Abstract
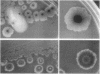
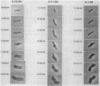
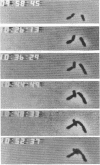
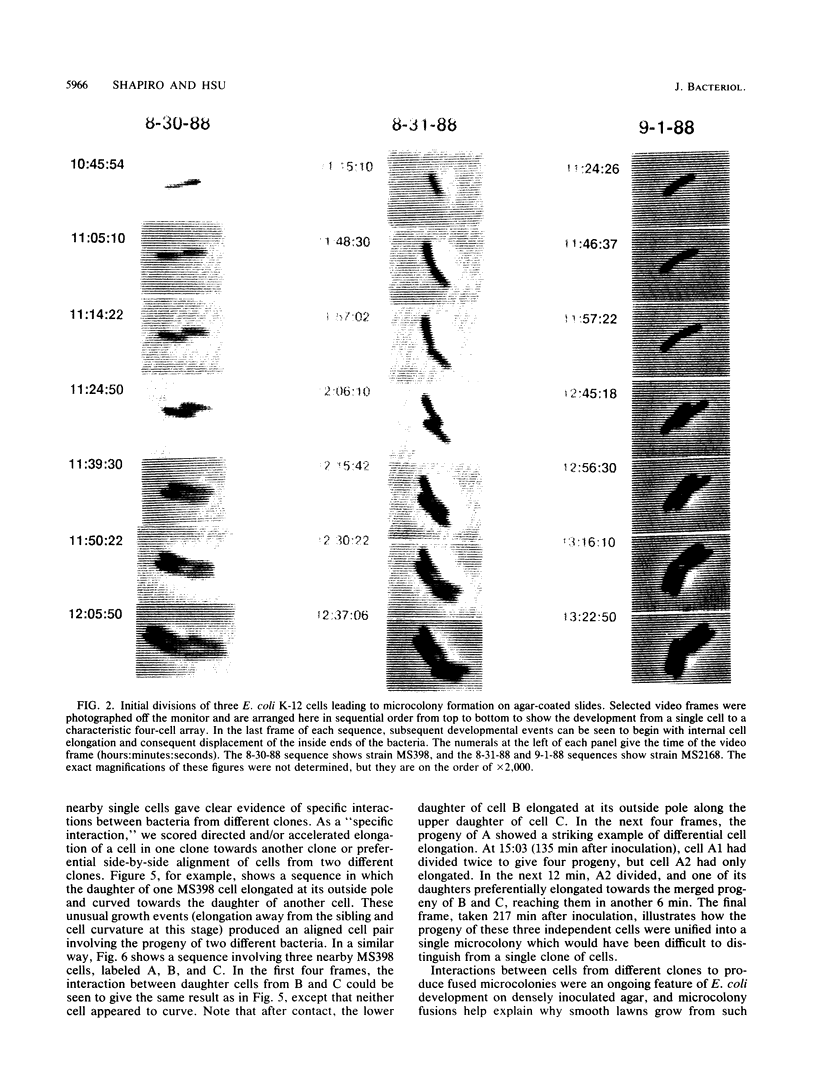
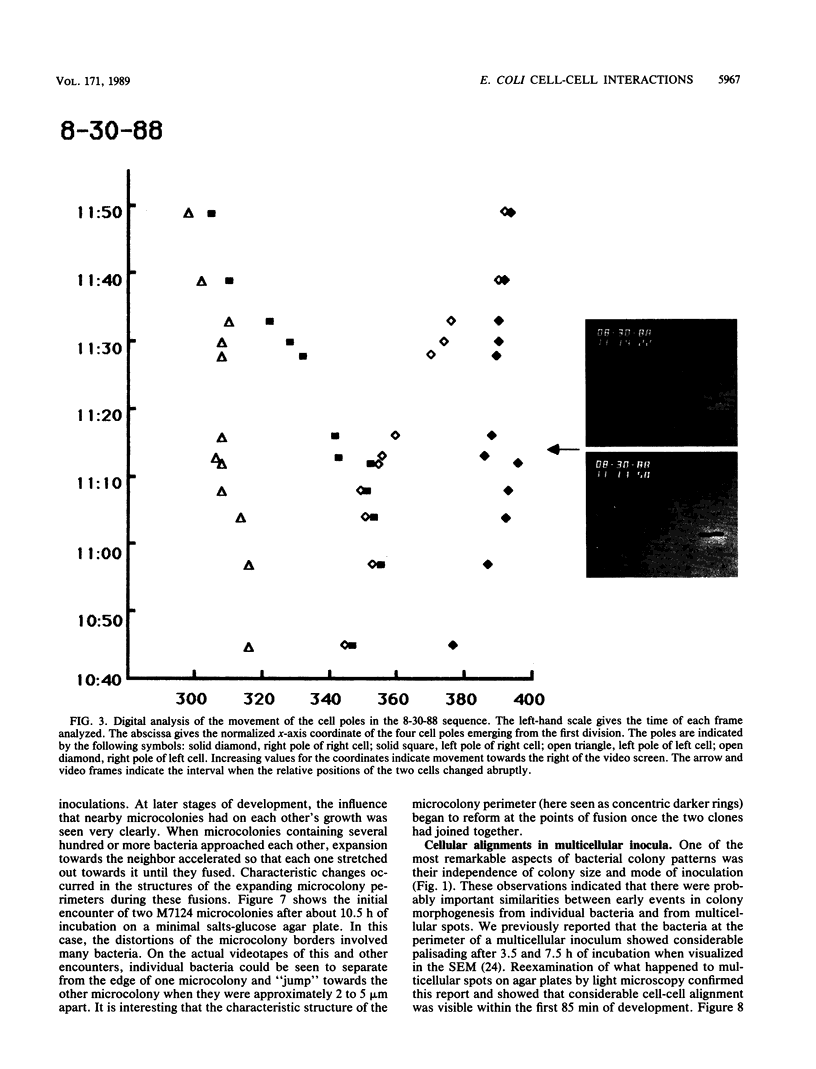
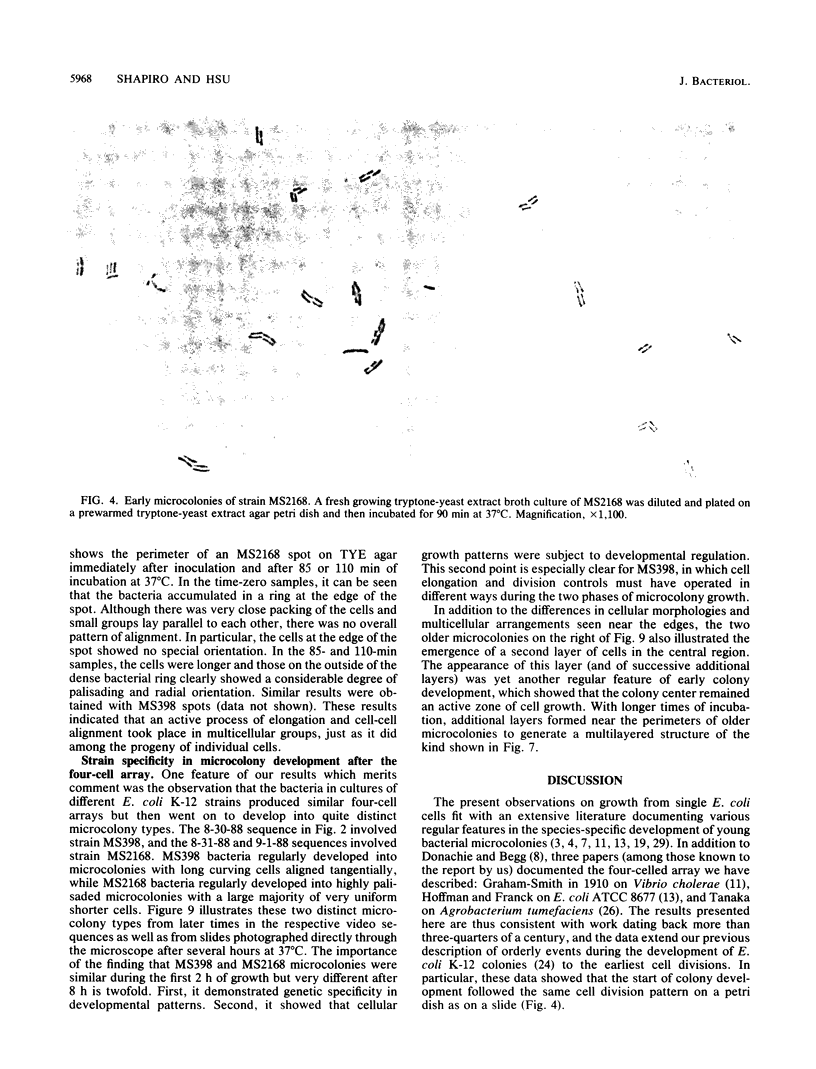
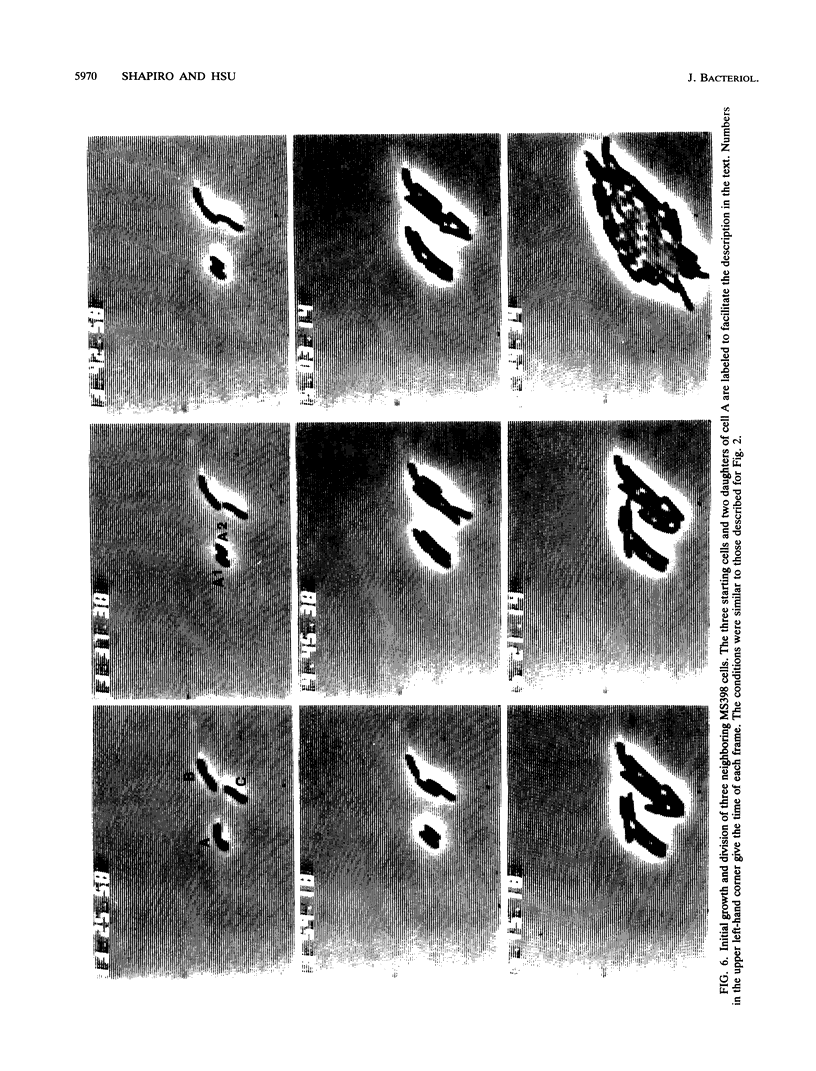
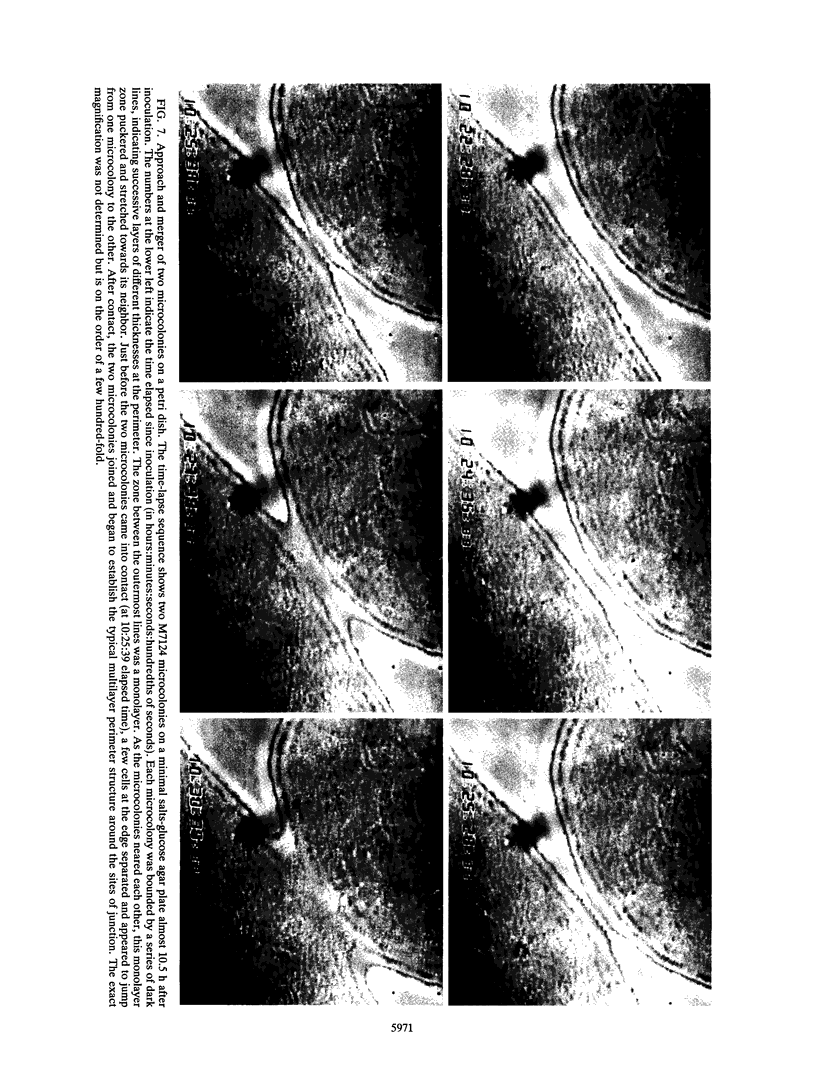
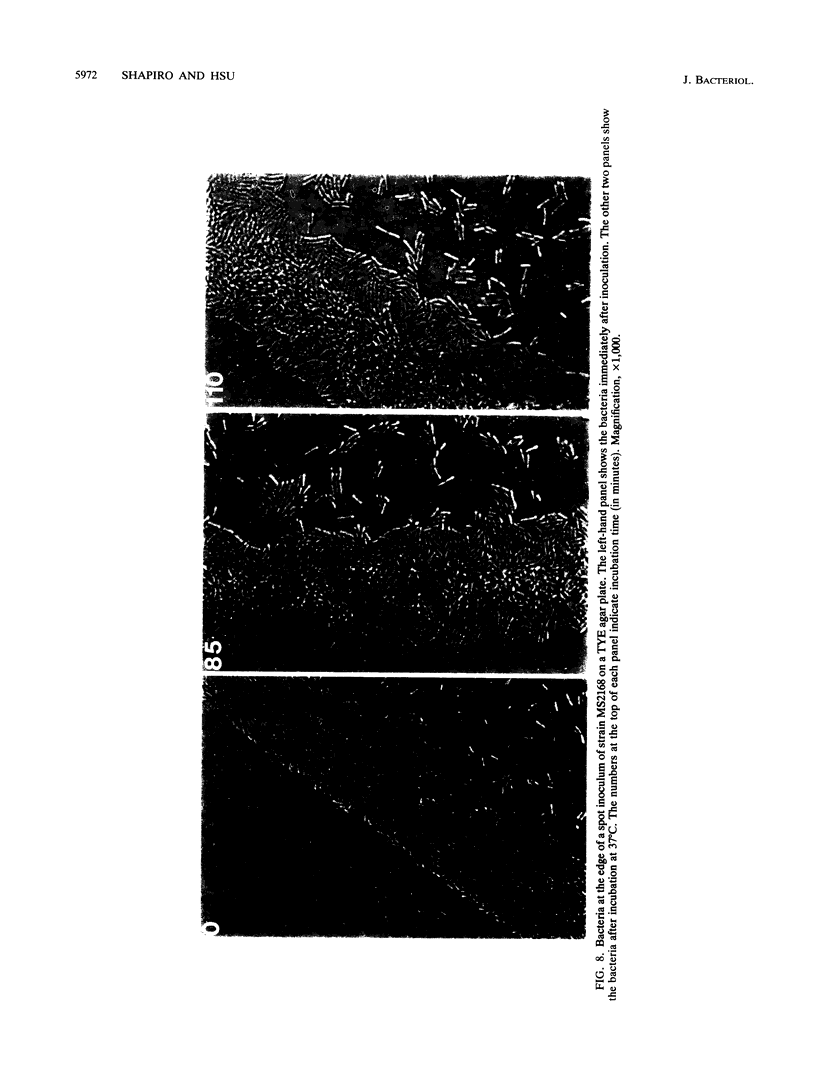
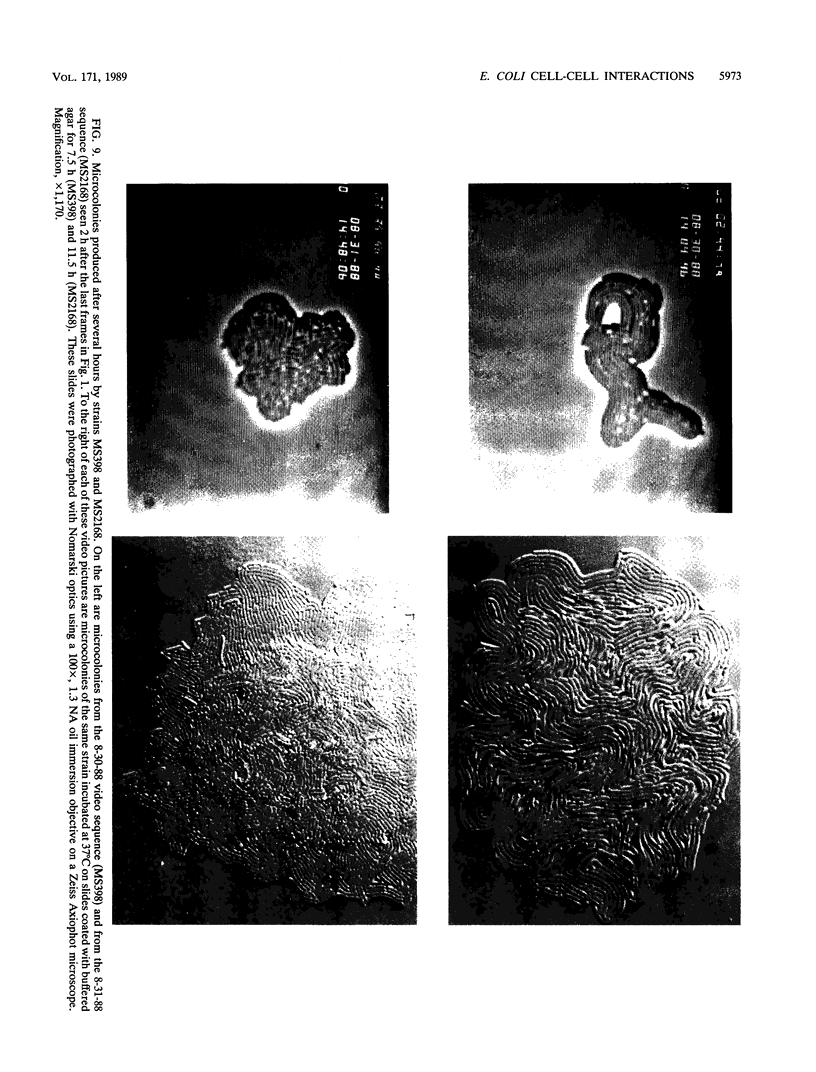
The high degree of organization in mature bacterial colonies suggests specific interactions between the cells during colony development. We have used time-lapse video microscopy to find evidence for cell-cell interactions. In its initial stages, Escherichia coli K-12 colony morphogenesis displayed control of the geometry of cell growth and involved intimate side-by-side associations. When microcolonies developed from isolated single bacteria, a directed process of elongation and division resulted in the appearance of a symmetrical four-cell array. When growth began with separate but nearby bacteria, the daughters of different cells elongated towards each other and also lined up side by side. Interactions between microcolonies containing several hundred or more bacteria were visible several hours later. Control of cell morphogenesis at later stages of microcolony development was strain specific. These results show that E. coli K-12 cells respond to each other and adjust their cellular morphogenesis to form multicellular groups as they proliferate on agar.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremer E., Silhavy T. J., Weinstock G. M. Transposition of lambda placMu is mediated by the A protein altered at its carboxy-terminal end. Gene. 1988 Nov 15;71(1):177–186. doi: 10.1016/0378-1119(88)90089-3. [DOI] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J. Growth of the bacterial cell. Nature. 1970 Sep 19;227(5264):1220–1224. doi: 10.1038/2271220a0. [DOI] [PubMed] [Google Scholar]
- Gottlieb D. I. GABAergic neurons. Sci Am. 1988 Feb;258(2):82–89. doi: 10.1038/scientificamerican0288-82. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Losick R. Extracellular control of spore formation in Bacillus subtilis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4369–4373. doi: 10.1073/pnas.85.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN H., FRANK M. E. Form and internal structure of cellular aggregations in early Escherichia coli microcultures. J Gen Microbiol. 1961 Jul;25:353–364. doi: 10.1099/00221287-25-3-353. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D. Control of multicellular development: Dictyostelium and Myxococcus. Annu Rev Genet. 1986;20:539–566. doi: 10.1146/annurev.ge.20.120186.002543. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Nelligan J., Costerton J. W. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982 Dec;66(6):1339–1341. doi: 10.1161/01.cir.66.6.1339. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Organization of developing Escherichia coli colonies viewed by scanning electron microscopy. J Bacteriol. 1987 Jan;169(1):142–156. doi: 10.1128/jb.169.1.142-156.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. Scanning electron microscope study of Pseudomonas putida colonies. J Bacteriol. 1985 Dec;164(3):1171–1181. doi: 10.1128/jb.164.3.1171-1181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. The use of Mudlac transposons as tools for vital staining to visualize clonal and non-clonal patterns of organization in bacterial growth on agar surfaces. J Gen Microbiol. 1984 May;130(5):1169–1181. doi: 10.1099/00221287-130-5-1169. [DOI] [PubMed] [Google Scholar]