Abstract
Two infected Sf-9 cell cultures were monitored on-line by multi-frequency permittivity measurements using the Fogale BIOMASS SYSTEM® and by applying different off-line methods (CASY®1, Vi-CELL™, packed cell volume) to measure the biovolume and the mean diameter of the cell population. During the growth phase and the early infection phase the measured permittivity at the working frequency correlated well with the different off-line methods for the biovolume. We found a value of 0.67 pF cm−1 permittivity per unit of total biovolume (CASY) (μL mL−1). After the maximum value in the permittivity was reached, i.e. when the viability of the cultures decreased significantly, we observed different time courses for the biovolume depending on the applied method. The differences were compared and could be explained by the underlying measurement principles. Furthermore, the characteristic frequency (fC) was calculated from the on-line scanning permittivity measurements. The fC may provide an indication of changes in cell diameter and membrane properties especially after infection and could also be an indicator for the onset of the virus production phase. The changes in fC were qualitatively explained by the underlying equation that is correlating fC and the properties of the cell population (cell diameter, intracellular conductivity and capacitance per membrane area).
Keywords: Baculovirus, Biomass, Biovolume, Capacitance, Characteristic frequency, Insect cell culture, On-line monitoring, Permittivity, Scanning spectroscopy, Sf-9
Introduction
Biomass is the key parameter in any animal cell culture process (Ducommun et al. 2001) and its reliable on-line estimation is of great interest. Different approaches for the measurement of biomass in biotechnological processes were described in the literature (Sonnleitner et al. 1992) and an overview focusing on various techniques to on-line monitor the concentration of animal cells was given elsewhere (Ducommun et al. 2001). Capacitive measurements were already described 10 years ago as ‘probably the best available’ in-situ technique compared to other methods (Olsson and Nielsen 1997) because such measurements correlate biomass with the membrane enclosed volume fraction (biovolume) (Harris et al. 1987). The usefulness of this technique in animal cell culture processes has been described in recent years by a number of researchers (Noll and Biselli 1998; Ducommun et al. 2002; Cannizzaro et al. 2003; Ansorge et al. 2007).
The correlation of capacitive measurements and biovolume can be easily demonstrated by investigating infected Sf-9 cultures. The insect cell baculovirus expression system has become one of the most widely used systems for routine production of recombinant proteins (Schmid 1996; Kost et al. 2005). After infection with the baculovirus the cells undergo large physiological changes resulting in cell enlargement which has been used to monitor the infection’s success (Schopf et al. 1990; Schmid et al. 1994; Taticek and Shuler 1997; Chico and Jäger 1998). Increasing cell size is probably caused by the synthesis of new cell material following the baculoviral infection (Kamen et al. 1996).
The on-line monitoring of infected insect cell cultures using capacitive measurements has already been described in the literature (Kamen et al. 1996; Zeiser et al. 1999, 2000). In this work, we used the newly developed BIOMASS SYSTEM® from Fogale Nanotech (Nîmes, France) and monitored infected Sf-9 cell cultures by multi-frequency measurements. To our knowledge, this has not been described so far. The multi-frequency measurements of the permittivity were used to shape the β-dispersion and to calculate the characteristic frequency, a parameter that was expected to give information about physiological changes in the cell population. The fC calculations could indeed be used to predict physiological changes and important time points during the fermentation process.
Furthermore, we compare the on-line permittivity measurements at the working frequency with different off-line methods that measure the biovolume (CASY®, Vi-CELL™ XR, packed cell volume) and explain the deviating patterns with the underlying measurement principles.
Theoretical background on dielectric properties of biological cells
The basic theory behind capacitive measurements was described elsewhere (Pethig and Kell 1987; Foster and Schwan 1989; Markx and Davey 1999). In general, one exploits the relationship of permittivity and increasing frequency when measuring the permittivity of a cell suspension. By increasing the frequency from 0.1 to 10 MHz a characteristic drop in the permittivity can be observed that is caused by the polarization of the cellular membranes. This drop in the permittivity is called the β-dispersion (Fig. 1). It is for spherical cells mathematically defined by three parameters (Δε, fC, α) and the equation of Schwan:
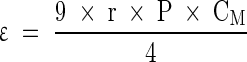 |
with
Fig. 1.
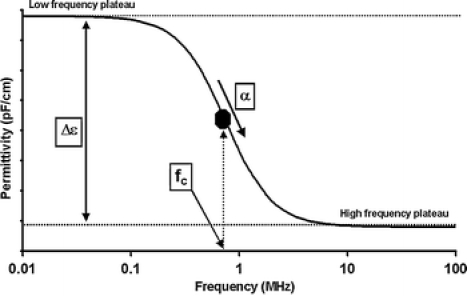
The β-dispersion for spherical cells within the frequency range of 0.1–10 MHz. The β-dispersion is mathematically defined by three parameters (Δε, fC, α) that are dependent on the properties of the cell population
ε: permittivity (F m−1)
r: cell radius (m)
P: volume fraction of the cells (biovolume)
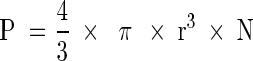 |
N: cell density (m−3)
CM: Capacitance per membrane area (F m−2)
The β-dispersion can in itself be described by several parameters. One of them is the fC which is defined by the simplified equation
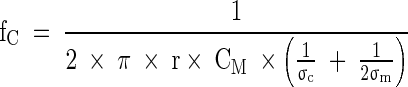 |
with
σc: conductivity of the cytoplasm/intracellular conductivity (mS cm−1)
σm: conductivity of the medium (mS cm−1)
and is consequently changing with cell size (r), cell state (σc) and the properties of the cellular membrane CM.
In our study, the fC was calculated from the permittivity scanning data within the frequency range of 0.3–10 MHz by modeling the β-dispersion and applying the upper equation to the dataset.
Another parameter describing the β-dispersion is the α or Cole–Cole α. It is an empirical parameter that is describing the decrease in the capacitance/permittivity with increasing frequency. It can assume values between 0 and 1 whereby 0 is describing a steep fall in the permittivity and 1 is describing an infinitely shallow fall in the permittivity. The parameter is therefore not a direct measure of the slope. α is believed to increase when the distribution in cell electrical properties widens in the cell population (Davey 1993).
Experimental conditions
Cell culture setup
Sf-9 fermentations were performed in duplicate using an autoclavable bench top 2 L Biostat MCD system (B. Braun Biotech GmbH, Melsungen, Germany) operated at 1.2 L working volume. For both runs the same baculovirus stock was used with a multiplicity of infection (MOI) of ∼10. Cultivation was started with an inoculation cell density of 6 × 105 viable cells mL−1. Temperature (27 °C), pH (6.3), dissolved oxygen (30% air saturation) and agitation (100 rpm, 2 pitched blade impellers, 2 blades each, 45°) were measured and controlled. Cells were cultivated in SF-1 medium, a hydrolysate based insect cell medium described in the literature (Schlaeger 1996). Oxygen transfer was accomplished by using surface aeration only and oxygen uptake rates were obtained from measurements of the oxygen molar fraction in the inlet gas flow and a measured KLa of 1 h−1.
Depending on the stage of the process, samples were taken from the culture twice or three times per day. 5 mL of sample was typically used for the described off-line analyses.
The data discussed in detail represent the first run where more off-line analyses were performed.
Biovolume, cell number and cell size determinations
Hemacytometer
The Trypan Blue exclusion method is one of the classical methods for the estimation of cellular viability (Cook and Mitchell 1989) and we routinely used a 0.05% (w/v) solution of Trypan Blue (Sigma-Aldrich, MO, 0.4% (w/v) cell culture tested, diluted for use with PBS to a final concentration of 0.05% (w/v)) for the staining in a Neubauer hemacytometer. For each sample, two dilutions of 100 μL sample with 100 μL reagent were performed. For each dilution, the average of two counts was taken, whereby one count represents the number of cells contained in four quadrants of the hemacytometer.
CASY®1
A CASY®1 device (Schärfe System GmbH, Reutlingen, Germany) was used to determine the mean cell diameter and the ‘total biovolume (CASY)’. A measuring range of 8–40 μm was routinely used to calculate the parameters mean and most frequent cell diameter and total biovolume (CASY). Each value represents the mean of three measurements per sample. Samples were diluted by a factor of 100 or 1,000 (depending on cell density) with an isotonic solution of about 335 mOsm kg−1 before analysis. The analyzed volume of one diluted sample was 1.2 mL. When taking all three repeated measurements into account, we measured around 6,000–20,000 particles for each sample to get statistically reliable data.
Vi-CELL™ XR
The Vi-CELL™ XR (Beckman Coulter, Fullerton, CA) that is based upon the Trypan Blue method was employed as a cell counting device and for the measurement of viability and cell size. The concentration of the Trypan Blue solution that is used by the Vi-CELL is 0.4% (w/v). The system then automatically dilutes the sample 1:2 with the dye solution before measurement. Depending on the cell density of the samples, around 500–2,000 cells were analyzed per measurement.
Fogale Biomass System®
The commercial in situ autoclavable DN 12 probe was employed for the 2 L bioreactor. The signal was zeroed under cultivation conditions in cell free medium before inoculation. The moving average for the smoothing of the signal was set to a 3 min integration delay and the recording period to 12 min as suggested by the manufacturer.
The equipment is using the β-dispersion phenomenon to measure the viable cell density or biovolume.
According to Schwan’s equation a higher cell radius r causes a higher amplitude Δε also when the volume fraction P (biovolume) remains constant. Because of the measurement principle of the Fogale BIOMASS SYSTEM® this coherence is negligible and the permittivity signal is proportional to the biovolume. The system is applying a dual frequency method with a high frequency f2 at 10 MHz and a working frequency f1 in the critical frequency region of the β-dispersion. The permittivity signal given by the Fogale BIOMASS SYSTEM® ΔεFOGALE is the result of the difference in permittivities measured at f1 and f2. As the measuring frequency f1 is adjustable it is possible to measure the ΔεFOGALE for any given cell type in the region of the fC (in this case 0.7–1.1 MHz) and not in the low-frequency range of Schwan’s postulation. This provides the advantage that a constant ΔεFOGALE response can be observed when the cell radius is changing at a constant biovolume. ΔεFOGALE is henceforth linear to the biovolume even in the case of cell size changes (Fogale Nanotech 2004).
During our experiments Fogale has provided an additional software for the frequency scanning over the β-dispersion range. A total of 20 frequencies from 0.3 to 10 MHz were applied by the Fogale BIOMASS SYSTEM® and the corresponding permittivities were measured to establish a complete spectrum of the cell suspension. It took approximatively 1 min to complete a spectrum and the software was recording the spectra every 12 min.
For each spectrum the software determines the three parameters Δε, fC, and α according to the β-dispersion model described above.
Packed cell volume (PCV)
The total biovolume (PCV) was measured by using disposable PCV measurement tubes (Techno Plastics Products AG, Trasadingen, Switzerland). Up to 1 mL of the culture sample was centrifuged for 3 min at 2500 g and the PCV in μL was estimated (Stettler et al. 2006). As the graduation on the plastic tube was calibrated in 0.5 μL the measured value had to be estimated by interpolation.
Results and discussion
The results part is divided in three sections. The first section presents the data of the permittivity measurements (ΔεFOGALE) and compares the obtained off- and on-line data. The second part shows the calculated fC and compares it with the cell size measurements and in a third and final part data from duplicate runs are compared to demonstrate the reproducibility of the employed system.
On-line permittivity data, oxygen uptake rate and off-line biovolume measurements
Figure 2A displays data for a representative baculovirus-infected Sf-9 culture whereby virus addition took place during the exponential growth phase at 2.2 × 106 viable cells mL−1 after about 50 h cultivation time (time of infection, TOI). The cell counts given by the Vi-CELL™ system and the hemacytometer are depicted in the graph. Whereas the total cell count is similar for both measurements, the viable cell count (Vi-CELL) shows lower counts than the hemacytometer. This was the case in particular after virus infection. It is in agreement with our observation that the Vi-CELL™ system underestimates cell viability of non-infected and especially baculovirus-infected Sf-9 cells. Possible reasons for this observation might be the higher concentration of Trypan Blue (0.4% (w/v)) compared to our standard hemacytometer method (0.05% (w/v)) and/or the different and potentially harsher mixing procedure by the automated system.
Fig. 2.
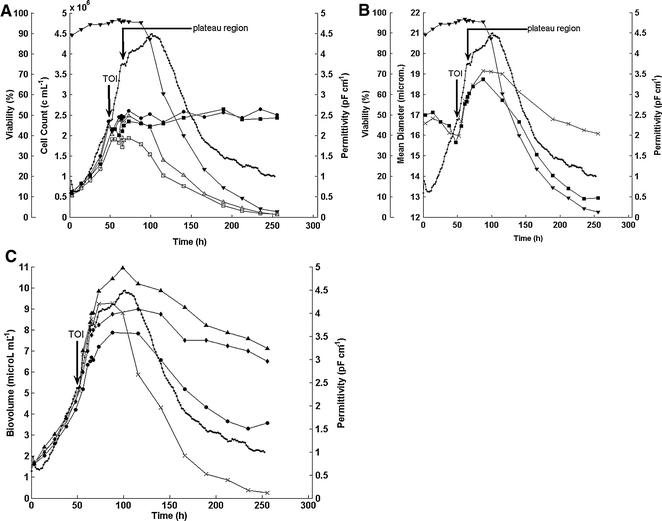
(A) Representative pattern of a baculovirus-infected Sf-9 culture (fermentation 1): Hemacytometer counts (total cell count (●), viable cell count (△), viability (hema) (▼), Vi-CELL cell counts (total cell count (■), viable cell count (□)), permittivity (·) over time. (B) Mean cell diameter (CASY (■), Vi-CELL(×)), viability (hema) (▼) and permittivity (·) of a baculovirus-infected Sf-9 culture (fermentation 1). (C) Total volume (CASY), packed cell volume, total biovolume (Vi-CELL), viable biovolume (Vi-CELL), permittivity over time for an infected Sf-9 culture (fermentation 1). (·) permittivity, (×) viable biovolume (Vi-CELL), (▴) total biovolume (Vi-CELL), (•) total biovolume (CASY), (◆) total biovolume (PCV)
After infection the permittivity is increasing even faster than during the exponential growth phase whereas the viable and total cell counts remain nearly constant until about 100 h cultivation time. Quite noticeable is a plateau region for the permittivity from about 12 to 20 h post-infection (Fig. 2A and B marked by black arrow) followed by a subsequent further increase in signal.
After 100 h cultivation time the maximum of the permittivity is reached and viability is strongly decreasing (Fig. 2A and B). The total cell count remains constant until the end of the fermentation at about 250 h. During the whole time course nearly no cell debris was generated and even stained cells kept optically intact membranes (microscopic observation).
The cell size data (CASY®1, Vi-CELL™) and the viability (hema) for this run are shown in Fig. 2B. After infection, the mean cell diameter is increasing by 20% or 3–4 μm whereas the permittivity increases by about 80%. This is in agreement with the equation of Schwan as the diameter contributes to the permittivity to the power of three. Furthermore, the plateau in the permittivity is accompanied by a temporary leveling off of cell size during this period (Fig. 3A). The two different methods of measuring the cell diameter give quite similar results although in the later stages of the culture the Vi-CELL™ mean diameter is not decreasing in the same way as the CASY®1 mean diameter. Generally, it could be observed by microscopic observation that the cell size change in the later stages of the fermentation does not correlate with the extensive reduction measured by the CASY®1 system.
Fig. 3.
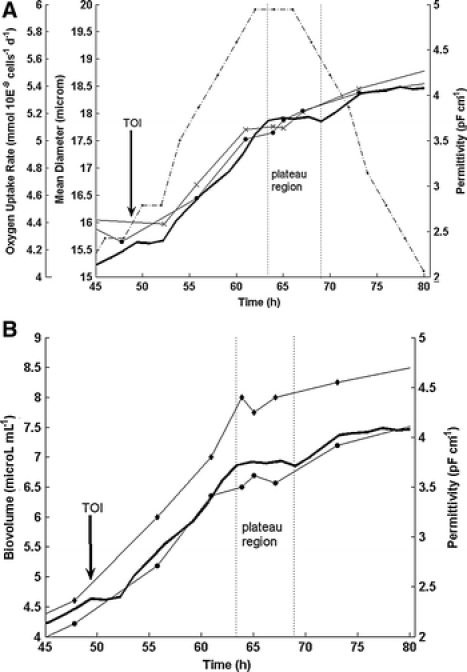
(A) Permittivity (solid line), mean cell diameter (Vi-CELL) (×), mean cell diameter (CASY) (●) and oxygen uptake rate (·, dash dotted line) for the plateau region of fermentation 1. (B) Permittivity (solid line), total biovolume (PCV) (◆) and total biovolume (CASY) (●) for the plateau region of fermentation 1
To explain the differences in the cell size determinations observed for the two methods a closer look into the underlying techniques is needed. On the one hand the CASY®1 system is based on the principle of electrical resistance measurement. This method takes into account the integrity of the cell membrane in the sense that a disrupted membrane or even an increase in membrane permeability leads to a higher conductivity of the cytoplasm and will result in a smaller measured cell size for the respective cell (Winkelmeier et al. 1993). On the other hand the Vi-CELL™ system employs optical analysis for the measurement of cell diameter which should not be influenced by changes in membrane integrity. This might explain why the patterns for the two mean cell diameters (Fig. 2B) are very similar in the first 100 h of the fermentation and start to deviate after the maximum in the permittivity. In particular the membrane permeability of infected Sf-9 cells changes in the later stages of the culture when viability is decreasing and produced virus gets released into the suspending medium (Zeiser et al. 2000).
As mentioned in the theoretical part, the Fogale BIOMASS SYSTEM® measures the membrane enclosed volume fraction or biovolume in a cell suspension. Hence, permittivity increases with increasing cell diameter although the total cell count remains constant.
The CASY®1-Counter calculates a value which gives the volume of all particles (cells) measured in a sample which is in this article referred to as the total biovolume (CASY). This value correlates well with the permittivity (Fig. 2C). The Vi-CELL™ system allows the measurement of the cell diameter of viable cells by separating stained from unstained (viable) cells. The measured total biovolume (Vi-CELL), i.e. the number of all cells counted by the system multiplied with their respective diameter, was calculated by assuming that all cells are spherical. The viable biovolume (Vi-CELL) was calculated accordingly by computing the number of all viable cells and the viable cell diameters. Due to the observation that the Vi-CELL™ system seems to underestimate the viable cell count of infected Sf-9 cells in our system we used the hemacytometer cell counts for the respective calculation of the biovolume corresponding to the different populations of viable (viable biovolume (Vi-CELL) and total cells (total biovolume (Vi-CELL)). Packed cell volume (PCV) measurements were used as an additional estimation for the total biovolume (PCV).
Figure 2C presents the good correlation of all these values and the permittivity until a certain point in time after infection. The viable biovolume (Vi-CELL) and the total biovolume (CASY) are both in good agreement with the on-line permittivity measurement over the whole time course of the cultivation.
Whereas the viable biovolume (Vi-CELL) decreases faster than the permittivity after the drop in viability, the total biovolume (CASY) shows a smaller decrease compared to the permittivity. The values for the total biovolume (Vi-CELL) as well as the total biovolume (PCV) show a very different pattern. After the maximum value both biovolume parameters are decreasing much less than the other three values. Interestingly, the total biovolume (CASY) and the viable biovolume (Vi-CELL) correlate in the best way with the permittivity over the whole time course of the cultivation. The permittivity is according to the equation of Schwan influenced by the changes in membrane state being a function of the CM. Although CM was originally reported to be a biological constant, recent studies reported changes with decreasing viability (Fehrenbach et al. 1992) and changes in the physiological state (Noll and Biselli 1998; Ansorge et al. 2007). This may explain the correlation with the total biovolume (CASY) as the two measurement techniques are both dependent on cell membrane properties. The good correlation with the viable biovolume (Vi-CELL) was expected since permittivity measurements take per definition only viable cells into account. However, stained cells still contribute to the signal which has been reported in different publications for various cell lines and yeast (Davey et al. 1993; Guan et al. 1998; Ducommun et al. 2002; Cannizzaro et al. 2003; Ansorge et al. 2007). In addition, we observed in this system only a marginal cell disintegration that might have been the result of using a bench top surface-aerated bioreactor system. Thus any hydrodynamic stress caused by bursting bubbles was eliminated. In conclusion, the difference in between the viable biovolume (Vi-CELL) and the permittivity after 100 h cultivation time was most likely caused by the presence of stained cells with membranes that are still contributing to the signal.
An interesting point in the time course is the plateau region in the permittivity that is observed at ca. 12–20 h post-infection. It was apparent in both performed fermentations although it was not as obvious in the second one (Fig. 5A). Figures 3A and B represent an enlarged view of the fermentation course around the time of infection highlighting the plateau region of fermentation 1. The plateau can also be observed with all the other off-line measurements. Figure 3B represents the correlation of the total biovolume (PCV) and the total biovolume (CASY) with the permittivity. The cell size values also level off at that time and the oxygen uptake rate reaches a maximum of ca. 6 mmol 10E−9 cells−1 day−1 (Fig. 3A). These values are in agreement with the data of other authors (Schopf et al. 1990; Schmid 1996; Schmid et al. 1994). The plateau region for the permittivity signal has likewise been described by others (Zeiser et al. 1999, 2000). Zeiser et al. further demonstrated that the plateau in the permittivity correlates to the maximum in the CO2 evolution rate (Zeiser et al. 2000). Furthermore, these authors and others observed that this time point corresponds to the first release and the production of budded virus (Ooi and Miller 1988; Wong et al. 1994). The appearance of the plateau before 20 h post-infection was interpreted as a good indicator for synchronous infection which can be assumed here as we used a comparatively high MOI of ∼10. With respect to the oxygen uptake rate our findings are also in agreement with the literature data stating that the maximal oxygen consumption after baculoviral infection is observed at 12–20 h post-infection (Kamen et al. 1996; Schmid 1996).
Fig. 5.
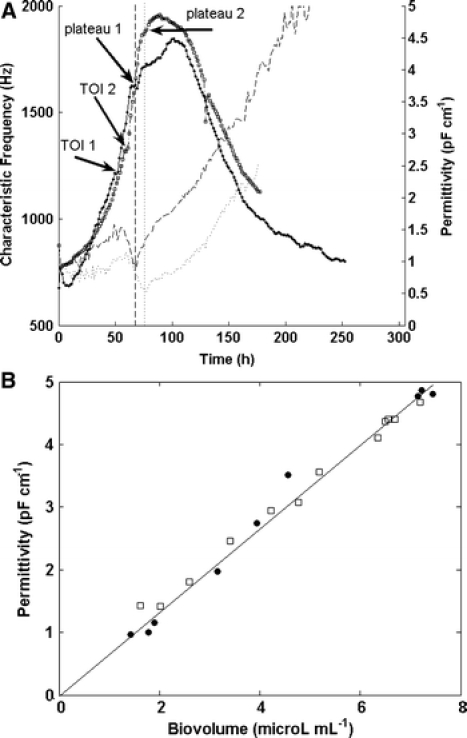
(A) Permittivity (fermentation 1 (□, dash dotted line), fermentation 2 (·)) and characteristic frequency (fermentation 1: dotted line, fermentation 2: dashed line) over time for two duplicate fermentations. (B) Correlation of permittivity and the total biovolume (CASY) for two duplicate fermentations (fermentation 1: □; fermentation 2: ●). Only the data up to the maximum in permittivity (around 100 h cultivation time) were plotted in this graph. Linear regression yields: permittivity = 0.6692 * biovolume; R2 = 0.985
Monitoring the infection process by scanning permittivity measurements
As mentioned in the theoretical part the multi-frequency measurement allowed the calculation of the fC. Figure 4 displays the fC data for the first fermentation run. Until the time of infection the fC increases from ∼0.7 to 0.9 MHz. This is in line with the simplified equation for the fC since the cell diameter is decreasing. The values for fC are not shown for the first few hours of cultivation because their calculation is subject to large errors due to the presence of insufficient biomass at the beginning of the culture. After viral infection fC starts to decrease with the cell diameter increasing substantially. This holds true until the time of the plateau region in the permittivity. Although the cell size is still increasing after the plateau region, we observe a minimum in fC followed by a subsequent increase from that time until the end of the culture. From then on, the trend in fC is not in agreement with the cell diameter data anymore. From viral infection until plateau region however, the change in fC is in agreement with the change in cell size following the respective equation, i.e. the increase in cell size is reflected by the decreasing fC. Assuming that this is the time of first virus release as reported by Zeiser et al. (1999, 2000) major changes of the cell membrane properties should arise that are also affecting fC. This might explain the changes of fC after the plateau phase that are not correlating with the cell diameter. In fact, what can be expected are either changes in CM or/and the intracellular conductivity (σc).
Fig. 4.
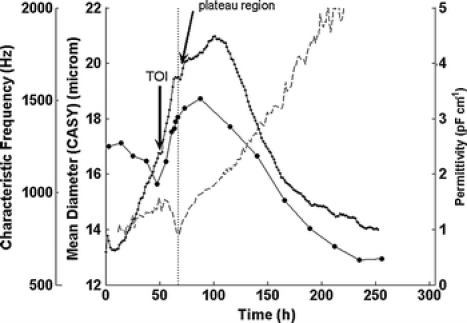
Permittivity (·), characteristic frequency (dashed line) and cell size (CASY) (●) over time (fermentation 1)
Reproducibility
Figures 5A shows an overlay of permittivity and fC for the duplicate Sf-9 fermentations. The results are generally comparable although in fermentation 2 the time of infection is delayed due to a slightly slower cell growth rate. However, the overall pattern for the permittivity signal is similar with a more distinct plateau region in fermentation 1. There is a good agreement of the on-line permittivity and the calculated frequency for both runs and our data demonstrated an excellent linear correlation between total biovolume (CASY) and permittivity until the maximum permittivity was reached (Fig. 5B). We determined a value of 0.67 pF cm−1 permittivity per unit of total biovolume (CASY) (μL mL−1). This value differs significantly from the values we found for the exponential growth phase of two different CHO cell lines that gave ∼0.90 and 0.75 pF cm−1 permittivity per unit of total biovolume (CASY) (μL mL−1), respectively (Ansorge et al. 2007). These results suggest that the dielectric properties are dependent on the employed cell line.
Outlook
Multi-frequency permittivity measurements offer a unique possibility of on-line monitoring physiological changes of cultured cells that may not be possible with comparable on-line measurements (e.g. optical density probes). With regards to the baculovirus insect cell expression system additional experiments need to prove the usefulness of these measurements and a correlation between the plateau region in the permittivity, the minimum in the fC and the time of first viral release.
Acknowledgements
The authors would like to acknowledge C. Ghommidh from Université Montpellier II and M. Biselli from the Aachen University of Applied Sciences (Department Juelich) for the stimulating and fruitful discussions and remarks, M. Foggetta, M. Siegrist, J.-M. Vonach and J.-C. von Bueren for the support with small scale experiments and the setup of the bioreactor system and H. Remy for the use and help with the CASY®1 system. S. Ansorge was supported from March to September 2004 by a Hoffmann-La Roche AG fellowship and the presented results form a part of his diploma thesis at the Aachen University of Applied Sciences.
Abbreviations
- α
Cole–Cole alpha
- CM
Capacitance per membrane area (F m−2)
- Δε
Cell permittivity (pF cm−1)
- ΔεFOGALE
Permittivity difference at f1 and f2 (pF cm−1)
- fC
Characteristic frequency (Hz)
- hema
Hemacytometer
- KLa
Volumetric oxygen mass transfer coefficient (h−1)
- PBS
Phosphate buffered saline
- PCV
Packed cell volume (μL mL−1)
- TOI
Time of infection with baculovirus (h)
References
- Ansorge S, Esteban G, Ghommidh C, Schmid G (2007) Monitoring nutrient limitations by online capactitance measurements in batch and fed-batch CHO fermentations. In: Smith R (ed) Conference Proceedings to the 19th ESACT Meeting: Cell Technology for Cell Products. Springer, Dordrecht/NL, pp 723–726
- Cannizzaro C, Gugerli R, Marison I, von Stockar U (2003) On-line biomass monitoring of CHO perfusion culture with scanning dielectric spectroscopy. Biotechnol Bioeng 84:597–610 [DOI] [PubMed]
- Chico E, Jäger V (1998) Measurements of changes in cell size distribution to monitor Baculovirus infection of insect cells. In: Merten OW, Perrin P, Griffiths B (eds) New developments and new applications in animal cell technology. Kluwer Academic Publishers, Dordrecht, 329–331
- Cook JA, Mitchell JB (1989) Viability measurements in mammalian cell systems. Anal Biochem 179:1–7 [DOI] [PubMed]
- Davey CL (1993) The biomass monitor source book. Department of Biological Sciences, University of Wales, Aberystwyth
- Davey CL, Marckx GH, Kell DB (1993) On the dielectric method of monitoring cellular viability. Pure Appl Chem 65:1921–1926 [DOI]
- Ducommun P, Bolzonella I, Rhiel M, Pugeaud P, von Stockar U, Marison IW (2001) On-line determination of animal cell concentration. Biotechnol Bioeng 72:515–522 [DOI] [PubMed]
- Ducommun P, Kadouri A, von Stockar U, Marison IW (2002) On-line determination of animal cell concentration in two industrial high-density culture processes by dielectric spectroscopy. Biotechnol Bioeng 77:316–323 [DOI] [PubMed]
- Fehrenbach R, Comberbach M, Pêtre JO (1992) On-line biomass monitoring by capacitance measurement. J Biotechnol 23:303–314 [DOI] [PubMed]
- Fogale Nanotech (2004) Biomass System User Manual V 3.0
- Foster KR, Schwan HP (1989) Dielectric properties of tissues and biological materials: A critical review. Crit Rev Biomed Eng 17:25–104 [PubMed]
- Guan Y, Evans PM, Kemp RB (1998) Specific heat flow rate: an on-line monitor and potential control variable of specific metabolic rate in animal cell culture that combines microcalorimetry with dielectric spectroscopy. Biotechnol Bioeng 58:464–477 [DOI] [PubMed]
- Harris CM, Todd RW, Bungard SJ, Lovitt RW, Morris JG, Kell DB (1987) Dielectric permittivity of microbial suspensions at radio frequencies: a novel method for the real-time estimation of microbial biomass. Enzyme Microb Technol 9:181–186 [DOI]
- Kamen AA, Bédard C, Tom R, Perret S, Jardin B (1996) On-line monitoring of respiration in recombinant-baculovirus infected and uninfected insect cell bioreactor cultures. Biotechnol Bioeng 50:36–48 [DOI] [PubMed]
- Kost TA, Condreay JP, Jarvis DL (2005) Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol 23:567–575 [DOI] [PMC free article] [PubMed]
- Markx GH, Davey CL (1999) The dielectric properties of biological cells at radiofrequencies: applications in biotechnology. Enzyme Microb Technol 25:161–171 [DOI]
- Noll T, Biselli M (1998) Dielectric spectroscopy in the cultivation of suspended and immobilized hybridoma cells. J Biotechnol 63:187–198 [DOI] [PubMed]
- Olsson L, Nielsen J (1997) On-line and in situ monitoring of biomass in submerged cultivations. Trends Biotechnol 15:517–522 [DOI]
- Ooi BG, Miller LK (1988) Regulation of host RNA levels during baculovirus infection. Virology 166:515–523 [DOI] [PubMed]
- Pethig R, Kell DB (1987) The passive electrical properties of biological systems: their significance in physiology, biophysics and biotechnology. Phys Med Biol 32:933–970 [DOI] [PubMed]
- Schlaeger E-J (1996) Medium design for insect cell culture. Cytotechnology 20:57–70 [DOI] [PubMed]
- Schmid G (1996) Insect cell cultivation: growth and kinetics. Cytotechnology 20:43–56 [DOI] [PubMed]
- Schmid G, Wild N, Fountoulakis M, Gallati H, Gentz R, Ozmen L, Garotta G (1994) Production and characterization of soluble mouse and human interferon-g receptors (IFNg-R) from insect cells. In: Spier RE, Griffiths JB, Berthold W (eds) Conference Proceedings to the 12th ESACT Meeting: Animal cell technology: products for today, prospects for tomorrow. Butterworth-Heinemann, Oxford/UK, pp 625–632
- Schopf B, Howaldt MW, Bailey JE (1990) DNA distribution and respiratory activity of Spodoptera frugiperda populations infected with wild-type and recombinant Autographa californica nuclear polyhedrosis virus. J Biotechnol 15:169–186 [DOI] [PubMed]
- Sonnleitner B, Locher G, Fiechter A (1992) Biomass determination. J Biotechnol 25:5–22 [DOI] [PubMed]
- Stettler M, Jaccard N, Hacker D, De Jesus M, Wurm FM, Jordan M (2006) New disposable tubes for rapid and precise biomass assessment for suspension cultures of mammalian cells. Biotechnol Bioeng 95:1228–1233 [DOI] [PubMed]
- Taticek RA, Shuler ML (1997) Effect of elevated oxygen and glutamine levels on foreign protein production at high cell densities using the insect cell-baculovirus expression system. Biotechnol Bioeng 54:142–152 [DOI] [PubMed]
- Winkelmeier P, Glauner B, Lindl T (1993) Quantification of cytotoxicity by cell volume and cell proliferation. Alterna Lab Anim 21:269–280
- Wong TK, Nielsen LK, Greenfield PF, Reid S (1994) Relationship between oxygen uptake rate and time of infection of Sf9 insect cells infected with a recombinant baculovirus. Cytotechnology 15:157–167 [DOI] [PubMed]
- Zeiser A, Bédard C, Voyer R, Jardin B, Tom R, Kamen AA (1999) On-line monitoring of the progress of infection in Sf-9 insect cell cultures using relative permittivity measurements. Biotechnol Bioeng 63:122–126 [DOI] [PubMed]
- Zeiser A, Elias CB, Voyer R, Jardin B, Kamen AA (2000) On-line monitoring of physiological parameters of insect cell cultures during the growth and infection process. Biotechnol Prog 16:803–808 [DOI] [PubMed]


