Abstract
Ma Huang (equivalent to 0, 12.5, 25, or 50 mg/kg ephedrine) or ephedrine (0, 6.25, 12.5, 25 mg/kg) were administered as one bolus oral dose to male F344 rats with and without caffeine. The herbal medicine Ma Huang (ephedra) in combination with caffeine caused rapid clinical signs of toxicity including salivation, hyperactivity, ataxia, and eventually lethargy, and failure to respond to stimuli. When this syndrome of clinical signs emerged, animals were moribund sacrificed, and a histological analysis for heart lesions performed. Cardiotoxicity included hemorrhage, necrosis, and degeneration in the ventricles or interventricular septum within 2–4 hours after treatment with Ma Huang (ephedra)/caffeine or ephedrine (the principal active component in Ma Huang)/caffeine. There was a steep dose response curve for cardiotoxicity with minimal toxicity seen at levels of Ma Huang (equivalent to 12.5 mg/kg ephedrine) with caffeine. However, cardiotoxic lesions occurred in 28% of animals with Ma Huang dosages equivalent to 25 mg/kg ephedrine with 15 or 30 mg/kg caffeine, and in 90% of animals at Ma Huang exposures equivalent to 50 mg/kg ephedrine with 15 or 30 mg/kg caffeine. Cardiotoxic lesions occurred in 47% of animals in the 25 mg/kg ephedrine groups with caffeine at 7.25, 15, or 30 mg/kg. There was no statistical difference in the occurrence of cardiotoxic lesions when 15 or 30 mg/kg caffeine was combined with Ma Huang equivalent to 25 or 50 mg/kg ephedrine; likewise there was no statistical difference in the occurrence of cardiotoxic lesions when 7.25, 15, or 30 mg/kg caffeine was combined with 25 mg/kg ephedrine. These results show that the cardiotoxic effects of the herbal medicine, Ma Huang, are similar to that of ephedrine, the principal active ingredient in the herbal medicine. The combination of Ma Huang or ephedrine with caffeine enhanced the cardiotoxicity over that with the herbal medicine or the active ingredient alone.
Keywords: Cardiotoxicity, Ma Huang, ephedra, ephedrine, caffeine
Introduction
Ephedrine and caffeine are the primary active components in dietary supplements that contain Ma Huang (ephedra) and guarana-derived caffeine (Csajka et al., 2005). Use of these herbal medicines has been linked to cardiotoxicity in humans (Astrup et al., 1991; McBride et al., 2004; Naik and Freudenberger, 2004; Persky et al., 2004; Haller et al., 2005b; Peters et al., 2005; Vukovich et al., 2005).
Ma Huang dietary supplements are regulated under the 1994 Dietary Supplement and Health Education Act (DSHEA), an act which does not require that manufacturers of a dietary supplement provide safety data to the U.S. Food and Drug Administration before marketing the product (U.S. Food and Drug Administration, 1995). However, based on accumulated data on the cardiotoxicty of ephedra containing supplements, the U.S. Food and Drug Administration issued a rule banning the sale of these supplements in 2004 (U.S. Food and Drug Administration, 2004), In 2005, U.S. courts (U.S. Federal District Court, 2005) notes that additional “dose specific analysis” is need to fully understand the adverse effects of ephedrine-containing products.
We have previously reported that the combination treatment of ephedrine and caffeine rapidly caused cardiotoxicity in rats (Howden et al., 2005; Nyska et al., 2005). The treatment-related changes included increases in heart rate, blood pressure, and temperature (Howden et al., 2005), and interstitial hemorrhage, with degeneration of myofibers in the subendocardial myocardium of the ventricles and interventricular septum (Nyska et al., 2005). In this study, we compare the cardiotoxicity of ephedrine and caffeine to that of herbal medicine Ma Huang (ephedra) and caffeine in the F344 rat based on clinical and histopathologic findings. The cardiotoxic effects are reported for Ma Huang (ephedra) and ephedrine dosages that are 10–20 times human oral ephedrine exposure levels based on a mg/m2 body surface area comparison.
Methods and Materials
Chemicals and Dose Formulation
Dosing solutions consisted of ephedrine hydrochloride (Sigma-Aldrich, St. Louis, Mo; >99% L-ephedrine), ephedrine hydrochloride-plus-caffeine (Pfaltz and Bauer, Waterbury, CT), Ma Huang (Jinke Group, USA, Inc.), Ma Huang-plus-caffeine, or caffeine prepared in 0.5% methyl-cellulose (Figure 1). Controls received 0.5% methyl cellulose alone (Figure 1). Ma Huang contained ~4% by weight of ephedrine in the crude extract (National Toxicology Program, 2004). Dosing solutions were prepared so that 5 mL of the solution/kg body weight delivered ephedrine/caffeine dosages of: 0/0; 6.25/0; 12.5/0; 25/0; 12.5/7.25; 25/7.25; 0/15; 12.5/15; 25/15; 0/30; 6.25/30; 12.5/30; 25/30 mg/kg. Ma Huang dosing solutions were prepared so that 5 mL/kg delivered Ma Huang (ephedrine equivalents)/caffeine dosages of: 0/0; 312.5(12.5)/0; 625(25)/0; 1250(50)/0; 0/15; 312.5(12.5)/15; 625(25)/12; 1250(50)/15; 0/30; 312.5(12.5)/30; 625(25)/30; 1250(50)/30; 0/45; 312.5(12.5)/45 mg/kg (National Toxicology Program, 2004).
Figure 1.
Chemical structure of ephedrine and caffeine.
Animals and Study Design
Male F344/N rats (Taconic Laboratory, Germantown, NY), 15–16 weeks of age, received a single oral gavage dose of the stock solutions at 5 ml/kg to 20 animals per treatment group. Rats were housed 5 per cage in polycarbonate cages and fed NTP-2000 wafer feed (Zeigler Brothers, Inc., Gardners, PA) ad libitum. At approximately 11 pm on the day prior to dosing, feed was removed so that animals would be dosed on an empty stomach (~8–10 am), and then returned to cages containing feed once the dose had been administered. Animals were observed continuously after dosing for clinical signs of toxicity, and clinical signs of toxicity were recorded at 2 and 4 hours after dosing or immediately prior to moribund sacrifice. The experiment was conducted at an AAALAC-accredited facility, and animal handling and husbandry met all NIH guidelines (Institute of Laboratory Animal Resources, 1996).
Pathology Procedures
Terminally sacrificed (~4 hours after dosing) and moribund animals were sacrificed with CO2. At necropsy, all organs and tissues were examined for grossly visible lesions. The heart was fixed in 10% neutral-buffered formalin, processed, and trimmed into 2 longitudinal halves; both sides were embedded in paraffin, sectioned to a thickness of 4–6 μm, and stained with hematoxylin and eosin (H&E) for microscopic examination. Sections of the heart contained muscle of both ventricles, interventricular septum and atria (Morawietz et al., 2004).
The criteria for grading lesion severity used was as previously reported (Howden et al., 2005; Nyska et al., 2005), which, in turn were consistent with NTP criteria for non-neoplastic lesions (Shackelford et al., 2002). As stated by Shackelford et al., a grade of Minimal (+1) consists of a lesion that affects up to 10% of a tissue and can vary from being barely noticeable to one considered minor enough to warrant the lowest assignable grade, a grade of Mild (+2) consists of a lesion that affects 11–20% of a tissue and is noticeable but not a prominent feature of the tissue, while a grade of Moderate (+3) consists of a lesion that affects 21–40% of a tissue and is a prominent feature of the tissue.
Statistical Methods
Cochran-Mantel-Haenszel (CMH) trend tests were performed to relate the level of ephedrine or Ma Huang (or ephedrine equivalents) with the lesion incidence at each level of caffeine (Fleiss, 1973). CMH tests were also performed for caffeine at each level of ephedrine and Ma Huang administered. Fisher’s exact tests were used to compare each dosed group with its corresponding control group (Gart et al., 1979).
Results
Mortality and Clinical Observations
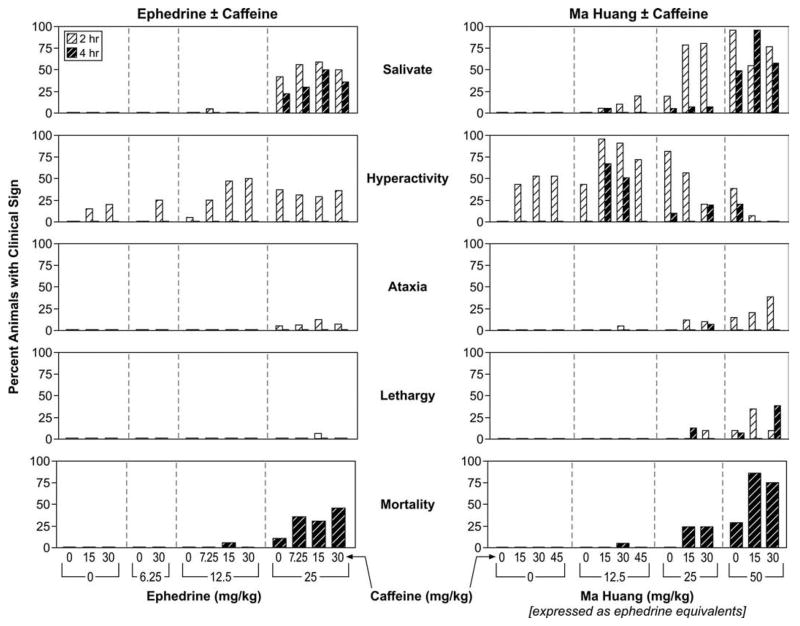
Clinical signs of toxicity were seen in rats receiving a combination of ephedrine/caffeine or Ma Huang/caffeine (Figure 2) and included salivation, hyperactivity, ataxia, and/or lethargy. Clinical signs of toxicity increased when ephedrine or Ma Huang exposure was combined with caffeine exposure. All animals that were moribund sacrificed were lethargic and recumbent.
Figure 2.
Clinical signs of toxicity from ephedrine or Ma Huang administered alone and in combination with caffeine.
Treatment-Related Cardiac Lesions
Treatment-related cardiotoxicity was observed in most of the moribund sacrificed animals and consisted of hemorrhage, degeneration, and necrosis (Tables 1 and 2. These lesions were of minimal severity and a magnification of 10× or greater was generally needed to see these changes (Figure 3), and involved up to 10% of the myocardium. Myofiber necrosis was the most commonly observed change. After ephedrine treatment, particularly in animals receiving combinations of ephedrine and caffeine, 96% (24/25) of moribund sacrificed animals had at least 1 of 3 treatment-related cardiotoxic lesions (i.e., hemorrhage, degeneration, or necrosis), while only 6% (15/235) of animals surviving until to terminal sacrifice (~4 hours) had cardiotoxic lesions. Similarly, after Ma Huang exposures, 98% (49/50) of the animals that were moribund sacrificed because of clinical toxicity had cardiotoxic lesions, while only 6% (14/230) of animals living to terminal sacrifice (~4 hours) had cardiotoxic lesions.
Table 1.
Incidence of treatment-related lesions (Necrosis, Degeneration, and Hemorrhage) and survival in the ephedrine + caffeine-treated groups.
| Ephedrine(mg/kg)
|
||||
|---|---|---|---|---|
| Caffeine (mg/kg) | 0 | 6.25 | 12.5 | 25 |
| 0 | ||||
| Hemorrhage | 0/20 | 1/20 | 0/20 | 2/20† |
| Necrosis | 0/20** | 0/20 | 0/20 | 3/20† |
| Degeneration | 0/20* | 0/20 | 0/20 | 2/20†† |
| Combineda | 0/20** | 1/20 | 0/20 | 4/20† |
| Survivalb | 20/20 | 20/20 | 20/20 | 18/20 (2 hr 42 min)c |
| 7.25 | ||||
| Hemorrhage | 0/20 | 3/20 | ||
| Necrosis | 0/20 | 8/20 | ||
| Degeneration | 0/20 | 5/20 | ||
| Combined | 0/20 | 9/20 | ||
| Survival | 20/20 | 13/20 (2 hr 6 min) | ||
| 15 | ||||
| Hemorrhage | 1/20 | 1/20 | 3/20 | |
| Necrosis | 0/20** | 1/20 | 7/20** | |
| Degeneration | 1/20* | 1/20 | 5/20 | |
| Combined | 2/20** | 1/20 | 9/20* | |
| Survival | 20/20 | 19/20 (1 hr 38 min) | 14/20 (2 hr 4 min) | |
| 30 | ||||
| Hemorrhage | 1/20** | 1/20 | 1/20 | 6/20* |
| Necrosis | 0/20** | 0/20 | 0/20 | 10/20**† |
| Degeneration | 0/20** | 0/20 | 0/20 | 9/20**† |
| Combined | 1/20** | 1/20 | 1/20 | 10/20**† |
| Survival | 20/20 | 20/20 | 20/20 | 11/20 (1 hr 47 min) |
Combined (Hemorrhage or Necrosis or Degeneration or a combination of these lesions).
Survival = Percentage of animals that survived to terminal sacrifice at 4 hours.
Mean survival time (hours:minutes) among moribund sacrifices.
Statistical Results:
In ephedrine = 0 column, significant trend with ephedrine; in ephedrine > 0 columns, significant pairwise difference from no ephedrine at
p < 0.05;
p < 0.01.
In caffeine = 0 row, significant trend with caffeine; in caffeine > 0 rows, significant pairwise difference from no caffeine at † p < 0.05;
p < 0.01.
Table 2.
Incidence of treatment-related lesions (Necrosis, Degeneration, and Hemorrhage) and survival in the Ma-Huang + caffeine-treated groups.
| Ma-Huang (mg/kg) (and equivalent ephedrine doses (mg/kg)
|
||||
|---|---|---|---|---|
| Caffeine (mg/kg) | 0 | 312.5 (12.5) | 625 (25) | 1250 (50) |
| 0 | ||||
| Hemorrhage | 1/20 | 0/20 | 0/20† | 2/20†† |
| Necrosis | 0/20** | 0/20 | 0/20† | 7/20**†† |
| Degeneration | 0/20 | 0/20 | 0/20† | 1/20†† |
| Combineda | 1/20** | 0/20 | 0/20† | 7/20*†† |
| Survivalb | 20/20 | 20/20 | 20/20 | 14/20 (3 hr 14 min)c |
| 15 | ||||
| Hemorrhage | 0/20** | 0/20 | 1/20 | 10/20**†† |
| Necrosis | 0/20** | 0/20 | 6/20*†† | 19/20**†† |
| Degeneration | 0/20** | 1/20 | 0/20 | 10/20**†† |
| Combined | 0/20** | 1/20 | 6/20*†† | 19/20**†† |
| Survival | 20/20 | 20/20 | 15/20 (2 hr 9 min) | 2/20 (2 hr 14 min) |
| 30 | ||||
| Hemorrhage | 1/20** | 3/20 | 3/20 | 12/20**†† |
| Necrosis | 0/20** | 1/20 | 5/20*† | 17/20**†† |
| Degeneration | 0/20** | 0/20 | 2/20 | 9/20**†† |
| Combined | 1/20** | 4/20 | 5/20† | 17/20**†† |
| Survival | 20/20 | 19/20 (3 hr 23 min) | 15/20 (2 hr 40 min) | 5/20 (2 hr 8 min) |
| 45 | ||||
| Hemorrhage | 1/20 | 1/20 | ||
| Necrosis | 0/20 | 0/20 | ||
| Degeneration | 0/20 | 0/20 | ||
| Combined | 1/20 | 1/20 | ||
| Survival | 20/20 | 20/20 | ||
Combined (Hemorrhage or Necrosis or Degeneration or a combination of these lesions).
Survival = Percentage of animals that survived to terminal sacrifice at 4 hours.
Mean survival time (hours:minutes) among moribund sacrifices.
Statistical Results:
In ephedrine = 0 column, significant trend with ephedrine; in ephedrine > columns, significant pairwise difference from no ephedrine at
p < 0.05;
p < 0.01
In caffeine = 0 row, significant trend with caffeine; in caffeine >0 rows, significant pairwise difference from no caffeine at † p < 0.05;
p < 0.01.
Figure 3.
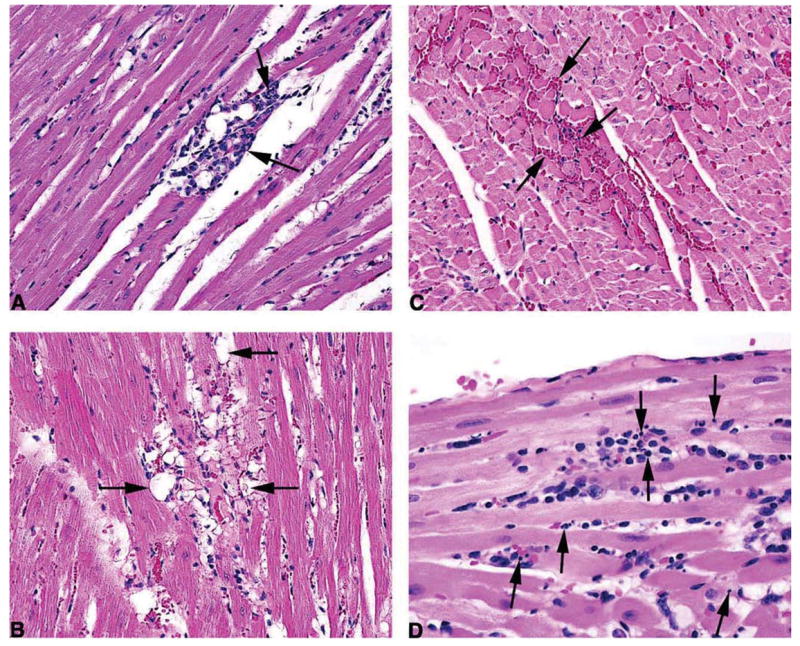
Treatment-related cardiotoxic lesions. (A) Cardiomyopathy, minimal (25 mg/kg ephedrine and 7.25 mg/kg caffeine). This change is considered to be an incidental background change. Note a single small focus of mononuclear cell (lymphocytes and histiocytes) infiltration, associated with variable myofiber degeneration, necrosis and loss. H&E, 20×. (B) Degeneration, minimal (12.5 mg/kg ephedrine and 15 mg/kg caffeine), 20×. Note a focus of myocardial fibers with clear, vacuolated cytoplasm. These vacuoles varied from large vacuoles, which distended the myofiber. H&E, 20×. (C) Hemorrhage, minimal (25 mg/kg). Note small foci in which free red blood cells filling the spaces between adjacent myocardial fibers. H&E, 20×. (D) Necrosis, minimal (25 mg/kg ephedrine and 30 mg/kg caffeine), 40×. Note the presence of numerous minute clusters of deeply basophilic fragments of nuclear debris, mixed with some macrophages. H&E, 40×.
Hemorrhage (Figure 3B) consisted of one to few, generally small foci in which free red blood cells filled the spaces between adjacent myocardial fibers. Degeneration (Figure 3C) areas contained numerous scattered myocardial fibers with clear vacuolated cytoplasm. In some fibers the vacuolation had replaced nearly all of the normal cytoplasm. These vacuolated fibers appeared to be most common in the left ventricle and the interventricular septum. It also appeared that in general these vacuolated fibers occurred in hearts that also had necrosis. Some degree of myocardial fiber vacuolation was seen even in control hearts. However, the degree of vacuolation in hearts from treated animals contained a substantially greater degree of vacuolation than was seen in controls and, thus, the degree of vacuolation was treatment-related. There was also variable hyalinization and increased eosinophilia of the sarcoplasm in hearts from treated animals.
Necrosis (Figure 3D) consisted of numerous, minute clusters of deeply basophilic fragments of nuclear debris scattered diffusely within the myocardium. The interventricular septum appeared to be most commonly involved, followed by the left ventricular wall, right ventricular wall, and sometimes the left papillary muscle. Commonly the nuclear fragments were seen lying between adjacent myocardial fibers or occasionally within a fiber, and were unaccompanied by a cellular inflammatory reaction, giving the impression of an acute process. At other points nuclear fragments were mixed with some macrophages, and sometimes bits of eosinophilic material that appeared to be myocardial fiber cytoplasm were observed lying within a clear space between two fibers, giving the impression that there may have been focal loss of a preexisting fiber.
Occasionally, a myocardial fiber adjacent to the cluster of nuclear fragments was hyalinized and deeply eosinophilic indicating it was undergoing coagulative necrosis. The prominent distinguishing feature of necrosis was the presence of diffusely scattered fragmented nuclei with little or no accompanying inflammatory cell reaction. In contrast, cardiomyopathy consisted of a smaller number of larger foci most of which, except for those consisting of early coagulation necrosis of myocardial fibers, contained prominent inflammatory cell infiltrate. The exact cell undergoing nuclear fragmentation could not be determined with certainty. In some cases it appeared to be myocardial cells, and in other cases possibly interstitial cells, endothelial cells, or inflammatory cells located in the space between adjacent fibers. The hemorrhage seen in treated animals could have been an indicator of endothelial damage.
Treatment-related cardiotoxic lesions occurred in the 25 mg/kg ephedrine groups with caffeine (Table 1), in the 1250 mg/kg Ma Huang (equivalent to 50 mg/kg ephedrine) groups with or without caffeine, and in the 625 mg/kg Ma Huang (equivalent ot 25 mg/kg ephedrine) groups with caffeine (Table 2).
In addition to the treatment-related lesions, there was a background of cardiomyopathy (Figure 3A) characteristic of the F344 rat (Ruben et al., 2000). These lesions occurring in the ventricle, interventricular septum, and/or the papillary muscle begin with foci of coagulative necrosis of myocardial fibers, characterized by deeply eosinophilic, hyalinized cytoplasm with pyknotic nuclei, followed by infiltration with macrophages and lymphocytes, and occasionally a few neutrophils. Based upon this, the diagnosis of cardiomyopathy was applied when multiple, small, scattered foci consisting initially of necrotic, sometimes misshapen fibers with hyalinized, hypereosinophilic, cytoplasm, and pyknotic or fragmented nuclei, were present. As lesion development continued the necrotic foci were infiltrated with macrophages that were sometimes accompanied by a few lymphocytes and occasionally a few neutrophils. The macrophages phagocytized the necrotic fibers and sometimes could be seen to contain eosinophilic bits of phagocytized fibers. Necrotic fibers were sometimes seen adjacent to these foci of inflammatory cells and that change appeared to represent a continuation of the degenerative process causing enlargement of the lesion.
There was no statistical difference in the occurrence of cardiotoxic lesions between 15 and 30 mg/kg caffeine combined with Ma Huang equivalent to 25 or 50 mg/kg ephedrine (p > .60); likewise, there was no statistical difference in the occurrence of cardiotoxic lesions between 7.25, 15, and 30 mg/kg caffeine combined with 25 mg/kg ephedrine (p > .30). However, when used in combination with Ma Huang equivalent to 25 or 40 mg/kg ephedrine, 15 or 30 mg/kg caffeine significantly increased (p < 0.05) the occurrence of cardiotoxic lesions compared to when no caffeine was added. Likewise, in combination with 25 mg/kg ephedrine, 30 mg/kg caffeine significantly (p < 0.05) increased the occurrence of cardiotoxic lesions compared to when no caffeine was added.
Discussion
In this study, we compare the cardiotoxic effects of ephedrine and caffeine with those of Ma Huang and caffeine. Clinical signs of toxicity in the male F344 rat began within 1 hour of exposure to ephedrine/caffeine or Ma Huang/caffeine, and included ataxia, hyperactivity, and eventually failure to respond to stimuli, resulting in moribund sacrifice. While clinical toxicity occurred with ephedrine alone (25 mg/kg), the magnitude of the response increased with the addition of caffeine. With Ma Huang (equivalent to 25 or 50 mg/kg of ephedrine), the magnitude of clinical toxicity also increased with the addition of caffeine. The lowest dose of Ma Huang alone to cause clinical toxicity leading to moribund sacrifice was equivalent to an exposure of 25 mg/kg ephedrine.
Ma Huang and ephedrine cardiotoxicity, with and without caffeine, induced cardiotoxic lesions of hemorrhage, necrosis, and degeneration. The treatment-related lesions were of minimal severity regardless of dose level. This is consistent with previous studies where we found that ephedrine/caffeine related heart toxicity (as measured by functional changes) is not always accompanied by the presence of severe cardiotoxic lesions (Howden et al., 2005). The addition of caffeine enhanced the incidence of treatment-related cardiotoxic lesions of the herbal medicine or ephedrine, although caffeine alone did not induce significant cardiotoxic lesions. Treatment-related histopathologic findings of minimal severity were considered significant.
Very small lesions may significantly affect the function of the heart and produce profound clinical signs (and even death). It is not known whether these treatment-related heart lesions are secondary to myocardial ischemia or hyperthermia or other clinical toxicity (Howden et al., 2005), or a primary effect of the test articles. Treatment-related heart lesions were found primarily in animals showing clinical signs of toxicity followed by moribund sacrifice, and not in the ephedrine/Ma Huang groups of rats living until terminal sacrifice (~4 hours after treatment). Treatment-related heart lesions were not found in any control rats in the ephedrine study and in only 1 control rat (hemorrhage) in the Ma-Huang study. This suggests a very strong connection between the clinical signs of lethargy and recumbancy and the myocardial degeneration and necrosis.
Ephedra sinica extract commonly used in dietary supplements consists of ephedrine, pseudoephedrine, methyle-phedrine, norpseudoephedrine, and norephedrine, of which ephedrine is the principal alkaloid component. A typical ephedrine-alkaloid containing dietary supplement dose contains ~20 mg ephedrine and ~200 mg of guarana-derived caffeine (Haller et al., 2004; Jacob et al., 2004). Ephedrine alkaloids are rapidly absorbed with maximum plasma concentration (tmax) occurring within 2.4 hours and an ephedrine plasma half-life of ~6.1 hours (Wilkinson and Beckett, 1968; White et al., 1997; Haller et al., 2002b). A typical human ephedrine/caffeine dosage, is ~0.3 mg ephedrine/kg body weight (11 mg/m2 body surface area) (Nyska et al., 2005).
In this study, ephedrine cardiotoxicity was studied over a broader dose range than in our previous study where the exposure level of 25 mg/kg (~130 mg/m2 body surface area) is ~12 times human exposure based on a mg/m2 body surface area comparison (Nyska et al., 2005). Ephedrine doses of 6.25, 12, or 25 mg/kg are ~3–12 times human exposure levels (mg/m2 body surface area comparison).
Ephedrine plasma levels in humans taking a typical ephedrine-containing herbal medicine are reported to be approximately 60–150 ng/ml plasma at 1–2 hours after drug administration (Vanakoski et al., 1993; Haller et al., 2002a; Csajka et al., 2005; Haller et al., 2005a, 2005b). Analysis of a few 25 mg/kg ephedrine rat plasma samples 1–2 hours after administration showed ephedrine plasma levels of ~1000–2000 ng/ml (National Toxicology Program, 2006). More complete toxicokinetic studies are required to compare human and rat ephedrine plasma levels and parameters.
One hypothesis for the mechanism of ephedrine and caffeine cardiotoxicity is that these chemicals work together to cause rapid death by increasing calcium levels and thereby altering electrical and contraction properties of the heart (Figure 4). Ephedrine binds to α/β adrenergic membrane receptors, causing release of catecholamines, followed by increase in inositol 1,4,5-triphosphate (IP3). IP3 binds to receptors in the endoplasmic reticular, resulting in calcium release from intracellular stores, depolarization and contraction of vessels, leading to ischemia. Caffeine stimulates ryonodine receptors (RyR) and Ca release from intracellular stores, also contributing to depolarization and contraction of vessels (Vansal and Feller, 1999; Braun et al., 2001; Broad et al., 2001; Luo et al., 2001a, 2001b; Wellman et al., 2003; Burt, 2004; Luo et al., 2005). Results of this study showed that treatment-related cardiotoxicity occurs after administration of either ephedrine (25 mg/kg) or Ma Huang (equivalent to 25 mg/kg ephedrine) and increases in severity with the addition of caffeine. These studies show that toxicology studies may be important in understanding side effects from herbal medicines.
Figure 4.
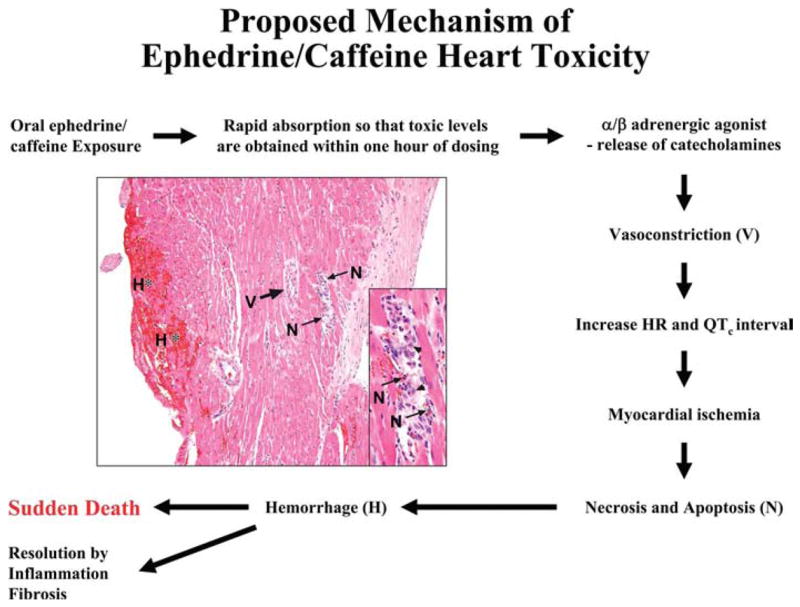
Proposed mechanism of ephedrine cardiotoxicity (cardiotoxicity at 25 mg/kg ephedrine and 30 mg/kg caffeine is depicted in figure).
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. We thank Battelle Columbus where the animals for this study were dosed and housed under NIEHS contract N01-ES-65406. The statistical analysis was conducted under NIEHS contract N01-ES-55547 to Constella Group, Durham, NC 27713.
References
- Astrup A, Toubro S, Cannon S, Hein P, Madsen J. Thermogenic synergism between ephedrine and caffeine in healthy volunteers: a double-blind, placebo-controlled study. Metabolism. 1991;40:323–9. doi: 10.1016/0026-0495(91)90117-f. [DOI] [PubMed] [Google Scholar]
- Braun FJ, Broad LM, Armstrong DL, Putney JW., Jr Stable activation of single Ca2+ release-activated Ca2+ channels in divalent cation-free solutions. J Biol Chem. 2001;276:1063–70. doi: 10.1074/jbc.M008348200. [DOI] [PubMed] [Google Scholar]
- Broad LM, Braun FJ, Lievremont JP, Bird GSJ, Kurosaki T, Putney JW., Jr Role of the phospholipase C-inositol 1,4,5-triphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J Biol Chem. 2001;276:15945–52. doi: 10.1074/jbc.M011571200. [DOI] [PubMed] [Google Scholar]
- Burt RP. Depletion of Ca from intracelluar stores potentiates spontaneous contractions of the rat portal ven. Eur J Clin Pharmacol. 2004;496:109–18. doi: 10.1016/j.ejphar.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Csajka C, Haller CA, Benowitz NL, Verotta D. Mechanistic pharmacokinetic modelling of ephedrine, norephedrine and caffeine in healthy subjects. Br J Clin Pharmacol. 2005;59(3):335–45. doi: 10.1111/j.1365-2125.2005.02254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss JL. Statistical methods for rates and proportions. John Wiley & Sons; New York: 1973. [Google Scholar]
- Gart JJ, Chu KC, Tarone RE. Statistical issues in interpretation of chronic bioassay tests for carcinogenicity. JNCI. 1979;62:957–74. [PubMed] [Google Scholar]
- Haller CA, Benowitz NL, Jacob P., 3rd Hemodynamic effects of ephedra-free weight-loss supplements in humans. Am J Med. 2005a;118(9):998–1003. doi: 10.1016/j.amjmed.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Haller CA, Duan M, Benowitz NL, Jacob PI. Concentrations of ephedra alkaloids and caffeine in commercial dietary supplements. J Anal Toxicol. 2004;28:145–51. doi: 10.1093/jat/28.3.145. [DOI] [PubMed] [Google Scholar]
- Haller CA, Jacob P, 3rd, Benowitz NL. Pharmacology of ephedra alkaloids and caffeine after single-dose dietary supplement use. Clin Pharmacol Ther. 2002a;71(6):421–32. doi: 10.1067/mcp.2002.124523. [DOI] [PubMed] [Google Scholar]
- Haller CA, Jacob P, Benowitz NL. Short-term metabolic and hemodynamic effects of ephedra and guarana combinations. Clin Pharmacol Ther. 2005b;77(6):560–71. doi: 10.1016/j.clpt.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Haller CA, Jacob PI, Benowitz NL. Pharmacology of ephedra alkaloids and caffeine after single-dose dietary supplement use. Clin Pharmacol Ther. 2002b;71:421–32. doi: 10.1067/mcp.2002.124523. [DOI] [PubMed] [Google Scholar]
- Howden R, Hanlon PR, Petranka JG, Kleeberger S, Bucher J, Dunnick J, Nyska A, Murphy E. Ephedrine plus caffeine causes age-dependent cardiovascular responses in Fischer 344 rats. Am J Physiol Heart Circ Physiol. 2005;288(5):H2219–24. doi: 10.1152/ajpheart.01164.2004. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Jacob PI, Haller CA, Duan M, Yu L, Peng M, Benowitz NL. Determination of ephedra alkaloid and caffeine concentrations in dietary supplements and biological fluids. J Anal Toxicol. 2004;28:152–9. doi: 10.1093/jat/28.3.152. [DOI] [PubMed] [Google Scholar]
- Luo D, Broad LM, Bird GSJ, Putney JW., Jr Mutual antagonism of calcium entry by capacitative and arachidonic acid-mediate calcium entry pathways. J Biol Chem. 2001a;276:20186–9. doi: 10.1074/jbc.M100327200. [DOI] [PubMed] [Google Scholar]
- Luo D, Broad LM, Bird GSJ, Putney JW., Jr Signaling pathways underlying muscarinic receptor-induced [Ca2+]i oscillations in HEK293 cells. J Biol Chem. 2001b;276:5613–21. doi: 10.1074/jbc.M007524200. [DOI] [PubMed] [Google Scholar]
- Luo D, Sun H, Xiao RP, Han Q. Caffeine induced Ca2+ release and capacitative Ca2+ entry in human embryonic kidney (HEK293) cells. Eur J Clin Pharmacol. 2005;509:109–15. doi: 10.1016/j.ejphar.2004.12.038. [DOI] [PubMed] [Google Scholar]
- McBride BF, Karapanos AK, Krudysz A, Kluger J, Coleman CI, White CM. Electrocardiographic and hemodynamic effects of a multicomponent dietary supplement containing ephedra and caffeine: a randomized controlled trial. JAMA. 2004;291(2):216–21. doi: 10.1001/jama.291.2.216. [DOI] [PubMed] [Google Scholar]
- Naik SD, Freudenberger RS. Ephedra-associated cardiomyopathy. Ann Pharmacother. 2004;38(3):400–3. doi: 10.1345/aph.1D408. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. Ephedrine, Ma Huang, and caffeine combination; special chemical analysis report; December 17, 2004.2004. [Google Scholar]
- National Toxicology Program (2006). Ephedrine + Ma Huang + Caffeine combination in Male Fischer 344 rat plasma: biological sample analysis. RTI under NIH Contract No N01-ES-65554. Chem Task No. CHEM08401.
- New York Times. Time to ban ephedra. 2005 April 21; 2005. [Google Scholar]
- Nyska A, Murphy E, Foley JF, Collins BJ, Petranka J, Howden R, Hanlon P, Dunnick JK. Acute hemorrhagic myocardial necrosis and sudden death of rats exposed to a combination of ephedrine and caffeine. Toxicol Sci. 2005;83(2):388–96. doi: 10.1093/toxsci/kfi034. [DOI] [PubMed] [Google Scholar]
- Persky AM, Berry NS, Pollack GM, Brouwer KL. Modelling the cardiovascular effects of ephedrine. Br J Clin Pharmacol. 2004;57(5):552–62. doi: 10.1111/j.1365-2125.2003.02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters CM, O’Neill JO, Young JB, Bott-Silverman C. Is there an association between ephedra and heart failure? a case series. J Card Fail. 2005;11(1):9–11. doi: 10.1016/j.cardfail.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Ruben Z, Arceo RJ, Bishop SP, Elwell MR, Kerns WD, Mesfin GM, Sandusky GE, Van Vleet JF. Non-proliferative lesions of the heart and vasculature in rats. STP/ARP/AFIP; Washington, DC: 2000. [Google Scholar]
- Shackelford C, Long G, Wolf J, Okerberg C, Herbert R. Qualitative and quantitative analysis of nonneoplastic lesions in toxiciology studies. Toxicol Pathol. 2002;30:93–6. doi: 10.1080/01926230252824761. [DOI] [PubMed] [Google Scholar]
- U. S. Food and Drug Administration. Dietary supplement health and education act of 1994. 1995 http://wwwcfsanfdagov/~dms/dietsupphtml.
- U. S. Food and Drug Administration. Final rule declaring dietary supplements containing ephedrine alkaloids adulterated because they present unreasonable risk; final rule. Fed Reg. 2004;69:6788–854. [PubMed] [Google Scholar]
- U.S. Federal District Court U. 2005 http://wwwutduscourtsgov/documents/profpagehtml.
- Vanakoski J, Stromberg C, Seppala T. Effects of a sauna on the pharmacokinetics and pharmacodynamics of midazolam and ephedrine in health young women. Eur J Clin Pharmacol. 1993;45:377–81. doi: 10.1007/BF00265959. [DOI] [PubMed] [Google Scholar]
- Vansal SS, Feller DR. Direct effecs of ephedrine isomers on human B-adrenergic receptor subtypes. Biochem Pharmacol. 1999;58:807–10. doi: 10.1016/s0006-2952(99)00152-5. [DOI] [PubMed] [Google Scholar]
- Vukovich MD, Schoorman R, Heilman C, Jacob P, 3rd, Benowitz NL. Caffeine-herbal ephedra combination increases resting energy expenditure, heart rate and blood pressure. Clin Exp Pharmacol Physiol. 2005;32(1–2):47–53. doi: 10.1111/j.1440-1681.2005.04152.x. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Miller DK, Ho DH. Noradrenergic modulation of ephedrine-induced hypophagia. Synapse. 2003;48:18–24. doi: 10.1002/syn.10182. [DOI] [PubMed] [Google Scholar]
- White LM, Gardner SF, Gurley BJ, Marx MA, Wang PL, Estes E. Pharmacokinetics and cardiovascular effects of Ma-Huang (Ephedra sinica) in normotensive adults. J Clin Pharmacol. 1997;37:116–22. doi: 10.1002/j.1552-4604.1997.tb04769.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson GR, Beckett AH. Absorption, metabolism, and excretion of the ephedrines in man II. Journal of Pharmaceutical Sciences. 1968;57:1933–8. doi: 10.1002/jps.2600571122. [DOI] [PubMed] [Google Scholar]