Abstract
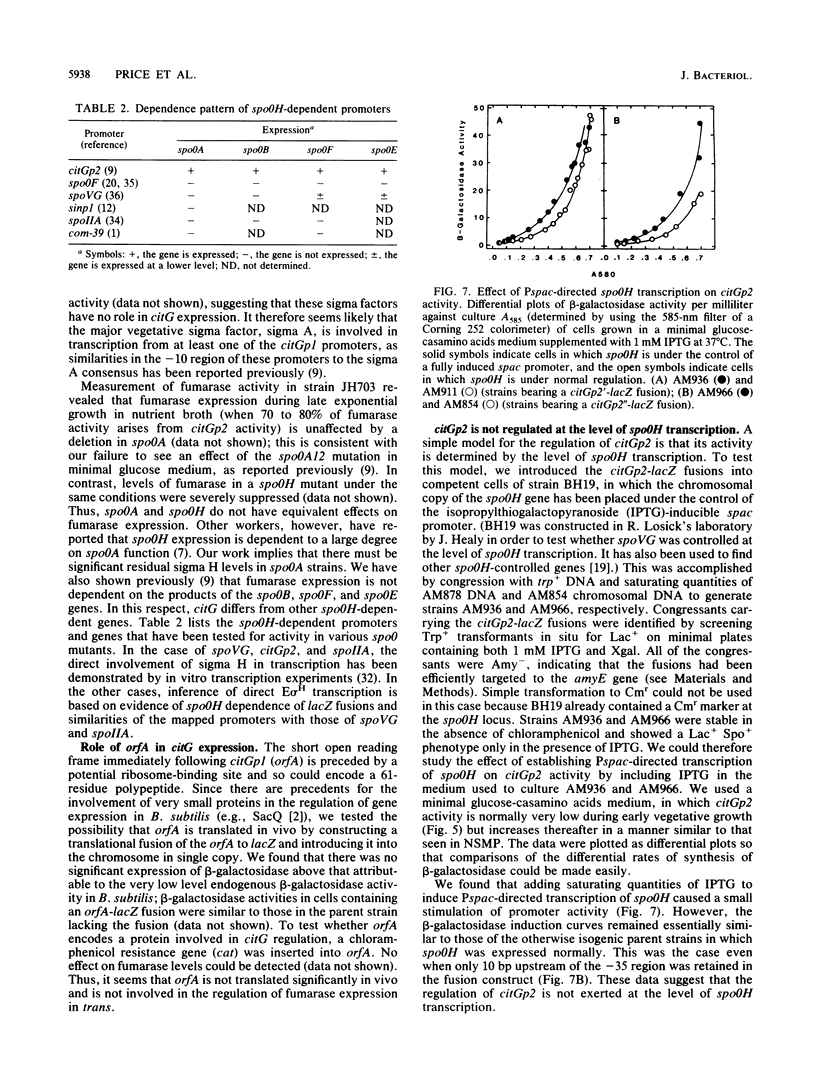
The fumarase gene (citG) of Bacillus subtilis is transcribed from two promoter regions, citGp1 and citGp2 (P1 and P2); the P2 promoter is used by the E sigma H form of RNA polymerase. In order to study the role of P1 and P2 in citG expression, the promoter region and various deletion derivatives that effectively separate P1 and P2 were fused to the Escherichia coli beta-galactosidase gene (lacZ) and introduced into the chromosome in single copy at the amyE locus. P1 functioned to provide a relatively low and stable basal level of fumarase activity throughout growth. In contrast, P2 activity was found to vary over at least a 50-fold range and was responsible for regulating fumarase activity during growth and sporulation in a rich medium and in response to changes in carbon source. To further investigate the role of sigma H in fumarase regulation, citGp2-lacZ fusions were introduced into a strain in which the expression of the chromosomal spoOH gene was under the control of the isopropylthiogalactopyranoside-inducible spac promoter. Induction of pspac did not lead to P2 induction, suggesting that citG expression is not regulated at the level of spoOH transcription.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano M., Hahn J., Dubnau D. Expression of competence genes in Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):3110–3117. doi: 10.1128/jb.169.7.3110-3117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amory A., Kunst F., Aubert E., Klier A., Rapoport G. Characterization of the sacQ genes from Bacillus licheniformis and Bacillus subtilis. J Bacteriol. 1987 Jan;169(1):324–333. doi: 10.1128/jb.169.1.324-333.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banner C. D., Moran C. P., Jr, Losick R. Deletion analysis of a complex promoter for a developmentally regulated gene from Bacillus subtilis. J Mol Biol. 1983 Aug 5;168(2):351–365. doi: 10.1016/s0022-2836(83)80023-0. [DOI] [PubMed] [Google Scholar]
- Berman M. L., Jackson D. E. Selection of lac gene fusions in vivo: ompR-lacZ fusions that define a functional domain of the ompR gene product. J Bacteriol. 1984 Aug;159(2):750–756. doi: 10.1128/jb.159.2.750-756.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carter H. L., 3rd, Moran C. P., Jr New RNA polymerase sigma factor under spo0 control in Bacillus subtilis. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9438–9442. doi: 10.1073/pnas.83.24.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J. A general method for fusion of the Escherichia coli lacZ gene to chromosomal genes in Bacillus subtilis. J Gen Microbiol. 1986 Nov;132(11):2953–2966. doi: 10.1099/00221287-132-11-2953. [DOI] [PubMed] [Google Scholar]
- Feavers I. M., Price V., Moir A. The regulation of the fumarase (citG) gene of Bacillus subtilis 168. Mol Gen Genet. 1988 Mar;211(3):465–471. doi: 10.1007/BF00425702. [DOI] [PubMed] [Google Scholar]
- Ferrari F. A., Nguyen A., Lang D., Hoch J. A. Construction and properties of an integrable plasmid for Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1513–1515. doi: 10.1128/jb.154.3.1513-1515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur N. K., Cabane K., Smith I. Structure and expression of the Bacillus subtilis sin operon. J Bacteriol. 1988 Mar;170(3):1046–1053. doi: 10.1128/jb.170.3.1046-1053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Mathews J. L. Chromosomal location of pleiotropic negative sporulation mutations in Bacillus subtilis. Genetics. 1973 Feb;73(2):215–228. doi: 10.1093/genetics/73.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Igo M., Lampe M., Ray C., Schafer W., Moran C. P., Jr, Losick R. Genetic studies of a secondary RNA polymerase sigma factor in Bacillus subtilis. J Bacteriol. 1987 Aug;169(8):3464–3469. doi: 10.1128/jb.169.8.3464-3469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaacks K. J., Healy J., Losick R., Grossman A. D. Identification and characterization of genes controlled by the sporulation-regulatory gene spo0H in Bacillus subtilis. J Bacteriol. 1989 Aug;171(8):4121–4129. doi: 10.1128/jb.171.8.4121-4129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandoski M., Dubnau E., Smith I. Transcriptional regulation of the spo0F gene of Bacillus subtilis. J Bacteriol. 1986 Nov;168(2):870–877. doi: 10.1128/jb.168.2.870-877.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles J. S., Guest J. R. Complete nucleotide sequence of the fumarase gene (citG) of Bacillus subtilis 168. Nucleic Acids Res. 1985 Jan 11;13(1):131–140. doi: 10.1093/nar/13.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A., Feavers I. M., Guest J. R. Characterization of the fumarase gene of Bacillus subtilis 168 cloned and expressed in Escherichia coli K12. J Gen Microbiol. 1984 Nov;130(11):3009–3017. doi: 10.1099/00221287-130-11-3009. [DOI] [PubMed] [Google Scholar]
- Moir A. The isolation of lambda transducing phages carrying the citG and gerA genes of Bacillus subtilis. J Gen Microbiol. 1983 Feb;129(2):303–310. doi: 10.1099/00221287-129-2-303. [DOI] [PubMed] [Google Scholar]
- Murray J. A. HCC ligation: rapid and specific DNA construction with blunt ended DNA fragments. Nucleic Acids Res. 1986 Dec 22;14(24):10118–10118. doi: 10.1093/nar/14.24.10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohné M. Regulation of the dicarboxylic acid part of the citric acid cycle in Bacillus subtilis. J Bacteriol. 1975 Apr;122(1):224–234. doi: 10.1128/jb.122.1.224-234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primrose S. B., Ehrlich S. D. Isolation of plasmid deletion Mutants and study of their instability. Plasmid. 1981 Sep;6(2):193–201. doi: 10.1016/0147-619x(81)90066-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotsu H., Henner D. J. Construction of a single-copy integration vector and its use in analysis of regulation of the trp operon of Bacillus subtilis. Gene. 1986;43(1-2):85–94. doi: 10.1016/0378-1119(86)90011-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tatti K. M., Carter H. L., 3rd, Moir A., Moran C. P., Jr Sigma H-directed transcription of citG in Bacillus subtilis. J Bacteriol. 1989 Nov;171(11):5928–5932. doi: 10.1128/jb.171.11.5928-5932.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J., Dubnau E., Ramakrishna N., Smith I. Bacillus subtilis spo0H gene. J Bacteriol. 1984 Feb;157(2):405–412. doi: 10.1128/jb.157.2.405-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. J., Howard M. G., Piggot P. J. Regulation of transcription of the Bacillus subtilis spoIIA locus. J Bacteriol. 1989 Feb;171(2):692–698. doi: 10.1128/jb.171.2.692-698.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S., Yoshikawa H., Kawamura F., Takahashi H., Yamamoto T., Kobayashi Y., Saito H. The effect of spo0 mutations on the expression of spo0A- and spo0F-lacZ fusions. Mol Gen Genet. 1986 Oct;205(1):28–33. doi: 10.1007/BF02428029. [DOI] [PubMed] [Google Scholar]
- Zuber P., Losick R. Use of a lacZ fusion to study the role of the spoO genes of Bacillus subtilis in developmental regulation. Cell. 1983 Nov;35(1):275–283. doi: 10.1016/0092-8674(83)90230-1. [DOI] [PubMed] [Google Scholar]
- Zuberi A. R., Feavers I. M., Moir A. Identification of three complementation units in the gerA spore germination locus of Bacillus subtilis. J Bacteriol. 1985 May;162(2):756–762. doi: 10.1128/jb.162.2.756-762.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



