Abstract
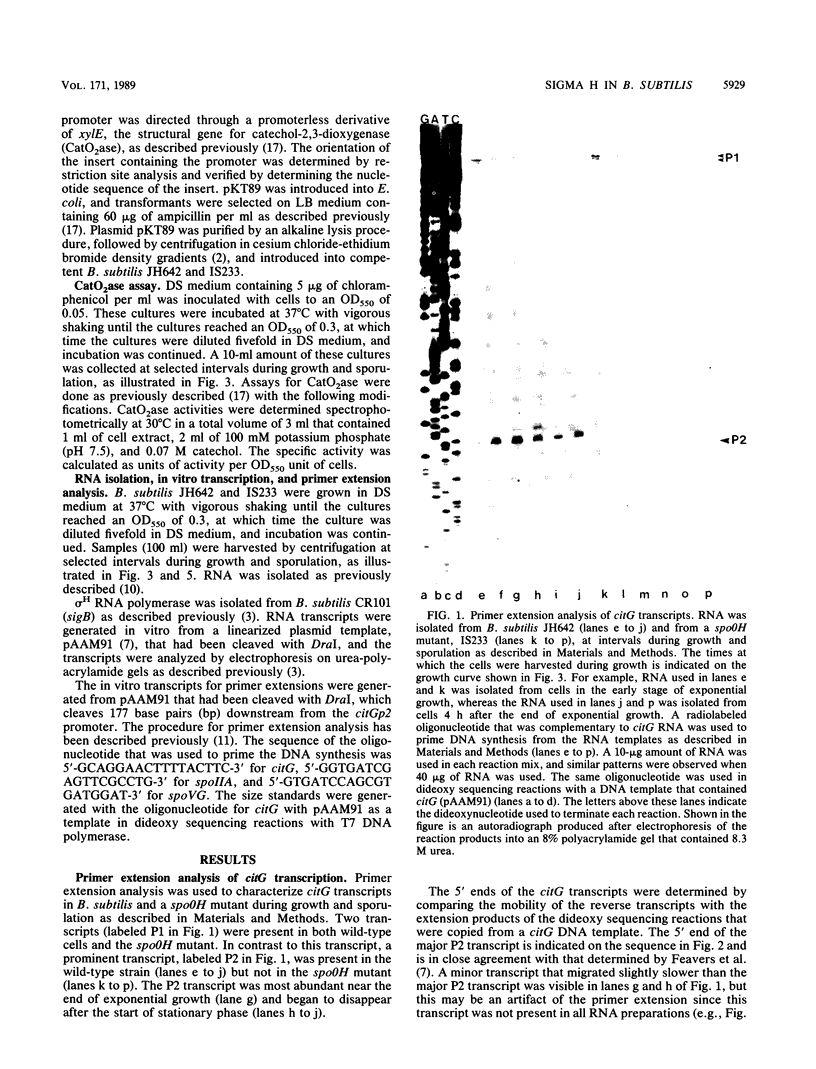
The RNA polymerase sigma factor sigma H is essential for the onset of endospore formation in Bacillus subtilis. sigma H also is required for several additional stationary-phase-specific responses, including the normal expression of several genes that are required for the development of competence for DNA uptake. It is necessary to identify the genes that are transcribed by sigma H RNA polymerase (E sigma H) in order to understand the role of this sigma factor during the transition from exponential growth to stationary phase. Feavers et al. (Mol. Gen. Genet. 211:465-471, 1988) proposed that citG, the structural gene for fumarase, is transcribed from two promoters, one of which (citGp2 [P2]) may be used by E sigma H. It is likely that the citGp2 promoter is used by E sigma H because we found that this promoter was used accurately in vitro by E sigma H and directed expression of xylE in vivo. This xylE expression was dependent on spo0H, the structural gene for sigma H, and was independent of the citGp1 promoter. Comparison of the nucleotide sequences of several sigma H-dependent promoters showed that these sequences were similar at two regions approximately 10 and 35 base pairs upstream from the start points of transcription. These sequences may signal recognition of these promoters by E sigma H. Primer extension analyses were used to examine transcription from three sigma H-dependent promoters during growth and sporulation. The citGp2 promoter appeared to be active during the middle and late stages of exponential growth, whereas activation of the spoIIA promoter was delayed until after the end of exponential growth. Evidently, promoters used by E sigma H can display different temporal patterns of expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano M., Hahn J., Dubnau D. Expression of competence genes in Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):3110–3117. doi: 10.1128/jb.169.7.3110-3117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter H. L., 3rd, Moran C. P., Jr New RNA polymerase sigma factor under spo0 control in Bacillus subtilis. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9438–9442. doi: 10.1073/pnas.83.24.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter H. L., 3rd, Wang L. F., Doi R. H., Moran C. P., Jr rpoD operon promoter used by sigma H-RNA polymerase in Bacillus subtilis. J Bacteriol. 1988 Apr;170(4):1617–1621. doi: 10.1128/jb.170.4.1617-1621.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau E., Weir J., Nair G., Carter L., 3rd, Moran C., Jr, Smith I. Bacillus sporulation gene spo0H codes for sigma 30 (sigma H). J Bacteriol. 1988 Mar;170(3):1054–1062. doi: 10.1128/jb.170.3.1054-1062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feavers I. M., Price V., Moir A. The regulation of the fumarase (citG) gene of Bacillus subtilis 168. Mol Gen Genet. 1988 Mar;211(3):465–471. doi: 10.1007/BF00425702. [DOI] [PubMed] [Google Scholar]
- Ferrari E., Howard S. M., Hoch J. A. Effect of stage 0 sporulation mutations on subtilisin expression. J Bacteriol. 1986 Apr;166(1):173–179. doi: 10.1128/jb.166.1.173-179.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán P., Westpheling J., Youngman P. Characterization of the promoter region of the Bacillus subtilis spoIIE operon. J Bacteriol. 1988 Apr;170(4):1598–1609. doi: 10.1128/jb.170.4.1598-1609.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaacks K. J., Healy J., Losick R., Grossman A. D. Identification and characterization of genes controlled by the sporulation-regulatory gene spo0H in Bacillus subtilis. J Bacteriol. 1989 Aug;171(8):4121–4129. doi: 10.1128/jb.171.8.4121-4129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney T. J., Kirchman P. A., Moran C. P., Jr Gene encoding sigma E is transcribed from a sigma A-like promoter in Bacillus subtilis. J Bacteriol. 1988 Jul;170(7):3058–3064. doi: 10.1128/jb.170.7.3058-3064.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T., Yoshitake J., Kato C., Usami R., Horikoshi K. Cloning of a developmentally regulated element from alkalophilic Bacillus subtilis DNA. J Bacteriol. 1985 Jan;161(1):158–163. doi: 10.1128/jb.161.1.158-163.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandoski M., Dubnau E., Smith I. Transcriptional regulation of the spo0F gene of Bacillus subtilis. J Bacteriol. 1986 Nov;168(2):870–877. doi: 10.1128/jb.168.2.870-877.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Youngman P., Piggot P. J. Genetics of endospore formation in Bacillus subtilis. Annu Rev Genet. 1986;20:625–669. doi: 10.1146/annurev.ge.20.120186.003205. [DOI] [PubMed] [Google Scholar]
- Price V. A., Feavers I. M., Moir A. Role of sigma H in expression of the fumarase gene (citG) in vegetative cells of Bacillus subtilis 168. J Bacteriol. 1989 Nov;171(11):5933–5939. doi: 10.1128/jb.171.11.5933-5939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray C., Hay R. E., Carter H. L., Moran C. P., Jr Mutations that affect utilization of a promoter in stationary-phase Bacillus subtilis. J Bacteriol. 1985 Aug;163(2):610–614. doi: 10.1128/jb.163.2.610-614.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh A., Banner C. D., Losick R., Fitz-James P. C. Identification of a new developmental locus in Bacillus subtilis by construction of a deletion mutation in a cloned gene under sporulation control. J Bacteriol. 1981 Oct;148(1):341–351. doi: 10.1128/jb.148.1.341-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall J., Losick R. Cloned Bacillus subtilis DNA containing a gene that is activated early during sporulation. Cell. 1977 Aug;11(4):751–761. doi: 10.1016/0092-8674(77)90289-6. [DOI] [PubMed] [Google Scholar]
- Wu J. J., Howard M. G., Piggot P. J. Regulation of transcription of the Bacillus subtilis spoIIA locus. J Bacteriol. 1989 Feb;171(2):692–698. doi: 10.1128/jb.171.2.692-698.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P., Healy J., Carter H. L., 3rd, Cutting S., Moran C. P., Jr, Losick R. Mutation changing the specificity of an RNA polymerase sigma factor. J Mol Biol. 1989 Apr 20;206(4):605–614. doi: 10.1016/0022-2836(89)90569-x. [DOI] [PubMed] [Google Scholar]
- Zuber P., Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]