Abstract
Establishing tissue cultures derived from deep-sea multicellular organisms has been extremely difficult because of the serious damage they sustain upon decompression and exposure to the high temperature of surface seawater. We developed a novel pressure-stat aquarium system for the study of living deep-sea multicellular organisms under pressure. Using this system, we have succeeded in maintaining a variety of deep-sea multicellular organisms under pressure and atmospheric conditions after gradual, slow decompression. Furthermore, we successfully cultivated and freeze-stocked pectoral fin cells of the deep-sea eel Simenchelys parasiticus collected at a depth of 1,162 m under atmospheric pressure conditions. This review describes novel capture and maintenance devices for deep-sea organisms and cell culture studies of the organisms under atmospheric and pressure conditions.
Keywords: Cell culture, Deep-sea fish, Deep-sea shrimp, Hydrostatic pressure, Piezostat aquarium system
The deep sea represents a large portion of the total volume of the oceans and this extreme environment is characterized by the absence of light, low temperature, and high hydrostatic pressure. After the advent of deep-sea vessels opened the door to research on deep-sea organisms, many have been isolated and characterized (Horikoshi and Tsujii 1999). Although increasing attention has been paid to deep-sea animals, no group succeeded in culturing them before 2000 (Koyama and Aizawa, 2000). There are three major obstacles to molecular and cellular biological studies of deep-sea multicellular organisms under atmospheric pressure. The first is the development of devices for capturing and maintaining them, because most succumb as a result of decompression and exposure to the high-temperature surface seawater (Koyama and Aizawa 2000; Koyama et al. 2002, 2005a). The second is acclimation of the captured organisms to atmospheric conditions. The third is culture and freeze-storage of cells extracted from deep-sea organism tissues under atmospheric conditions.
We solved those three problems by developing a novel piezostat aquarium system to capture and maintain deep-sea organisms (Koyama et al. 2002, 2003a, b, 2005a) and succeeded in the cultivation and freeze-storage of pectoral fin cells from the living deep-sea eel Simenchelys parasiticus (habitat depth, 366–2,630 m; Nakabo 2000) at atmospheric pressure (Koyama et al. 2003a, b, 2005b).
This review describes novel capture and maintenance devices for deep-sea organisms and subsequent cell culture studies of the organisms under atmospheric pressure.
Capture and maintenance of deep-sea multicellular organisms under pressure
Three main approaches have been used to collect deep-sea multicellular organisms for study. The first is remote collection with trawls, corers, and grabs. However, the animals are often injured during decompression. The second is collection by submersibles with a suction sampler (Hashimoto et al. 1992) or a manipulator (Koyama and Aizawa 2000), but most of the organisms succumb as the result of decompression and exposure to the high-temperature surface seawater. The third method is capture of the organisms in pressure-retaining traps and maintaining them in the laboratory for study within the traps (Yayanos 1978; Macdonald and Gilchrist 1978; Wilson and Smith 1985; reviewed in Smith and Baldwin 1997). Because no piezostat systems were developed for the traps, most organisms could not be kept alive in the pressure-retaining devices for long periods in early studies (Yayanos 1978; Macdonald and Gilchrist 1978; Wilson and Smith 1985).
We developed a novel piezostat aquarium system (Koyama et al. 2002; Fig. 1) to study the behavior of abyssal organisms. The aquarium system was designed for suction capture of deep-sea organisms with a submersible servomotor (Fig. 2). After capture of the organisms, the openings in the inner lids are closed by a spring mechanism that removes the pins (Fig. 2). The calculated minimum-resistance pressure limit of the aquarium is 30 MPa, and the inner seawater can escape through a pressure control valve (Fig. 2) when the inner pressure increases to greater than 20 MPa. Therefore, the aquarium system maintains an inner pressure of 20 MPa (corresponding to a depth of 2,000 m) even when the submersible surfaces from the deepest sea bottom. The organisms are fed on bait worms, and the compressed seawater is circulated and exchanged. Large waste particles such as uneaten bait worms are removed by a filter in the feed box (Fig. 2). Small waste products from the deep-sea fish are eliminated with the exchange of seawater. Pressure is maintained at ±0.1–0.2 MPa during a 4-month period when the compressed sea water supply is set at the rate of 36 mL min−1. The temperature inside the pressure aquarium is monitored and controlled by a TE electrode sensor and a cooler that cools to 5 °C when the ambient room temperature is higher than 30 °C.
Fig. 1.

Photographs of the piezostat aquarium system. (Right) Piezostat maintenance system. (Left) Suction capture system connected to the RV Hyperdolphin
Fig. 2.
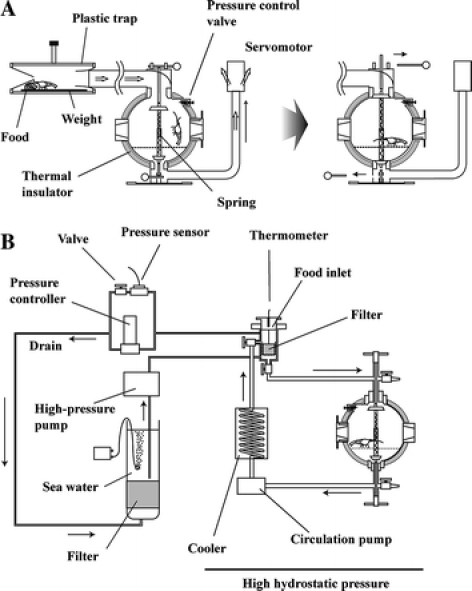
Schematic illustration of the piezostat aquarium system. (a) Suction capture system. (b) Piezostat maintenance system
Using the piezostat aquarium system, we succeeded in capturing and maintaining a variety of deep-sea organisms such as deep-sea fish, deep-sea shrimp, deep-sea bivalves, and deep-sea snails under pressure for a approximately 2 months (Koyama 2003).
Induction of hatching of deep-sea organisms after full decompression
Development of the piezostat aquarium system paved the way for the study of living deep-sea organisms under pressure. In a subsequent study, we attempted to breed abyssal organisms using the system in our land laboratory.
Environmental cues for the reproduction of shallow-water animals include a wide variety of factors such as bright sunlight, lunar period, presence of phytoplankton, rainstorms, time of sunrise and sunset, temperature change, and wave action (Fig. 3; reviewed in Giese and Kanatani 1987). Most deep-sea multicellular organisms investigated to date appear to undergo asynchronous gametogenesis, because there are fewer environmental cues in deep-sea habitats (reviewed in Van Dover 2000). Valuable observations have been made of the spawning behavior of the hydrothermal vent tubeworm Riftia pachyptila on the East Pacific Rise (Van Dover 1994) and of the vesicomyid clam Calyptogena soyoae at cold seeps in Sagami Bay, Japan (Fujiwara et al. 1998; Momma et al. 1995). In the vesicomyid clam C. soyoae, spawning was synchronized with natural temperature (Momma et al. 1995) and induced by an artificial temperature increment of in situ water using the submersible Shinkai 2000 carrying a polycarbonate dome with a halogen light (Fujiwara et al. 1998). Using the piezostat aquarium system, we investigated whether gametogenesis is induced in abyssal organisms by alterations in exterior environmental factors such as pressure, temperature, and/or artificial light exposure (Koyama et al. 2005a).
Fig. 3.
Environmental cues used by shallow-water animals to synchronize reproductive processes
Three specimens of the deep-sea shrimp Alvinocaris sp. were collected using a trap with the piezostat aquarium system (Figs. 1 and 2) from an ocean depth of 1,157 m at cold-seep sites off Hatsushima Island in Sagami Bay, Japan (Koyama et al. 2005a). The deep-sea shrimp were maintained at a temperature of 4.5 °C with fluctuation of ±0.3 °C at hydrostatic pressure of 11.5 MPa (corresponding to a depth of 1,130 m). Two of the three Alvinocaris sp. specimens (Fig. 4) contained eggs at capture, and we investigated the physical parameters that triggered spawning and/or hatching of the shrimp eggs. To trigger spawning and/or hatching, we altered such environmental factors as pressure, temperature, and illumination in the pressure-stat aquarium system. However, temperature increases up to 9 °C, light exposure (60 lux), or lunar phase did not induce spawning and/or hatching of the Alvinocaris sp. specimens.
Fig. 4.
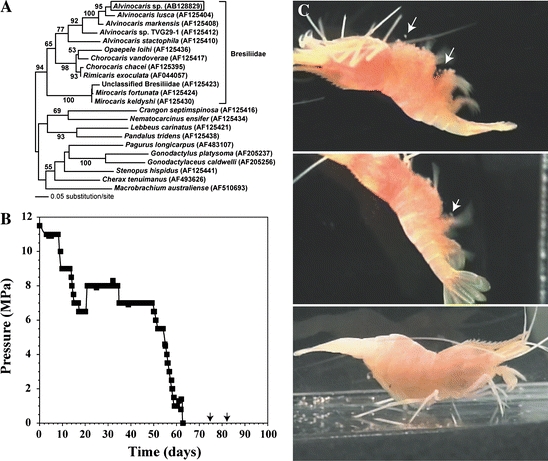
Survival of deep-sea Alvinocaris sp. specimens during decompression and larval hatching at atmospheric pressure. (a) Phylogenetic position of Alvinocaris sp. inferred from nucleotide sequences of the COI gene. Numbers are bootstrap values for nodes supported by more than 50% (100 replicates). Bar indicates 0.05 substitution per site. (b) Pressure in the aquarium system. Arrows indicate hatching of deep-sea mother shrimp. (c) Hatching of deep-sea shrimp after full decompression. Arrows indicate the time immediately after hatching
Neither hatching nor spawning was observed during 50 days of rearing even when the aquarium pressure was varied between 11.5 and 6.5 MPa, corresponding to a depth of 1,130 and 640 m, respectively. After 10 and 16 days of full decompression, corresponding to 73 and 79 days of rearing, respectively, hatching of the shrimp larvae occurred while the two original specimens continued to swim (Fig. 4). The Alvinocaris sp. larvae metamorphosed after 15 days of rearing and a pair of compound eyes developed in each (Fig. 5). The larvae were fed commercially purchased plankton and more than 80% remained alive after 50 days at atmospheric pressure. After 74 days of rearing, all of the larvae died due to an outbreak of mold. No molting was observed during those 74 days.
Fig. 5.

Photographs of deep-sea shrimp larva. (a) Day 0 and (b) day 50 of the larval stage. Scale bars indicate 1 mm
We used an one-variable-at-a-time program to investigate the triggering effects of pressure, temperature, and illumination on shrimp spawning and hatching, because it is extremely difficult to collect numerous gravid deep-sea shrimp with eggs. Future research will elucidate the details of these effects if more gravid deep-sea shrimp with eggs can be captured.
Tissue culture of deep-sea multicellular organsisms
Establishment of tissue cultures derived from deep-sea multicellular organisms has been difficult because of the serious damage they sustain upon decompression and exposure to high temperatures. Therefore, almost all deep-sea multicellular organisms collected at depths exceeding 1,000 m died within a half-day. Even if deep-sea creatures can be kept alive for a short period, contamination by microorganisms hinders their primary culture. Nevertheless, we maintained a primary culture of the deep-sea clam Calyptogena soyoae collected at depths of 1,180, 1,148, and 1,386 m, although only for a short time (Koyama and Aizawa 2000). Several researchers have attempted primary cell cultures of shallower marine clams, although they also were maintained only for a short period (Li and Stewart 1966; Cecil 1969; Odintsova and Khomenko 1991; Takeuchi et al. 1999).
We attempted cell culture of collected deep-sea organisms using the piezostat aquarium system after gradual, slow decompression (Koyama et al. 2002, 2003a, b, 2005b). The deep-sea eel Simenchelys parasiticus (Fig. 6; habitat depth, 366–2,630 m; Nakabo 2000) captured from an ocean depth of 1,162 m survived under atmospheric pressure for 5 days after gradual, slow decompression (Koyama et al. 2003a, b). Using the living deep-sea eel S. parasiticus, we successfully cultivated pectoral fin cells in salt-enriched L-15 medium supplemented with fetal bovine serum (FBS) at atmospheric pressure (Koyama et al. 2003a, b). We confirmed that the deep-sea eel cells passaged more than 20 times and succeeded in freeze-storage of the cells under atmospheric conditions (Koyama et al. 2005b). Figure 7 shows the rate of cell proliferation of the deep-sea eel cells at 15 °C under atmospheric pressure. Reaching the M phase, in which nuclear division and cytoplasmic division occur, requires about 1 h when the deep-sea eel cells are at atmospheric pressure (Fig. 7). Based on the results shown in Fig. 7, the doubling time of the deep-sea eel cells was 170 h. When the FBS concentration increased from 10 to 20% (v/v) in the medium, the cell proliferation rate also increased by approximately 2.2-fold (Fig. 7). FBS contained growth factor(s) and therefore enhanced the speed of cell growth with a doubling time of 77 h.
Fig. 6.

Photograph of a deep-sea eel S. parasiticus. Inner pressure and temperature in the piezostat aquarium system were set at 10 MPa and 5 °C, respectively, with a seawater exchange rate of 36 mL min−1
Fig. 7.
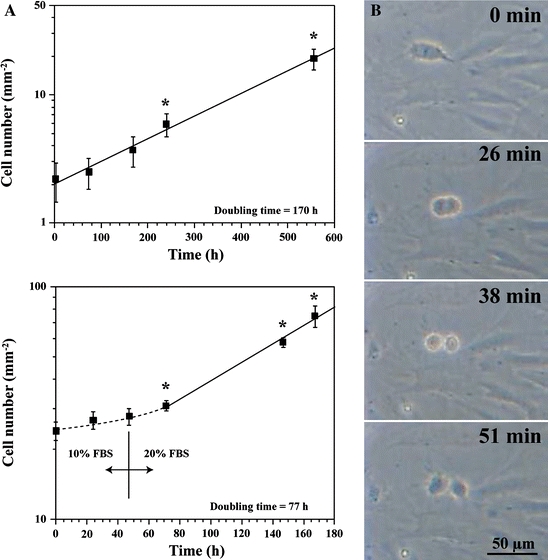
Deep-sea eel cell growth under atmospheric conditions at 15 °C. (a) Proliferation of deep-sea eel cells. Values shown are expressed as mean ± SEM (n = 10; *p < 0.05 compared with time 0 h by Student’s t-test). (b) Mitotic deep-sea eel cell
To investigate the M phase of deep-sea eel cells under pressure, we observed the cell division process using an optical pressure chamber system (Koyama et al. 2001, 2005b). Figure 8 shows real-time observations of deep-sea eel cells under pressure. Most animal cells including those of deep-sea eels require approximately 1 h to reach the M phase at atmospheric pressure (Fig. 7; Koyama et al. 2003a, b). Based on our microscopic observations, deep-sea eel cells require about 4 h to reach the M phase under pressure of 20 MPa (Fig. 8). At pressure of 30 MPa, the morphology of deep-sea eel cells began to change after 2 days of cultivation (Fig. 8). It remains unclear why it takes the deep-sea eel cells require so long to reach the M phase in the optical pressure chamber at 20 MPa (Fig. 8). Hydrostatic pressure increases the Km and decreases the Vmax of enzymatic reactions (Siebenaller and Somero 1979; reviewed in Gibbs 1997); therefore, cells under pressure may require a longer time to reach the M phase compared with cells under atmospheric conditions.
Fig. 8.
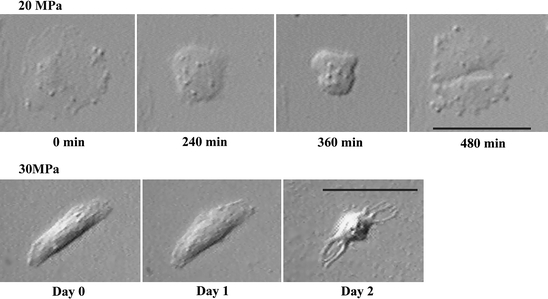
Real-time observation of cultured deep-sea eel cells under pressure. Deep-sea eel cells were seeded on a 2-mm-thick cover glass coated with poly-d-lysine and grown to a cell density of 30–70 cells 3.14 mm−2 for 3–7 days at 15 °C in a humidified atmosphere. The cells on the cover glass were placed in an optical pressure chamber system (Koyama et al. 2001) and exposed to hydrostatic pressure. The median perfusion speed was set at 6 μL h−1. Bars indicate 50 μm
Next, we examined the survival rates of deep-sea eel cells after exposure to high hydrostatic pressure and compared them with the rates of conger eel and mouse 3T3-L1 cells. Cell viability was investigated using double staining with both calcein-AM and EthD-1. Using the double-staining technique, living cells emit the green fluorescence of calcein due to esterase activity. On the other hand, dead cells generate a bright red fluorescence because EthD-1 enters cells with damaged membranes. More than 100 cells were assayed by counting the percentage of living cells. Figure 9 shows the survival rates of mouse 3T3-L1, conger eel cells, and deep-sea eel cells after pressurization. The majority (>95%) of mouse 3T3-L1 cells remained alive at pressure of up to 60 MPa (Fig. 9). At pressure of 70 MPa, more than 70% of mouse 3T3-L1 cells were damaged or died based on the bright red fluorescence of EthD-1 (Fig. 9). No living mouse 3T3-L1 cells were found at 100 MPa (Fig. 9). Conger eel cells at 25 °C remained alive at pressure of up to 60 MPa (Fig. 9). All of the conger eel cells were damaged or died at 130 MPa (Fig. 9). Conger eel cells at 15 °C began to sustain damage or die at pressure of only 5 MPa (Fig. 9). Although no living mouse and conger eel cells were found at pressure of 130 MPa, all deep-sea eel cells remained alive until the pressure reached 150 MPa (Fig. 9). All of the living deep-sea eel cells detached from the growth surface at 175 MPa. At 200 MPa, all deep-sea eel cells collapsed and died (Fig. 9). The deep-sea eel cells thus survived at higher pressure than did the mouse and conger eel cells.
Fig. 9.
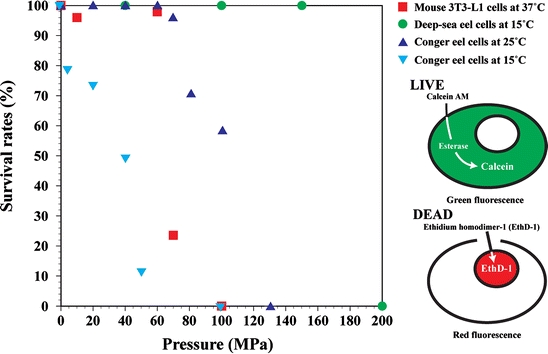
Survival rates of mouse, conger, and deep-sea eel cells. Each cell type was grown to 1–5 × 10−3 cells cm−2 and subjected to pressure ranging from 5 to 200 MPa for 20 min. After pressurization, the viability of more than 100 cells was investigated
Next, we examined the depolymerization of actin and tubulin filaments after the application of hydrostatic pressure and compared the results among deep-sea eel, conger eel, and mouse 3T3-L1 cells (Figs. 10 and 11). Each cell type was grown to 1–5 × 103 cells/cm2 and then exposed to hydrostatic pressure varying from 40 to 130 MPa for 20 min. After pressurization, the change in the distribution of actin and tubulin in the cells was examined. In mouse 3T3-L1 and conger eel cells, hydrostatic pressure of 40 MPa induced disruption of the cytoskeletal organization with profound changes in cell shape (Fig. 10). Meanwhile, little change in cell shape with depolymerization of actin and tubulin filaments was observed in deep-sea eel cells at hydrostatic pressure of 40 MPa (Fig. 11). Most microtubules and some actin filaments in deep-sea eel cells began to be disrupted at 100 MPa (Fig. 11). At 130 MPa, all of the microtubules and a majority of the actin filaments depolymerized in the deep-sea eel cells (Fig. 11). We observed few profound cell shape changes in the pressurized deep-sea eel cells compared with the mouse and conger eel cells. From the results shown in Figs. 9–11, the deep-sea eel-derived cells had greater pressure tolerance than conger eel and mouse 3T3-L1 cells.
Fig. 10.
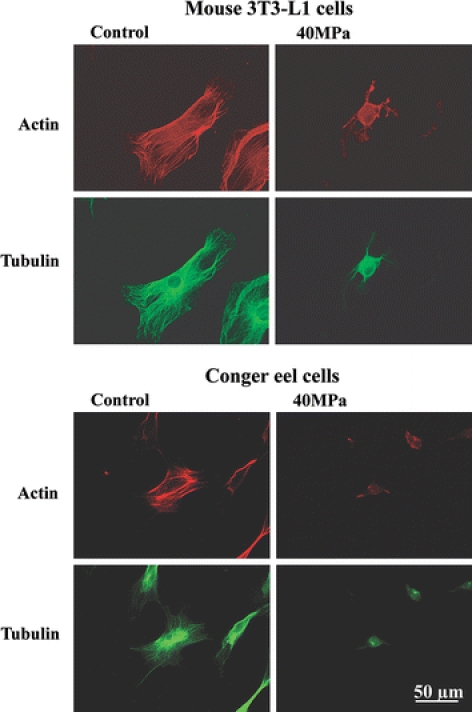
Pressure-induced actin and tubulin depolymerization in mouse and conger eel cells. Each cell type was subjected to pressure of 40 MPa for 20 min. The temperature of the conger eel and mouse cells was set at the growth optima of 25 and 37 °C, respectively
Fig. 11.
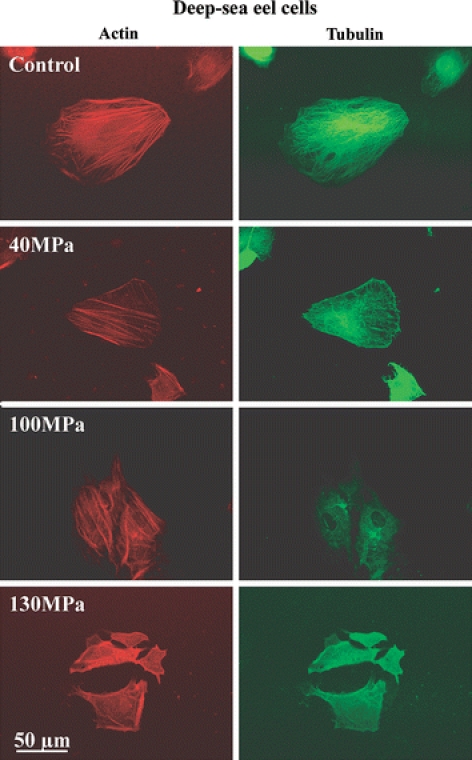
Actin and tubulin filaments in deep-sea eel cells at high pressure. Deep-sea eel cells were subjected to pressure ranging from 40 to 130 MPa for 20 min at 15 °C
Future study
We developed a novel piezostat aquarium system to capture and maintain deep-sea organisms. Using this system, we succeeded in the cultivation and freeze-storage of pectoral fin cells from the living deep-sea eel S. parasiticus at atmospheric pressure and demonstrated that S. parasiticus-derived pectoral fin cells have greater pressure tolerance than conger eel and mouse 3T3-L1 cells. In future studies, we will improve the rust resistance of the piezostat aquarium system against hydrogen sulfide and attempt to clarify the conundrum of the molecular and cellular biological characteristics of deep-sea chemoautotrophs.
Acknowledgments
The author is grateful to Dr. Masuo Aizawa (Tokyo Institute of Technology) and Dr. Koki Horikoshi (JAMSTEC) for helpful support and advice. The author also thanks Dr. Tetsuya Miwa for the gift of a deep-sea fish photograph.
References
- Cecil JT (1969) Mitosis in cell cultures from cardiac tissue of the surf clam Spisula solidissima. J Invest Pathol 14:407–410 [DOI] [PubMed]
- Fujiwara Y, Tsukahara J, Hashimoto J et al (1998) In situ spawning of a deep-sea vesicomyid clam: evidence for an environmental cue. Deep-Sea Res I 45:1881–1889 [DOI]
- Gibbs AG (1997) Biochemistry at depth. In: Randall DJ, Farrell AP (eds) Deep-sea fishes. Academic Press, San Diego, pp 239–277
- Giese AC, Kanatani H (1987) Maturation and spawning. In: Giese AC, Pearse JS, Pearse VB (eds) General aspects: seeking unity in diversity. Reproduction of marine invertebrates, vol. 9. Blackwell Scientific Publications and Boxwood Press, Palo Alto and Pacific Grove, California, pp 251–329
- Hashimoto J, Fujikura K, Aoki T et al (1992) Development of a suction sampler (slurp gun) for deep sea organisms. Proc JAMSTEC Symp Deep Sea Res 8:367–372
- Horikoshi K, Tsujii K (1999) Extremophiles in deep-sea environments. Springer, Tokyo
- Koyama S (2003) Acclimatization for deep-sea multicellular organisms under atmospheric pressure. Biosci Ind 61:36–37
- Koyama S, Aizawa M (2000) Tissue culture of the deep-sea bivalve Calyptogena soyoae. Extremophiles 4:385–389 [DOI] [PubMed]
- Koyama S, Miwa T, Sato T et al (2001) Optical chamber system designed for microscopic observation of living cells under extremely high hydrostatic pressure. Extremophiles 5:409–415 [DOI] [PubMed]
- Koyama S, Miwa T, Horii M et al (2002) Pressure-stat aquarium system designed for capturing and maintaining deep-sea organisms. Deep-Sea Res I 49:2095–2102 [DOI]
- Koyama S, Horii M, Miwa T et al (2003a) Tissue culture of the deep-sea eel Simenchelys parasiticus collected at 1,162 m. Extremophiles 7:245–248 [DOI] [PubMed]
- Koyama S, Horii M, Miwa T et al (2003b) Erratum. Extremophiles 7:340 [DOI] [PubMed]
- Koyama S, Nagahama T, Ootsu N et al (2005a) Survival of deep-sea shrimp (Alvinocaris sp.) during decompression and larval hatching at atmospheric pressure. Mar Biotechnol 7:272–278 [DOI] [PubMed]
- Koyama S, Kobayashi H, Inoue A et al (2005b) Effects of the piezo-tolerance of cultured deep-sea eel cells on survival rates, cell proliferation, and cytoskeletal structures. Extremophiles 9:449–460 [DOI] [PubMed]
- Li MF, Stewart JE (1966) In vitro cultivation of cells of the oyster, Crassostrea virginica. J Fish Res BD Canada 23:595–599
- Macdonald AG, Gilchrist I (1978) Further studies on the pressure tolerance of deep-sea crustacea, with observations using a new high-pressure trap. Mar Biol 45:9–21 [DOI]
- Momma H, Mitsuzawa K, Kaiho Y et al (1995) Long-term deep sea floor observation off Hatsushima Island in Sagami Bay—one year in the Calyptogena soyoae clam colony. JAMSTEC J Deep Sea Res 11:249–268
- Nakabo T (2000) Fishes of Japan with pictorial keys to the species, 2nd edn. Tokai University Press, Tokyo
- Odintsova NA, Khomenko AV (1991) Primary cell culture from embryos of the Japanese scallop Mizuchopecten yessoensis (bivalvia). Cytotechnology 6:49–54 [DOI] [PubMed]
- Siebenaller JF, Somero GN (1979) Pressure-adaptive differences in the binding and catalytic properties of muscle-type (M4) lactate dehydrogenases of shallow- and deep-living marine fishes. J Comp Physiol B 129:295–300 [DOI]
- Smith KL Jr, Baldwin RJ (1997) Laboratory and in situ methods for studying deep-sea fishes. In: Randall DJ, Farrell AP (eds) Deep-sea fishes. Academic Press, San Diego, pp 351–378
- Takeuchi Y, Inoue K, Miki D et al (1999) Cultured mussel foot cells expressing abyssal protein genes. J Exp Zool 283:131–136 [DOI]
- Van Dover CL (1994) In situ spawning of hydrothermal vent tubeworms (Riftia pachyptila). Biol Bull 186:134–135 [DOI] [PubMed]
- Van Dover CL (2000) The ecology of deep-sea hydrothermal vents. Princeton University Press, Princeton, New Jersey
- Wilson RR Jr, Smith KL Jr (1985) Live capture, maintenance and partial decompression of a deep-sea grenadier fish (Coryphaenoides acrolepis) in a hyperbaric trap-aquarium. Deep-Sea Res 32:1571–1582 [DOI]
- Yayanos AA (1978) Recovery and maintenance of live amphipods at a pressure of 580 bars from an ocean depth of 5,700 m. Science 200:1056–1059 [DOI] [PubMed]


