Abstract
Therapeutic antibody IgG1 has two N-linked oligosaccharide chains bound to the Fc region. The oligosaccharides are of the complex biantennary type, composed of a trimannosyl core structure with the presence or absence of core fucose, bisecting N-acetylglucosamine (GlcNAc), galactose, and terminal sialic acid, which gives rise to structural heterogeneity. Both human serum IgG and therapeutic antibodies are well known to be heavily fucosylated. Recently, antibody-dependent cellular cytotoxicity (ADCC), a lytic attack on antibody-targeted cells, has been found to be one of the critical effector functions responsible for the clinical efficacy of therapeutic antibodies such as anti-CD20 IgG1 rituximab (Rituxan®) and anti-Her2/neu IgG1 trastuzumab (Herceptin®). ADCC is triggered upon the binding of lymphocyte receptors (FcγRs) to the antibody Fc region. The activity is dependent on the amount of fucose attached to the innermost GlcNAc of N-linked Fc oligosaccharide via an α-1,6-linkage, and is dramatically enhanced by a reduction in fucose. Non-fucosylated therapeutic antibodies show more potent efficacy than their fucosylated counterparts both in vitro and in vivo, and are not likely to be immunogenic because their carbohydrate structures are a normal component of natural human serum IgG. Thus, the application of non-fucosylated antibodies is expected to be a powerful and elegant approach to the design of the next generation therapeutic antibodies with improved efficacy. In this review, we discuss the importance of the oligosaccharides attached to the Fc region of therapeutic antibodies, especially regarding the inhibitory effect of fucosylated therapeutic antibodies on the efficacy of non-fucosylated counterparts in one medical agent. The impact of completely non-fucosylated therapeutic antibodies on therapeutic fields will be also discussed.
Keywords: Therapeutic antibody; N-linked Fc oligosaccharide; Core-fucosylation; α-1,6-fucosyltransferase (FUT8) knockout; Chinese hamster ovary (CHO); ADCC; FcγRIIIa binding; Human plasma IgG
Introduction
Most of the current therapeutic antibodies that have been licensed and developed as medical agents are human IgG1 isotype including mouse/human chimeric, humanized and human IgG1. Human IgG1 is a glycoprotein bearing two N-linked biantennary complex-type oligosaccharides bound to the antibody constant region (Fc), in which the majority of the oligosaccharides are core-fucosylated (Mizuochi et al. 1982; Harada et al. 1987; Rademacher et al. 1988; Jefferis 2001), and it exercises effector functions of antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) through the interaction of the Fc with either leukocytes receptors (FcγRs) or complement. Some therapeutic antibodies can mediate direct apoptosis to the target cells as well. The efficacy of therapeutic antibodies results from specificity for the target antigen and the antibody effector functions, which are activated by the formation of immune complexes (Fig. 1). Recently, therapeutic antibodies have been shown to improve overall survival as well as time to disease progression in a variety of human malignancies such as breast, colon and haematological cancers (de Bono and Rowinsky 2002; Forero and Lobuglio 2003; Grillo-Lopez 2003; Vogel and Franco 2003) and genetic analysis of FcγR polymorphisms of cancer patients has clearly demonstrated that ADCC is one of the major anti-neoplasm mechanism responsible for clinical efficacy (Cartron et al. 2002; Anolik et al. 2003; Weng and Levy 2003; Dall’Ozzo et al. 2004; Gennari et al. 2004). The common features of antibody therapeutics, representing as high specificity to the target, long stability in blood, and high physiological functions induced effective clinical efficacy, are just about to be the features necessary for molecular-target based medicines. Thus, therapeutic antibodies now comprise the majority of recombinant proteins currently used in the clinic. A number of trials using therapeutic antibodies are ongoing, including more than 200 pre-clinical and 150 clinical studies (Reichert et al. 2005) and 17 types of recombinant monoclonal therapeutic antibodies have been approved in the U.S., and these agents represent a major new class of drugs (Table 1). It is generally expected that the indications for the use of therapeutic antibodies will be dramatically expanded in near future. Worldwide sales of total therapeutic antibodies have already exceeded 10 billion dollars in 2004 (Baker 2005).
Fig. 1.
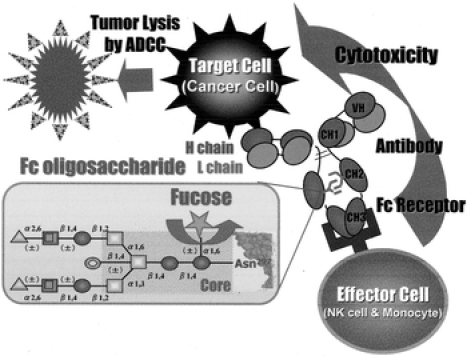
Schematic drawing of immune complex-induced effector function of ADCC. Antibody-coated tumor cells are killed by effector cells through the binding of the antibodies to Fc receptors on the effector cells. Complex-type N-linked Fc oligosaccharides (consists of GlcNAc (●), mannose (□), bisecting GlcNAc ( ), fucose (
), fucose ( ) galactose (
) galactose ( ), sialic acid (▲)) attached to the CH2 domains of the Fc affect the cellular cytotoxicity of ADCC
), sialic acid (▲)) attached to the CH2 domains of the Fc affect the cellular cytotoxicity of ADCC
Table 1.
Recombinant therapeutic antibodies on the US market
| Trade | Company | Type | Antigen | Indication | Approval date |
|---|---|---|---|---|---|
| Reopro® | Centocor/Lilly | Chimera | gpIIb/IIIa | Thrombosis | 12/24/1994 |
| Rituxan® | IDEC/Genentech/Roche | Chimera | CD20 | NHL | 11/26/1997 |
| Zenepax® | Roche | Humanized | IL-2R | Transplantation | 12/10/1997 |
| Simulect® | Novartis | Humanized | IL-2R | Transplantation | 05/12/1998 |
| Synagis® | MedImmune/Abott | Humanized | RSV | RSV infection | 06/19/1998 |
| Remicade® | Centocor/J&J | Chimera | TNF-α | RA, Chron’s | 08/24/1998 |
| Herceptin® | Genentech/Roche | Humanized | Her2 | Breast cancer | 09/25/1998 |
| Mylotarg® | Cellutech/AHP | Drug-conjugate | CD33 | AML | 05/17/2000 |
| Campath® | ILEX/Schering | Humanized | CD52 | B-CLL | 05/05/2001 |
| Zevalin® | IDEC/Schering | 90Y-conjugate | CD20 | NHL | 02/19/2002 |
| Humira® | Abott/CAT | Fully human | TNF-α | RA | 12/31/2002 |
| Xolair® | Genentech/Novartis/Tanox | Humanized | IgE | Allergic asthma | 06/20/2003 |
| Bexxar® | Corixa/SKB | 131I-conjugate | CD20 | NHL | 06/27/2003 |
| Raptiva® | Genentech/Xoma | Humanized | LFA-1 | Psoriasis | 10/27/2003 |
| Erbitux® | ImClone/BMS/Merck | Chimeric | EGFR | Colon cancer | 02/12/2004 |
| Avastin® | Genentech | Humanized | VEGF | Colon cancer | 02/26/2004 |
| Tysabri®a | Biogen-IDEC/Elan | Humanized | Integrinα4β1 | MS | 12/23/2004 |
RSV, Respiratory syncytial virus; NHL, non-Hodgkin lymphoma; AML, acute myeloid leukemia; B-CLL, B-cell chronic lymphocytic leukemia; RA, rheumatoid arthritis; MS, multiple sclerosis
aVoluntary suspension of Tysabri® marketing has been announced on Feb. 2005, and reintroduced on July 2006
Current feature of therapeutic antibodies
Although antibody therapeutics are currently recognized as new medicines that confer great benefits to patients suffering from various obstinate diseases, we also realize that these agents yield a serious issue of medical economy, so called “economical toxicity”. A common feature of therapeutic antibodies used for cancer treatment is that their anti-tumor efficacy requires high serum concentrations and continued therapy for several months. The treatment cycles thereby consume several grams of therapeutic antibody, resulting in a significant amount of drug needed and very high costs (Berinstein et al. 1998; Baselga and Albanell 2001; Goldenberg 1999). In fact, administration of a high dose (2–8 mg kg−1) of either anti-CD20 IgG1 rituximab (Rituxan®) or anti-Her2/neu IgG1 trastuzumab (Herceptin®) is required to keep the effective serum concentration of over 10 μg mL−1, which is more than 100-fold high administrated dose of cytokine therapy such as erythropoietin (EPO) and granulocyte colony-stimulating factor (G-CSF) regimens. The drug cost of one cycle medical treatment with such therapeutic antibodies amounts to being over 20 thousand dollars. This is one of the reasons why annual sales of rituximab and trastuzumab have exceed and almost reached 2 billion dollars, respectively, although the population of either non-Hodgkin’s lymphoma or breast cancer patients is limited; both patient groups are not major in whole cancer patients. As mentioned above, the indication of therapeutic antibodies will be more expanded in near future, and the economical situation of antibody therapy will soon no longer be viable, i.e., will be at risk of collapse. Moreover, there still remains the need to improve the efficacy of therapeutic antibodies such that they can be used to achieve not only remission, but also complete recovery from disease; many of the currently approved therapeutic antibodies remain unable to induce more than remission. Currently, numerous efforts to improve the efficacy of therapeutic antibodies are underway. These include the introduction of amino acid mutations in the Fc region (Shields et al. 2001; Lazar et al. 2006) and the co-administration of CpG as an adjuvant (Jahrsdorfer and Weiner 2003; van Ojik et al. 2003) or that of cytokines such as IL-2 (Friedberg et al. 2002) and GM-CSF (Stockmeyer et al. 2001). Among these efforts, the modification of N-linked Fc oligosaccharides, especially core-fucose removal, has been of note as a key of next generation therapeutic antibodies with improved efficacy (Shields et al. 2002; Shinkawa et al. 2003; Yamane-Ohnuki et al. 2004; Mori et al. 2004; Okazaki et al. 2004; Niwa et al. 2004a, b, 2005a, 2005b; Natsume 2005; Jefferis 2005; Iida et al. 2006; Kanda et al. 2006; Satoh et al. 2006).
Human serum IgG inhibits therapeutic antibody-induced ADCC
We should note that there is a huge discrepancy between the potency of therapeutic antibodies in vitro and in vivo, in terms of dosing therapeutic antibodies, especially anti-cancer antibodies. Cancer patients treated with therapeutic antibodies typically need to receive weekly doses of several hundred milligrams over several months for keeping the effective serum concentration of more than 10 μg mL−1 (Baselga and Albanell 2001; Berinstein et al. 1998; Goldenberg 1999). On the contrary, the maximal in vitro cellular cytotoxicity by ADCC of these therapeutic antibodies can be achieved at antibody concentrations of less than 10 ng mL−1, which is several orders of magnitude below the targeted serum concentrations (Lewis et al. 1993; Sliwkowski et al. 1999). This discrepancy, the low in vivo efficacy of therapeutic antibodies in contrast to the high in vitro ADCC, has been recently disclosed to be mainly due to competition between serum IgG and therapeutic antibodies for binding to FcγRIIIa on natural killer (NK) cells; endogenous human serum IgG inhibits ADCC induced by therapeutic antibodies (Vugmeyster and Howell 2004; Preithner et al. 2006). Human serum IgG dose not affect antigen binding by therapeutic antibodies however significantly impairs interaction of the antibodies with FcγRIIIa, and induce the inhibition of the ADCC of therapeutic antibodies. This phenomenon leads to the elucidation of necessity of the use of high doses in the clinical treatment, and also elucidates the molecular mechanism for the enhanced efficacy of non-fucosylated therapeutic antibodies in humans. Non-fucosylated therapeutic antibodies have much higher binding affinity for FcγRIIIa than fucosylated human serum IgG, which is a preferable character to conquer the interference by human plasma IgG (Iida et al. 2006; Satoh et al. 2006). Therefore, non-fucosylated therapeutic antibodies can evade the inhibitory effect of human plasma IgG on ADCC through their high FcγRIIIa bonding.
Fucosylated therapeutic antibodies spoil the non-fucosylated antibody-induced ADCC
More importantly, the enhanced ADCC of non-fucosylated therapeutic antibodies against a specific antigen has been shown to be inhibited in a dose-dependent manner by fucosylated antibodies against the same antigen in the case of both rituximab and trastuzumab in vitro and ex vivo (Iida et al. 2006; Satoh et al. 2006). Non-fucosylated therapeutic antibodies existing in the antibody mixtures, composed of non-fucosylated and fucosylated forms, do not exhibit activity equivalent to that of equal amounts of non-fucosylated therapeutic antibodies alone. This phenomenon reflects the fact that the maximum efficacy of both in vitro and ex vivo ADCCs of fucosylated therapeutic antibodies never reaches the level as high as that by non-fucosylated therapeutic antibodies, even when high doses of the fucosylated antibodies are employed. One of the major mechanisms for the inhibitory effect of fucosylated therapeutic antibodies on the ADCC activity of non-fucosylated counterparts is considered to be through the competition of the two antibodies for the antigens on target cells. Therapeutic antibodies have the same antigen binding ability irrespectively of core-fucosylation, and the density of the non-fucosylated antibodies binding on the target cells is thus reduced by fucosylated antibody occupation, which yields a similar effect to shed the target antigens from capture by the therapeutic agent possessing high ADCC activity, i.e., non-fucosylated therapeutic antibodies, even in human plasma. To overcome the interference of human plasma IgG, the target tumor cells need to be coated with therapeutic antibodies possessing higher binding affinity to FcγRIIIa than human plasma IgG. Hence, therapeutic antibodies consisting of only the non-fucosylated human IgG1 form (i.e., not including any of its fucosylated counterparts) is thought to be ideal.
Manufacturing of non-fucosylated therapeutic antibodies
All therapeutic antibodies currently licensed on the market are produced in rodent mammalian cell lines such as Chinese hamster ovary (CHO), mouse myeloma NS0 and SP2/0 and mouse hybridoma, and almost all of molecules produced by these cell lines have core-fucosylation in the Fc oligosaccharides (Schenerman et al. 1999; Kamoda et al. 2004) which means that ADCC of the products is unfortunately far from optimal. These cell lines retain the intrinsic α-1,6-fucosyltransferase (FUT8) enzyme activity responsible for the core-fucosylation of the Fc oligosaccharides. FUT8 catalyzes the transfer of fucose from GDP-fucose to the innermost GlcNAc in an α-1,6 linkage (Uozumi et al. 1996), and complete and irreversible inactivation of FUT8 function in antibody producing cells is essential to manufacture non-fucosylated therapeutic antibodies. A production process in which the core-fucosylation levels of the products vary depending on culture conditions should introduce severe clinical issues, and is therefore unacceptable from a practical industrial standpoint. Robust stable production of completely non-fucosylated therapeutic antibodies in a fixed quality has been achieved by the generation of a unique host cell line, in which the endogenous FUT8 gene is knocked out (Yamane-Ohnuki et al. 2004; Kanda et al. 2006). FUT8-knockout CHO/DG44 cells exhibit a morphology, growth kinetics, and productivity similar to those of the parent cells, and enable to stably produce non-fucosylated therapeutic antibodies with fixed quality and consistent ADCC activity of the ingredients in fed-batch serum-free cultures using bioreactors commonly employed in pharmaceutical industry. Thus, the application of non-fucosylated antibodies is expected to be a promising approach as a next-generation of therapeutic antibodies with improved efficacy even when administrated in low doses in humans in vivo (Satoh et al. 2006). Clinical trials using non-fucosylated antibody therapeutics are currently underway.
References
- Anolik JH, Campbell D, Felgar RE, Young F, Sanz I, Rosenblatt J, Looney RJ (2003) The relationship of FcgammaRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum 48:455–459 [DOI] [PubMed]
- Baker M (2005) Upping the ante on antibodies. Nat Biotechnol 23:1065–1072 [DOI] [PubMed]
- Baselga J, Albanell J (2001) Mechanism of action of anti-HER2 monoclonal antibodies. Ann Oncol 12(Suppl 1):S35–S41 [DOI] [PubMed]
- Berinstein NL, Grillo-Lopez AJ, White CA, Bence-Bruckler I, Maloney D, Czuczman M, Green D, Rosenberg J, McLaughlin P, Shen D (1998) Association of serum Rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol 9:995–1001 [DOI] [PubMed]
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H (2002) Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 99:754–758 [DOI] [PubMed]
- Dall’Ozzo S, Tartas S, Paintaud G, Cartron G, Colombat P, Bardos P, Watier H, Thibault G (2004) Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration–effect relationship. Cancer Res 64:4664–4669 [DOI] [PubMed]
- de Bono JS, Rowinsky EK (2002) The ErbB receptor family: a therapeutic target for cancer. Trends Mol Med 8(4 Suppl):S19–S26 [DOI] [PubMed]
- Forero A, Lobuglio AF (2003) History of antibody therapy for non-Hodgkin’s lymphoma. Semin Oncol 30:1–5 [DOI] [PubMed]
- Friedberg JW, Neuberg D, Gribben JG, Fisher DC, Canning C, Koval M, Poor CM, Green LM, Daley J, Soiffer R, Ritz J, Freedman AS (2002) Combination immunotherapy with rituximab and interleukin 2 in patients with relapsed or refractory follicular non-Hodgkin’s lymphoma. Br J Haematol 117:828–834 [DOI] [PubMed]
- Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, Castiglioni F, Villani L, Magalotti C, Gibelli N, Oliviero B, Ballardini B, Da Prada G, Zambelli A, Costa A (2004) Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res 10:5650–5655 [DOI] [PubMed]
- Goldenberg MM (1999) Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther 21:309–318 [DOI] [PubMed]
- Grillo-Lopez AJ (2003) Rituximab (Rituxan/MabThera): the first decade (1993–2003). Expert Rev Anticancer Ther 3:767–769 [DOI] [PubMed]
- Harada H, Kamei M, Tokumoto Y, Yui S, Koyama F, Kochibe N, Endo T, Kobata A (1987) Systematic fractionation of oligosaccharides of human immunoglobulin G by serial affinity chromatography on immobilized lectin columns. Anal Biochem 164:374–381 [DOI] [PubMed]
- Iida S, Misaka H, Inoue M, Shibata M, Nakano R, Yamane-Ohnuki N, Wakitani M, Yano K, Shitara K, Satoh M (2006) Nonfucosylated therapeutic IgG1 antibody can evade the inhibitory effect of serum immunoglobulin G on antibody-dependent cellular cytotoxicity through its high binding to FcγIIIa. Clin Cancer Res 12:2879–2887 [DOI] [PubMed]
- Jahrsdorfer B, Weiner GJ (2003) Immunostimulatory CpG oligodeoxynucleotides and antibody therapy of cancer. Semin Oncol 30:476–482 [DOI] [PubMed]
- Jefferis R (2001) Glycosylation of human IgG antibodies: relevance to therapeutic applications. BioPharm 14:19–26
- Jefferis R (2005) Glycosylation of recombinant antibody therapeutics. Biotechnol Prog 21:11–16 [DOI] [PubMed]
- Kamoda S, Nomura C, Kinoshita M, Nishiura S, Ishikawa R, Kakehi K, Kawasaki N, Hayakawa T (2004) Profiling analysis of oligosaccharides in antibody pharmaceuticals by capillary electrophoresis. J Chromatogr A 1050:211–216 [DOI] [PubMed]
- Kanda Y, Yamane-Ohnuki N, Sakai N, Yamano K, Nakano R, Inoue M, Misaka H, Iida S, Wakitani M, Konno Y, Yano K, Shitara K, Hosoi S, Satoh M (2006) Comparison of cell lines for stable production of fucose-negative antibodies with enhanced ADCC. Biotechnol Bioeng 94:680–688 [DOI] [PubMed]
- Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, Vielmetter J, Carmichael DF, Hayes RJ, Dahiyat BI (2006) Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA 103:4005–4010 [DOI] [PMC free article] [PubMed]
- Lewis GD, Figari I, Fendly B, Wong WL, Carter P, Gorman C, Shepard HM (1993) Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol Immunother 37:255–263 [DOI] [PMC free article] [PubMed]
- Mizuochi T, Taniguchi T, Shimizu A, Kobata A (1982) Structural and numerical variations of the carbohydrate moiety of immunoglobulin G. J Immunol 129:2016–2020 [PubMed]
- Mori K, Kuni-Kamochi R, Yamane-Ohnuki N, Wakitani M, Yamano K, Imai H, Kanda Y, Niwa R, Iida S, Uchida K, Shitara K, Satoh M (2004) Engineering Chinese hamster ovary cells to maximize effector function of produced antibodies using FUT8 siRNA. Biotechnol Bioeng 88:901–908 [DOI] [PubMed]
- Natsume A, Wakitani M, Yamane-Ohnuki N, Shoji-Hosaka E, Niwa R, Uchida K, Satoh M, Shitara K (2005) Fucose removal from complex-type oligosaccharide enhances the antibody-dependent cellular cytotoxicity of single-gene-encoded antibody comprising a single-chain antibody linked the antibody constant region. J Immunol Methods 306:93–103 [DOI] [PubMed]
- Niwa R, Shoji-Hosaka E, Sakurada M, Shinkawa T, Uchida K, Nakamura K, Matsushima K, Ueda R, Hanai N, Shitara K (2004a) Defucosylated anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T cell leukemia and lymphoma. Cancer Res 64:2127–2133 [DOI] [PubMed]
- Niwa R, Hatanaka S, Shoji-Hosaka E, Sakurada M, Kobayashi Y, Uehara A, Yokoi H, Nakamura K, Shitara K (2004b) Enhancement of the antibody-dependent cellular cytotoxicity of low-fucose IgG1 is independent of FcgRIIIa functional polymorphism. Clin Cancer Res 10:6248–6255 [DOI] [PubMed]
- Niwa R, Sakurada M, Kobayashi Y, Uehara A, Matsushima K, Ueda R, Nakamura K, Shitara K (2005a) Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density. Clin Cancer Res 11:2327–2336 [DOI] [PubMed]
- Niwa R, Natsume A, Uehara A, Wakitani M, Iida S, Uchida K, Satoh M, Shitara K (2005b) IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J Immunol Methods 306:151–160 [DOI] [PubMed]
- Okazaki A, Shoji-Hosaka E, Nakamura K, Wakitani M, Uchida K, Kakita S, Tsumoto K, Kumagai I, Shitara K (2004) Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcγRIIIa. J Mol Biol 336:1239–1249 [DOI] [PubMed]
- Preithner S, Elm S, Lippold S, Locher M, Wolf A, da Silva AJ, Baeuerle PA, Prang NS (2006) High concentrations of therapeutic IgG1 antibodies are needed to compensate for inhibition of antibody-dependent cellular cytotoxicity by excess endogenous immnunoglobulin G. Mol Immunol 43:1183–1193 [DOI] [PubMed]
- Rademacher TW, Parekh RB, Dwek RA (1988) Glycobiology. Annu Rev Biochem 57:785–838 [DOI] [PubMed]
- Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC (2005) Monoclonal antibody successes in the clinic. Nat Biotechnol 23:1073–1078 [DOI] [PubMed]
- Satoh M, Iida S, Shitara K (2006) Non-fucosylated therapeutic antibodies as next-generation therapeutic antibodies. Expert Opin Biol Ther 6:1161–1173 [DOI] [PubMed]
- Schenerman MA, Hope JN, Kletke C, Singh JK, Kimura R, Tsao EI, Folena-Wasserman G (1999) Comparability testing of a humanized monoclonal antibody (Synagis) to support cell line stability, process validation, and scale-up for manufacturing. Biologicals 27:203–215 [DOI] [PubMed]
- Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, Fox JA, Presta LG (2001) High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J Biol Chem 276:6591–6604 [DOI] [PubMed]
- Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH, Presta L (2002) Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem 277:26733–26740 [DOI] [PubMed]
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K (2003) The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 278:3466–3473 [DOI] [PubMed]
- Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA (1999) Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin). Semin Oncol 26:60–70 [PubMed]
- Stockmeyer B, Elsasser D, Dechant M, Repp R, Gramatzki M, Glennie MJ, van de Winkel JG, Valerius T (2001) Mechanisms of G-CSF- or GM-CSF-stimulated tumor cell killing by Fc receptor-directed bispecific antibodies. J Immunol Methods 248:103–111 [DOI] [PubMed]
- Uozumi N, Yanagidani S, Miyoshi E, Ihara Y, Sakuma T, Gao CX, Teshima T, Fujii S, Shiba T, Taniguchi N (1996) Purification and cDNA cloning of porcine brain GDP-L-Fuc:N-acetyl-beta-d-glucosaminide alpha1,6fucosyltransferase. J Biol Chem 271:27810–27817 [DOI] [PubMed]
- van Ojik HH, Bevaart L, Dahle CE, Bakker A, Jansen MJ, van Vugt MJ, van de Winkel JG, Weiner GJ (2003) CpG-A and B oligodeoxynucleotides enhance the efficacy of antibody therapy by activating different effector cell populations. Cancer Res 63:5595–5600 [PubMed]
- Vogel CL, Franco SX (2003) Clinical experience with trastuzumab (herceptin). Breast J 9:452–462 [DOI] [PubMed]
- Vugmeyster Y, Howell K (2004) Rituximab-mediated depletion of cynomolgus monkey B cells in vitro in different matrices: possible inhibitory effect of IgG. Int Immunopharmacol 4:1117–1124 [DOI] [PubMed]
- Weng WK, Levy R (2003) Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 21:3940–3947 [DOI] [PubMed]
- Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, Wakitani M, Niwa R, Sakurada M, Uchida K, Shitara K, Satoh M (2004) Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng 87:614–622 [DOI] [PubMed]


