflammatory response to Borrelia burgdorferi, the agent of Lyme disease: a possible role for suppressors of cytokine signaling 1 and 3. Infect Immun 74:5780–5789 [DOI] [PMC free article] [PubMed]
- He F, Morita H, Kubota A, Ouwehand AC, Hosoda M, Hiramatsu M, Kurisaki J (2005) Effect of orally administered non-viable Lactobacillus cells on murine humoral immune responses. Microbiol Immunol 49:993–997 [DOI] [PubMed]
- Hosono A, Lee J, Ametani A, Natsume M, Hirayama M, Adachi T, Kaminogawa S (1997) Characterization of a water-soluble polysaccharide fraction with immunopotentiating activity from Bifidobacterium adolescentis M101-4. Biosci Biotechnol Biochem 61:312–316 [DOI] [PubMed]
- Hosono A, Lee J, Ametani A, Natsume M, Hirayama M, Adachi T, Kaminogawa S (1998) Comparison of the immunopotentiating activity with structural characteristics among water-solubele polysaccharides isolated from the genus Bifidobacterium. Biosci Microflora 17:97–104
- Ibnou-Zekri N, Blum S, Schiffrin EJ, von der Weid T (2003) Divergent patterns of colonization and immune response elicited from two intestinal Lactobacillus strains that display similar properties in vitro. Infect Immun. 71:428–436 [DOI] [PMC free article] [PubMed]
- Kishi A, Uno K, Matsubara Y, Okuda C, Kishida T (1996) Effect of the oral administration of Lactobacillus brevis subsp. coagulans on interferon-alpha producing capacity in humans. J Am Coll Nutr 15:408–412 [DOI] [PubMed]
- Lee J, Ametani A, Enomoto A, Sato Y, Motosima H, Ike F, Kaminogawa S (1993) Screening for the immunopotentiating activity of the immune response by Bifidobacterium adolescentis M101-4. Biosci Biotechnol Biochem 57:2127–2132
- Li Y, Qu X, Yang H, Kang L, Xu Y, Bai B, Song W (2005) Bifidobacteria DNA induces murine macrophages activation in vitro. Cell Mol Immunol 2:473–478 [PubMed]
- Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, Bavari S, Klaenhammer TR (2005) Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci U S A 102:2880–2885 Epub 2005 Feb 2814 [DOI] [PMC free article] [PubMed]
- Nakanishi Y, Hosono A, Hiramatsu Y, Kimura T, Nakamura R, Kaminogawa S (2005) Characteristic immune response in Peyer’s patch cells induced by oral administration of Bifidobacterium components. Cytotechnology 47:69–77 [DOI] [PMC free article] [PubMed]
- Newton CA, Perkins I, Widen RH, Friedman H, Klein TW (2007) Role of Toll-like receptor 9 in Legionella pneumophila-induced interleukin-12 p40 production in bone marrow-derived dendritic cells and macrophages from permissive and nonpermissive mice. Infect Immun 75:146–151 Epub 2006 Oct 2023 [DOI] [PMC free article] [PubMed]
- Ohno H, Tsunemine S, Isa Y, Shimakawa M, Yamamura H (2005) Oral administration of Bifidobacterium bifidum G9-1 suppresses total and antigen specific immunoglobulin E production in mice. Biol Pharm Bull 28:1462–1466 [DOI] [PubMed]
- Ratajczak C, Duez C, Grangette C, Pochard P, Tonnel AB, Pestel J (2007) Impact of lactic acid bacteria on dendritic cells from allergic patients in an experimental model of intestinal epithelium. J Biomed Biotechnol 2007:71921 Epub 2007 Feb 71928 [DOI] [PMC free article] [PubMed]
- Shida K, Takahashi R, Iwadate E, Takamizawa K, Yasui H, Sato T, Habu S, Hachimura S, Kaminogawa S (2002) Lactobacillus casei strain Shirota suppresses serum immunoglobulin E and immunoglobulin G1 responses and systemic anaphylaxis in a food allergy model. Clin Exp Allergy 32:563–570 [DOI] [PubMed]
- Uehara A, Sugawara S, Takada H (2002) Priming of human oral epithelial cells by interferon-gamma to secrete cytokines in response to lipopolysaccharides, lipoteichoic acids and peptidoglycans. J Med Microbiol 51:626–634 [DOI] [PubMed]
- Whitaker RD, Batt CA (1991) Characterization of the heat shock response in Lactococcus lactis subsp. lactis. Appl Environ Microbiol 57:1408–1412 [DOI] [PMC free article] [PubMed]


