Abstract
Background
CF is characterised by a progressive decline in lung function; reductions in this decline are often used as a measure of success in clinical trials. With improvements in treatment it may be that there has been a temporal shift in the pattern of the disease.
Methods
318 patients born in five successive cohorts and attending a specialist clinic with at least two routine measurements of lung function made between the ages of 18 and 22 were included. The declines in their lung function were estimated and compared.
Results
The mean (SE) slopes for percentage predicted forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were −1.53 (0.36)% and −1.27 (0.34)%, respectively (NS). The annual deterioration in FEV1 was −2.49%, −1.99% −2.20%, −1.65%, and −0.65% from the earliest to the most recent birth cohort; a similar pattern was observed for changes in FVC. There were no differences between male and female patients. Patients infected with Pseudomonas had a greater average decline in FEV1 (−1.6% v −1.1%).
Conclusions
The rates of decline in lung function in young adults with CF have diminished with successive birth cohorts. This has important implications for the design of clinical studies in this disease.
Keywords: cystic fibrosis, lung function, young adults
Cystic fibrosis (CF) is a progressive condition in which pulmonary disease is the main determinant of morbidity and mortality. The progressive decline in pulmonary function with time has been a feature of CF lung disease in the past and seems to be inevitable in all patients. The annual rate of decline in forced expiratory volume in 1 second (FEV1) percentage predicted is a significant predictor of the risk of death,1 and the annual deterioration in FEV1 percentage predicted is considered a better parameter than single measurements for referral for lung transplantation.2,3 The mean rates of FEV1 decline reported in the literature are shown in table 1.
Table 1 Published rates of decline in percentage predicted FEV1 in cystic fibrosis.
| Reference | No of patients | Age (years) | Follow up (years) | Annual decline in FEV1 % predicted | |
|---|---|---|---|---|---|
| Mean | SE | ||||
| Corey7 | 132 | 5–27 | 7 | −1.87 (male) | NS* |
| −2.71 (female) | |||||
| Kerem8 | 39 | 7–40 | 2 | −2.2 | 1.67 |
| Kovesi9 | 325 | 4–28 | 15 | −1.25 | 0.14 |
| Konstan5 | 43 | 5–39 | 4 | −3.6 | 0.55 |
| Eigen10 | 95 | 6–14 | 4 | −1.5 | NS |
| Corey11 | 366 | 18–32 | 15 | −2.72 | NS |
| Davis4 | 215 | 2–5 | −2.3 | 0.28 | |
| Milla12 | 152 | 4 | −2.44 | NS | |
| Merkus13 | 52 | Children | 3.9 | −2.2 | NS |
| Merkus14 | 53 | Children | 3.8 | −1.8 | NS |
*Not stated.
In clinical trials, based on the assumption that CF causes a progressive decline in lung function, a reduction in this decline during the observation period is a common outcome measure.4,5,6 With improvements in treatment it may be that the pattern of CF pulmonary disease is not the same today as it was in the past. We set out to determine the rate of lung function change in a contemporary cohort of young adults with CF and to compare this with rates in earlier birth cohorts.
Methods
Population
The adult CF service at Royal Brompton Hospital cares for all patients aged 16 years or over, currently some 700. These are seen regularly in clinic and, since 1981, have undergone formal annual review with full lung function testing. Using attendance records of the last 10 years from the hospital lung function laboratory, we identified all adult CF patients (>18 years). 318 patients with at least two pulmonary function tests (in two calendar years) between the ages of 18 and 22 form the study base for this analysis. Tests dated from as early as 1978 to the end of 2004. The highest prebronchodilator FEV1 and forced vital capacity (FVC) were used to calculate the rates of changes. Information was also collected on year of birth, sex, height at ages 18 and 22 years, and chronic Pseudomonas infection (defined as Pseudomonas aeruginosa cultured in at least three consecutive sputum samples at least 1 month apart during the study period).
Statistical analysis
Percentage predicted values of FEV1 and FVC were calculated using the European community reference population. Annual changes in lung function were estimated using linear regression analysis. Lung function changes were distributed approximately normally and are expressed as mean (SE). We analysed time trends after categorising patients into successive 5 year birth cohorts and fitting “cohort” as a variable in a liner regression model; differences between cohorts were assessed using ANOVA.
Results
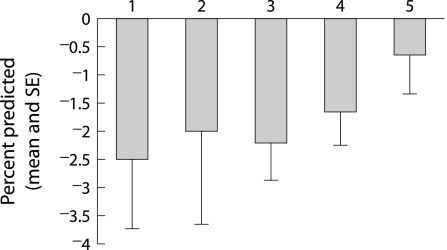
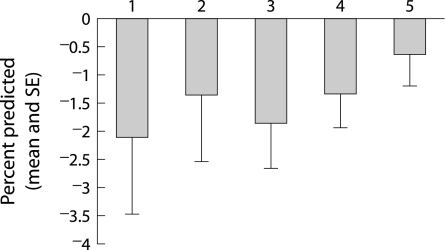
Between the ages of 18 and 22 years the mean (SE) annual changes in percentage predicted FEV1 and FVC among all patients were −1.53 (0.36)% and −1.27 (0.34)%. The rates of change tended to be greater in the earlier birth cohorts (table 2), but the trends were not statistically different (p = 0.28 and p = 0.35 for FEV1 and FVC, respectively). The trends were clearer, but again statistically not significant among patients born only after 1970 (figs 1 and 2).
Table 2 Mean height, pulmonary function, and FEV1 slope (percentage predicted) from age 18 to 22 years by birth cohort.
| Birth cohort | |||||
|---|---|---|---|---|---|
| 1960–4 | 1965–9 | 1970–4 | 1975–9 | 1980–4 | |
| Patients (n) | 17 | 28 | 62 | 113 | 98 |
| Measurements (n) | 40 | 76 | 167 | 349 | 314 |
| Mean height at age 18 (cm) | n/a | n/a | 167.1 | 167.2 | 167.4 |
| Mean FEV1 at 22 years (%) | 45.96% | 59.75% | 53.47% | 56.77% | 63.68% |
| FEV1 % predicted slope | −2.49% | −1.99% | −2.2% | −1.65% | −0.65% |
| Mean FVC at 22 years (%) | 68.13% | 78.02% | 69.85% | 75.18% | 81.8% |
| FVC % predicted slope | −2.1% | −1.35% | −1.86% | −1.34% | −0.64% |
Figure 1 Mean (SE) changes in percentage predicted FEV1 by birth cohort (numbers 1–5 refer to birth cohorts 1960–4, 1965–9, 1970–4, 1975–9, and 1980–4).
Figure 2 Mean (SE) changes in percentage predicted FVC by birth cohort (numbers 1–5 refer to birth cohorts 1960–4, 1965–9, 1970–4, 1975–9, and 1980–4).
There were fewer than 20 patients of either sex in the earlier two cohorts, so time trends by sex were analysed using only the later three cohorts. Among men born after 1970, the mean changes in FEV1 percentage predicted were −1.57% (1970–4 cohort), −0.57% (1975–9), and −0.91% (1980–4). These differences were not statistically significant (p = 0.66). Among women born within the same cohorts, the estimated changes in FEV1 percentage predicted were −2.48%, −2.98%, and −0.29%, respectively (p = 0.69).
There were no important differences between the different cohorts in mean height at age 18 (table 2) or 22 years (data not shown). Forty nine patients (15.4%) had no evidence of chronic P aeruginosa colonisation over the period of the study. Among these, the mean (SE) annual decline in FEV1 percentage predicted was −1.1 (0.8)%; patients infected with Pseudomonas had a higher mean decline (−1.61 (0.41)%). This difference was not statistically significant.
Discussion
Our findings indicate that the annual rate of decline in lung function among contemporary young adults with CF is very low, and is lower than among previous generations of adults with the disease. While it is possible that these apparent improvements occurred by chance, they are consistent with improvements in CF care and prognosis over the past 30 years.
There are several possible biases in our findings. Patients were selected on the basis of their having had lung function measurements at any age between 1995 and 2004, and we subsequently examined those measurements made when these patients were aged between 18 and 22 years. Patients were therefore selected by their survival and may have relatively mild disease. We suggest that this is likely to have resulted in an underestimate of the true rates of lung function decline in earlier cohorts and thus an underestimate of the true temporal differences. Furthermore, we cannot be sure that the indications for annual lung function testing remained the same throughout the period of our analysis. Analysis of the later three cohorts, all born after the period when routine testing was practised and in whom survival pressures are probably less marked, tended to confirm our overall findings.
The annual fall in FEV1 percentage predicted is believed to be associated with genotype, sex, pancreatic and nutritional status, and colonisation with mucoid strains of P aeruginosa.11,15,16 We were unable to show a difference between male and female patients in FEV1 decline; while this may be due to small numbers, similar findings have been reported in Swedish patients.17 Nor did we find any important difference in terms of height, which might be associated with nutritional status. We did not examine the effects of weight, but one study has suggested that children with CF who weigh more and who gain weight at an appropriate and uninterrupted rate have a better FEV1 trajectory.6 Our result with regard to the effect of P aeruginosa colonisation/infection on lung function decline is consistent with that of Schaedel et al.17 We were unable to demonstrate a statistically significant association between this and lung function decline, possibly because of the small number of non‐colonised patients. We did not identify the time at which the patients acquired Pseudomonas and cannot separate cause from effect as others have.18
Our results are similar to those of children in Toronto summarised in table 3,19 and to reports of patients treated with rhDNase.20 They are consistent with improving trends in survival and probably reflect better standards of care organised within specialist CF centres, and more aggressive approaches to treatment, especially with nebulised antibiotics and DNase. An important implication is that decline in lung function may now be too insensitive a measure for use in clinical trials of limited periods.
Table 3 Lung function decline in children with cystic fibrosis19.
| Birth cohort | ||||||
|---|---|---|---|---|---|---|
| 1960–4 | 1965–9 | 1970–4 | 1975–80 | 1980–4 | 1985–9 | |
| Follow up to | 1978 | 1983 | 1988 | 1993 | 1998 | 2003 |
| Study (n) | 132 | 120 | 114 | 90 | 90 | 108 |
| FEV1 slope | −2.1 | −2.6 | −2.5 | −1.8 | −1.1 | −0.8 |
In conclusion, the rate of decline of FEV1 in patients with CF is falling, in line with improved survival, and is now approaching zero. This is probably due to improved treatment. There are major implications for the future design of clinical trials.
Footnotes
Competing interests: none
References
- 1.Milla C E, Warwick W J. Risk of death in cystic fibrosis patients with severely compromised lung function. Chest 19981131230–1234. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbluth D B, Wilson K, Ferkol T.et al Lung function decline in cystic fibrosis patients and timing for lung transplantation referral. Chest 2004126412–419. [DOI] [PubMed] [Google Scholar]
- 3.Augarten A, Akons H, Aviram M.et al Prediction of mortality and timing of referral for lung transplantation in cystic fibrosis patients. Pediatr Transplant 20015339–342. [DOI] [PubMed] [Google Scholar]
- 4.Davis P B, Byard P J, Konstan M W. Identifying treatments that halt progression of pulmonary disease in cystic fibrosis. Pediatr Res 199741161–165. [DOI] [PubMed] [Google Scholar]
- 5.Konstan M W, Byard P J, Hoppel C L.et al Effect of high‐dose ibuprofen in patients with cystic fibrosis. N Engl J Med 1995332848–854. [DOI] [PubMed] [Google Scholar]
- 6.Ramsey B W, Dorkin H L, Eisenberg J D.et al Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med 19933281740–1746. [DOI] [PubMed] [Google Scholar]
- 7.Corey M, Levison H, Crozier D. Five‐ to seven‐year course of pulmonary function in cystic fibrosis. Am Rev Respir Dis 19761141085–1092. [DOI] [PubMed] [Google Scholar]
- 8.Kerem E, Corey M, Gold R.et al Pulmonary function and clinical course in patients with cystic fibrosis after pulmonary colonization with Pseudomonas aeruginosa. J Pediatr 1990116714–719. [DOI] [PubMed] [Google Scholar]
- 9.Kovesi T, Corey M, Levison H. Passive smoking and lung function in cystic fibrosis. Am Rev Respir Dis 19931481266–1271. [DOI] [PubMed] [Google Scholar]
- 10.Eigen H, Rosenstein B, FitzSimmons S.et al A multicenter study of alternate‐day prednisone therapy in patients with cystic fibrosis. J Pediatr 1995126515–523. [DOI] [PubMed] [Google Scholar]
- 11.Corey M, Edwards L, Levison H.et al Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr 1997131809–814. [DOI] [PubMed] [Google Scholar]
- 12.Milla C E, Warwick W J, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med 2000162891–895. [DOI] [PubMed] [Google Scholar]
- 13.Merkus P J, Tiddens H A, de Jongste J C. Annual lung function changes in young patients with chronic lung disease. Eur Respir J 200219886–891. [DOI] [PubMed] [Google Scholar]
- 14.Merkus P J, Govaere E S, Hop W H.et al Preserved diffusion capacity in children with cystic fibrosis. Pediatr Pulmonol 20043756–60. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbluth D B, Wilson K, Ferkol T.et al Lung function decline in cystic fibrosis patients and timing for lung transplantation referral. Chest 2004126412–419. [DOI] [PubMed] [Google Scholar]
- 16.Augarten A, Akons H, Aviram M.et al Prediction of mortality and timing of referral for lung transplantation in cystic fibrosis patients. Pediatr Transplant 20015339–342. [DOI] [PubMed] [Google Scholar]
- 17.Schaedel C, De Monestrol I, Hjelte L.et al Predictors of deterioration of lung function in cystic fibrosis. Pediatr Pulmonol 200233483–491. [DOI] [PubMed] [Google Scholar]
- 18.Kosorok M R, Zeng L, West S E.et al Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol 200132277–287. [DOI] [PubMed] [Google Scholar]
- 19.Xu W, Subbarao P, Corey M. Changing patterns of lung function decline in children with cystic fibrosis. J Cystic Fibrosis 20043S116 [Google Scholar]
- 20.Hodson M E, Koch C, Harries H K.et al The European Epidemiologic Registry of Cystic Fibrosis (ERCF). Does dornase alfa make any difference? In: Proceedings of the 22nd European CF Conference, Berlin, Germany. Berlin: European CF Conference, 199866