Abstract
Some of inositol derivatives have been reported to help the action of insulin stimulating glucose uptake in skeletal muscle cells. Rat L6 myotubes were employed in an attempt to develop an in vitro model system for investigation of the possible insulin-like effect of eight inositol derivatives, namely allo-inositol, d-chiro-inositol l-chiro-inositol, epi-inositol, muco-inositol, myo-inositol, scyllo-inositol and d-pinitol. At a higher concentration of 1 mM seven inositol derivatives other than myo-inositol were able to stimulate glucose uptake, while at 0.1 mM only d-chiro-inositol, l-chiro-inositol, epi-inositol and muco-inositol could induce glucose uptake, indicating their significant insulin-mimetic activity. Immunoblot analyses revealed that at least d-chiro-inositol, l-chiro-inositol, epi-inositol, muco-inositol and d-pinitol were able to induce translocation of glucose transporter 4 (GLUT4) to plasma membrane not only in L6 myotubes but also in skeletal muscles of rats ex vivo. These results demonstrated that L6 myotubes appeared efficient as an in vitro system to identify inositol derivatives exerting an insulin-like effect on muscle cells depending on the induced translocation of GLUT4.
Keywords: Glucose transporter 4, Glucose uptake, Inositol, L6 myotubes, Muscle cells
Introduction
Skeletal muscle is one of the major insulin-target tissues responsible for maintenance of whole body glucose homeostasis. It is well established that insulin stimulation of glucose uptake in skeletal muscle cells is mediated through translocation of glucose transporter 4 (GLUT4) from the endoplasmic reticulum to the plasma membrane (Klip and Ishiki 2005). It is also known that a defect in glucose transport efficiency and GLUT4 activity results in insulin resistance (Petersen and Shulman 2006). Thus, the action of insulin on muscle cells for glucose uptake depends on the induced translocation of GLUT4, but the mechanism regulating the translocation of GLUT4 has remained to be elucidated. Attempts have been made to develop efficient in vitro model systems for in-depth studies on the glucose uptake involving GLUT4 in muscle cells. However, such systems have been still awaited, since most of the established skeletal muscle cell lines reportedly show minimal insulin-dependent glucose uptake and translocation of GLUT4, and only the exceptions are rat L6 and mouse C2C12 myotubes (Nedachi and Kanzaki 2006). When stimulated by insulin, L6 myotubes appeared to exhibit glucose uptake to a larger extent than C2C12 cells (Sarabia et al. 1990), suggesting that at present L6 myotubes could be the most promising candidate as an efficient in vitro model system to investigate glucose uptake in muscle cells.
Inositol stands for 1,2,3,4,5,6-cyclohexanehexol, which is a class of compounds forming nine distinct stereoisomers through epimerization of the six hydroxyl groups (Fig. 1). Some of these derivatives have been reported to possess various biological functions for the treatment of diseases such as depression, panic disorder, polycystic ovary syndrome and so on (Benjamin et al. 1995; Iuorno et al. 2002; McLaurin et al. 2000). Furthermore, d-chiro-inositol (Kawa et al. 2003) and its 3-O-methyl form, d-pinitol (3-O-methyl-d-chiro-inositol; Fig. 1) (Bates et al. 2000; Weeks and Albany 2003; Ostlund and Sherman 1998), have been demonstrated to possess insulin-mimetic activity, and are also effective against insulin resistance. However, the effectiveness of the other inositol derivatives, differing only in the positions of the hydroxyl groups, has remained to be evaluated experimentally so far.
Fig. 1.
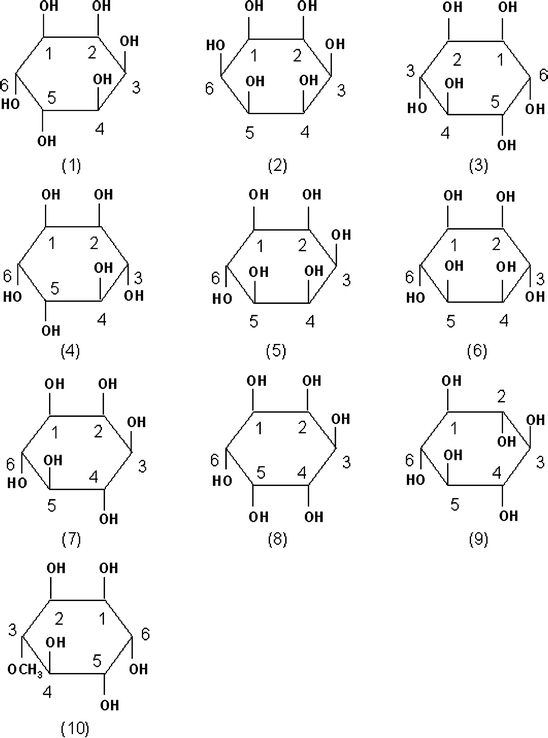
Structure of inositol derivatives. allo-Inositol (1), d-chiro-inositol (2), l-chiro-inositol (3), cis-inositol (4), epi-inositol (5), muco-inositol (6), myo-inositol (7), neo-inositol (8), scyllo-inositol (9) and d-pinitol (10). Carbon numbering is indicated in each structure
In this study, we attempted to develop an in vitro model system using L6 myotubes to investigate the possible stimulative effect of inositol derivatives on glucose uptake in the cultured cells depending on the induced translocation of GLUT4 to plasma membrane. In parallel an ex vivo experiment was performed to confirm that the effective inositol derivatives actually stimulated translocation of GLUT4 in the skeletal muscles of rat as well as in the in vitro system.
Materials and methods
Materials
Modified Eagle’s medium was obtained from Nissui Pharmaceutical (Tokyo, Japan). Fetal bovine serum was purchased from Equitech-Bio, Inc. (Kerrville, TX). allo-Inositol, d-chiro-inositol, l-chiro-inositol, epi-inositol, muco-inositol, myo-inositol, scyllo-inositol and d-pinitol (their structures are shown in Fig. 1) were supplied by Hokko Chemical Industry Co., Ltd (Tokyo, Japan). [1,2-3H(N)] 2-Deoxy-d-glucose (2DG) was purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO). Anti-GLUT4, anti-insulin receptor β-subunit (IRβ) and anti-goat IgG antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and ECL plus was from GE Healthcare Bio-Sciences Corp. (Piscataway, NJ).
Cell culture
L6 myoblasts were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum at 37 °C in a 5% CO2 atmosphere. Once the myoblasts were grown to confluence in 24-well plates, the medium was replaced with Dulbecco’s modified Eagle’s medium containing 2% fetal bovine serum to induce differentiation into myotubes. Fully differentiated myotubes were serum-starved for 18 h in Dulbecco’s modified Eagle’s medium containing 0.2% bovine serum albumin prior to experiments. The degree of the differentiation of L6 myotubes was confirmed by detection of the expression level of GLUT4 (data not shown).
Glucose uptake assay
L6 myotubes differentiated enough were exposed to 100 nM insulin or inositol derivatives (1 mM or 0.1 mM) in Krebs-Ringer HEPES (KRH) buffer (50 mM HEPES, pH 7.4, 137 mM NaCl, 4.8 mM KCl, 1.85 mM CaCl2 and 1.3 mM MgSO4) for 15 min followed by an additional incubation period of 5 min with 2DG (6.5 mM, 0.5 μCi). The cells were washed four times with ice-cold KRH buffer, lysed by adding 250 μL of 0.05 N NaOH, and then transferred to vials with scintillation cocktail. The radioactivity in the cells was measured by liquid scintillation counter. Non-specific uptake was measured using the cells pretreated with 20 μM cytochalasin B.
Preparation of membrane fraction from myotubes and tissues
A membrane fraction of L6 myotubes was prepared as described (Nishiumi and Ashida 2007). To perform the ex vivo experiment, skeletal muscle tissue was enucleated from hind legs of rats and chopped into small pieces, 100 mg of the chopped pieces was incubated in 3 mL KRH buffer with continuous shaking in the presence of inositol derivatives (1 mM) or insulin (100 nM) for 15 min at 37 °C, and then immediately washed twice with excess ice-cold KRH buffer. A membrane fraction of the muscle cells was prepared following a similar procedure as described (Nishiumi and Ashida 2007). Briefly, the skeletal muscle cells were homogenized in Buffer A (10 mM Tris–HCl, pH 7.8, 10 mM KCl, 1.5 mM MgCl2, 1 mM phenylmethylsulfonylfluoride, 0.5 mM dithiothreitol, 5 μg mL−1 aprotinin and 10 μg mL−1 leupeptin) containing 0.1% Nonidet P-40 and passed through 22-gauge needle three times. Homogenates were spun at 1,000g for 10 min at 4 °C. The pellet was resuspended in Buffer A and spun at 1,000g for another 10 min at 4 °C. Plasma membrane fraction was obtained by resuspending the resulting pellet in Buffer A containing 1% Nonidet P-40, and centrifuging at 10,000g for 20 min at 4 °C. The membrane fraction was subjected to the following immunoblot analysis. Animal treatment in this study conformed to the “Guidelines for the care and use of experimental animals, in Rokkodai Campus, Kobe University”.
Immunoblot analysis
Aliquots of the plasma membrane fraction (10 μg protein) were separated by 10% SDS-polyacrylamide gel electrophoresis, and transferred onto a PVDF membrane. The PVDF membrane was blocked with 1% (w/v) non-fat dry milk in TBST buffer containing 10 mM Tris–HCl, pH 8.0, 150 mM NaCl and 0.05% Tween-20, and incubated with anti-GLUT4 or anti-IRβ (as the internal control of the plasma membrane fraction) antibody for 1 h at room temperature. The membrane was further incubated with horseradish peroxidase-conjugated anti-goat IgG antibody for 1 h at room temperature in the same buffer, washed appropriately, and then immunoblot signals were obtained using ECL plus detection kit following the provided standard procedure.
Statistical analysis
Data are expressed as the means ± SE. Statistical significance was analyzed using Dunnett’s multiple comparison test, and a 0.05 level of the probability was used as the criterion of significance.
Results and discussion
Inositol derivatives stimulate glucose uptake in L6 myotubes
Peripheral tissue such as skeletal muscle is important to maintain the postprandial plasma glucose levels (Klip and Ishiki 2005). Rat L6 myotubes were employed as an in vitro system to investigate the effects of eight inositol derivatives on glucose uptake, which was monitored by increase in radioactivity of 2DG, non-metabolizable glucose analogue, incorporated into the cells. Seven inositol derivatives other than myo-inositol stimulated glucose uptake in L6 myotubes at 1 mM in the absence of added insulin (Table 1). Obviously myo-inositol failed to exert an effect on glucose uptake, which is consistent with the previous results that myo-inositol did not possess any hypoglycaemic effect (Ostlund and Sherman 1998; Ortmeyer et al. 1993). In addition, d-chiro-inositol, l-chiro-inositol, epi-inositol and muco-inositol showed a significant increase in the glucose uptake as compared to insulin (Table 1). Our results also showed that at 1 mM d-pinitol increased glucose uptake almost 50% over control (Table 1), coinciding with the previous observation (Bates et al. 2000).
Table 1.
Effect of inositol derivatives on glucose uptake in L6 myotubes
| Addition | Glucose uptakea |
|---|---|
| (nmol min−1 per 3.5 × 106 cells) | |
| None | 1.62 ± 0.04 |
| Insulin (100 nM) | 2.25 ± 0.09* |
| At 1 mM | |
| allo-Inositol | 2.22 ± 0.19* |
| d-chiro-Inositol | 2.53 ± 0.09*,** |
| l-chiro-Inositol | 2.82 ± 0.10*,** |
| epi-Inositol | 2.69 ± 0.16*,** |
| muco-Inositol | 2.71 ± 0.19*,** |
| myo-Inositol | 1.80 ± 0.08 |
| scyllo-Inositol | 2.35 ± 0.13* |
| Pinitol | 2.30 ± 0.16* |
| At 0.1 mM | |
| allo-Inositol | 1.67 ± 0.07 |
| d-chiro-Inositol | 1.98 ± 0.12* |
| l-chiro-Inositol | 2.14 ± 0.02* |
| epi-Inositol | 2.30 ± 0.05* |
| muco-Inositol | 2.31 ± 0.05* |
| myo-Inositol | 1.57 ± 0.04 |
| scyllo-Inositol | 1.68 ± 0.04 |
| Pinitol | 1.64 ± 0.08 |
aEach value represents the means ± SE of results from at least five independent assays
* Significantly different from the control group (None) by Dunnett’s test, p < 0.05
** Significantly different from the insulin addition group by Dunnett’s test, p < 0.05
We examined the effect at a lower dose of 0.1 mM inositol derivatives in L6 myotubes. At the lower dose, d-chiro-inositol, l-chiro-inositol, epi-inositol and muco-inositol were still effective to cause a significant increase in glucose uptake as compared to control (Table 1). But d-pinitol was not effective, although it has been demonstrated to possess insulin-mimetic activity (Kawa et al. 2003; Bates et al. 2000; Weeks and Albany 2003; Ostlund and Sherman 1998). This observation might coincide with the previous reports that d-pinitol did not exert a significant effect when treated at a lower dose (Bates et al. 2000; Weeks and Albany 2003). Interestingly, we found that even at this lower dose l-chiro-inositol, muco-inositol and epi-inositol were still effective almost similarly to insulin (Table 1). From these results, on top of the reported effective d-pinitol and d-chiro-inositol, we selected l-chiro-inositol, epi-inositol and muco-inositol for the further analysis.
Inositol derivatives stimulate translocation of GLUT4 to the plasma membrane
To determine whether translocation of GLUT4 might be actually a mechanism by which inositol derivatives increased glucose uptake in L6 myotubes, we next tried to detect translocation of GLUT4 in plasma membrane after the treatment with inositol derivatives by means of an immunoblot analysis of plasma membrane fraction prepared from L6 myotubes (Nishiumi and Ashida 2007). Since GLUT4 level was shown to be almost constant in the whole cell lysate, an increase in the amount of GLUT4 in a plasma membrane fraction could tell us that its translocation had occurred (Nishiumi and Ashida 2007). Treatment with insulin obviously increased the amount of GLUT4 in the plasma membrane fraction indicating the induced translocation of GLUT4 (Fig. 2a). And the three derivatives with the efficient activity to enhance glucose uptake at the lower dose (i.e. l-chiro-inositol, epi-inositol and muco-inositol; Table 1) were tested with reference to d-chiro-inositol and d-pinitol. The results indicated that l-chiro-inositol, epi-inositol and muco-inositol as well as d-chiro-inositol and d-pinitol induced translocation of GLUT4 to the plasma membrane to no less extent than insulin (Fig. 2a). Although by unknown reason difference in the amount of GLUT4 did not always seem to coincide with that in glucose uptake levels (Table 1), at this higher dose of 1 mM in vitro these inositol derivatives were proven to possess the activity in induction of accumulation of GLUT4 in plasma membrane of L6 myotubes.
Fig. 2.
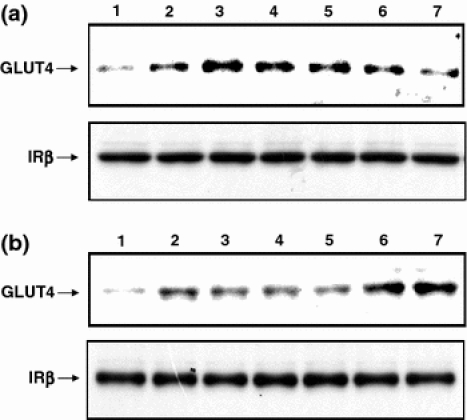
Effect of inositol derivatives on translocation of GLUT4 in L6 myotubes (a) and in skeletal muscles of rats ex vivo (b). Proteins contained in plasma membrane fraction were prepared from cells after treatment with inositol derivatives or insulin (in lane 1, basal; 2, insulin; 3, d-pinitol; 4, d-chiro-inositol; 5, l-chiro-inositol; 6, muco-inositol and 7, epi-inositol) were subjected to SDS-polyacrylamide gel electrophoresis, transferred to a PVDF membrane, and then GLUT4 was detected by immunoblotting as described in the text. The protein level of IRβ in the plasma membrane fraction was also detected and shown in each panel as the internal control
In addition we performed an ex vivo assay to confirm the translocation of GLUT4 occurring in skeletal muscles. When skeletal muscles of rats were treated with 100 nM insulin ex vivo, an increase in the amount of GLUT4 contained in the plasma membrane was observed (Fig. 2b), indicating the induced translocation of GLUT4 detected in this ex vivo system, which was in agreement with the previous report that insulin induces glucose uptake and translocation of GLUT4 in muscle cells (Khan and Pessin 2002). Furthermore, d-chiro-inositol, l-chiro-inositol, epi-inositol, muco-inositol and d-pinitol at 1 mM also appeared to induce translocation of GLUT4 to the plasma membrane no less efficiently than insulin (Fig. 2b). Thus, d-chiro-inositol, l-chiro-inositol, epi-inositol, muco-inositol and d-pinitol were shown to have the ability to stimulate translocation of GLUT4 enhancing glucose uptake in L6 myotubes in vitro and the induced translocation of GLUT4 in skeletal muscle cells was confirmed ex vivo as well. However, the abilities differed among the inositol derivatives (Table 1 and Fig. 2). Judging from our results (Fig. 2b), epi-inositol and muco-inositol stimulated translocation of GLUT4 to the plasma membrane somehow more efficiently than insulin did, which might be related to the results indicating their higher activity to induce glucose uptake in L6 muscle cells (Table 1). On the other hand, translocation of GLUT4 stimulated by d-chiro-inositol and d-pinitol was not as prominent as their insulin mimetic activities in vivo reported previously (Kawa et al. 2003; Bates et al. 2000; Weeks and Albany 2003; Ostlund and Sherman 1998). This inconsistency might be due to the difference in the conditions between in vivo and ex vivo.
In any case, all the results could lead us to conclude that these inositol derivatives, especially d-chiro-inositol, l-chiro-inositol, epi-inositol and muco-inositol, were shown to stimulate translocation of GLUT4 to plasma membrane in muscle cells in vitro as well as ex vivo. However, the mechanism for the effect of these inositol derivatives on glucose transport involving the induced translocation of GLUT4 remains still to be elucidated. It is expected that L6 myotubes will be a promising model system for the further studies.
Acknowledgments
We thank M. Yamaguchi, Hokko Chemical Industry Co., Ltd, and T. Yoshida, Fujicco Co., Ltd, for some of the inositol derivatives and generous supports. This work was supported in part by Fermentation and Metabolism Research Grant Awards from Japan Bioindustry Association to KY (2006).
Abbreviations
- 2DG
[1,2-3H(N)] 2-Deoxy-d-glucose
- GLUT4
Glucose transporter 4
- IRβ
Insulin receptor β-subunit
- KRH
Krebs-Ringer HEPES
References
- Bates SH, Jones RB, Bailey CJ (2000) Insulin-like effect of pinitol. Br J Pharmacol 130:1944–1948 [DOI] [PMC free article] [PubMed]
- Benjamin J, Agam G, Levine J, Bersudsky Y, Kofman O, Belmaker RH (1995) Inositol treatment in psychiatry. Psychopharmacol Bull 31:167–175 [PubMed]
- Iuorno MJ, Jakubowicz DJ, Baillargeon JP, Dillon P, Gunn RD, Allan G, Nestler JE (2002) Effects of D-chiro-inositol in lean women with the polycystic ovary syndrome. Endocr Pract 8:417–423 [DOI] [PubMed]
- Kawa JM, Taylor CG, Przybylski R (2003) Buckwheat concentrate reduces serum glucose in streptozotoxin-diabetic rats. J Agric Food Chem 51:7287–7291 [DOI] [PubMed]
- Khan AH, Pessin JE (2002) Insulin regulation of glucose uptake: a complex interplay of intracellular signaling pathways. Diabetologia 45:1475–1483 [DOI] [PubMed]
- Klip A, Ishiki M (2005) Recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology 146:5071–5078 [DOI] [PubMed]
- McLaurin J, Golomb R, Jurewicz A, Antel JP, Fraser PE (2000) Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid beta peptide and inhibit abeta-induced toxicity. J Biol Chem 275:18495–18502 [DOI] [PubMed]
- Nedachi T, Kanzaki M (2006) Regulation of glucose transporters by insulin and extracellular glucose in C2C12 myotubes. Am J Physiol Endocrinol Metab 291:E817–E828 [DOI] [PubMed]
- Nishiumi S, Ashida H (2007) Rapid preparation of a plasma membrane fraction from adipocytes and muscle cells: application to detection of translocated glucose transporter 4 on the plasma membrane. Biosci Biotechnol Biochem 71:2343–2346 [DOI] [PubMed]
- Ortmeyer HK, Huang LC, Zhang L, Hansen BC, Larner J (1993) Chiroinositol deficiency and insulin resistance. II. Acute effects of D-chiroinositol administration in streptozotocin-diabetic rats, normal rats given a glucose load, and spontaneously insulin-resistant rhesus monkeys. Endocrinology 132:646–651 [DOI] [PubMed]
- Ostlund RE, Sherman WR (1998) Pinitol and derivatives thereof for the treatment of metabolic disorders. US Patent 5,827,896, 27 Oct 1998
- Petersen KF, Shulman GI (2006) Etiology of insulin resistance. Am J Med 119:10S–16S [DOI] [PMC free article] [PubMed]
- Sarabia V, Ramlal T, Klip A (1990) Glucose uptake in human and animal muscle cells in culture. Biochem Cell Biol 68:536–542 [DOI] [PubMed]
- Weeks CE, Albany N (2003) Stimulating transport of glucose into animal tissue by the administration of pinitol. US Patent 6,518,318 B1, 11 Feb 2003


