Abstract
Background
The diagnosis of latent Mycobacteriumtuberculosis (MTB) infection with a tuberculin skin test (TST) in children is complicated by the potential influence of prior exposure to Bacille Calmette Geurin (BCG) vaccination or environmental mycobacteria. A whole blood assay has recently been developed to quantitatively measure interferon gamma (IFN‐γ) production by lymphocytes specific to the MTB antigens ESAT‐6 and CFP‐10, but its use and assessment in children has been limited. A study was undertaken to compare the performance of the whole blood IFN‐γ assay with the TST in diagnosing latent tuberculosis (TB) infection or TB disease in children in routine clinical practice.
Methods
One hundred and six children with a high risk of latent TB infection or TB disease were enrolled in the study. High risk was defined as contact with TB disease, clinical suspicion of TB disease, or recent arrival from an area of high TB prevalence. The whole blood IFN‐γ assay was undertaken in 101 children.
Results
Seventeen (17%) of the 101 assays yielded inconclusive results due to failure of positive or negative control assays. There was poor correlation between the whole blood IFN‐γ assay and the TST (kappa statistic 0.3) with 26 (70%) of the 37 children defined as latent TB infection by TST having a negative whole blood IFN‐γ assay. There were no instances of a positive whole blood IFN‐γ assay with a negative TST. Mitogen (positive) control IFN‐γ responses were significantly correlated with age (Spearman's coefficient = 0.53, p<0.001) and, in children with latent TB infection identified by TST, those with a positive IFN‐γ assay were older (median 12.9 v 6.92 years, respectively, p = 0.007). The whole blood IFN‐γ assay was positive in all nine children with TB disease.
Conclusion
There was poor agreement between the whole blood IFN‐γ assay and TST for the diagnosis of latent TB. The whole blood IFN‐γ assay may have lower sensitivity than the TST in diagnosing TB infection in children. A significant proportion of whole blood IFN‐γ assays fail when used as a screening assay in routine practice.
Keywords: tuberculosis, interferon gamma, tuberculin skin test, Mantoux, diagnosis, children
Globally, the burden of tuberculosis (TB) is immense with an estimated one third of the world's population infected and projections that almost 12 million new cases will occur annually by 2006.1 Accurate figures for the burden of childhood TB are not readily available, but children represent an increasing proportion of the total number of cases.2 In the majority of patients, primary infection with Mycobacterium tuberculosis (MTB) is contained by the host's immune system (establishing latent infection). The cytokine interferon gamma (IFN‐γ) produced by T cells has a critical role in the protective immunological response to primary infection.3,4,5 Clinical latency can persist for many years with a lifetime risk of reactivation to active disease of approximately 10%.3,6 However, in children, up to 50% of untreated infants and 15% of older children with latent TB infection develop disease within 2 years of being infected.7 Accurately identifying those who are latently infected is difficult as they remain asymptomatic. The tuberculin skin test (TST) is the established screening method for diagnosing latent TB infection in adults and children, but it has many potential problems. The specificity and sensitivity of this test is limited by prior Bacille Calmette Geurin (BCG) vaccination, previous exposure to environmental mycobacteria, and operator errors in placement and reading.8,9,10
A whole blood assay (QuantiFERON‐TB Gold, Cellestis Ltd, Victoria, Australia) has recently been developed and was approved by the US Food and Drug Administration in December 2004 as an in vitro test for TB in adults. The whole blood assay quantitatively measures IFN‐γ production by previously sensitised lymphocytes in response to the MTB specific proteins, early secretory antigenic target 6 (ESAT‐6) and culture filtrate protein 10 (CFP‐10).11 Potential advantages of the whole blood IFN‐γ assay over TST include being unaffected by previous BCG vaccination, the higher specificity of the test (ESAT‐6 and CFP‐10 are absent from almost all environmental mycobacteria), the requirement for only one patient visit, and the exclusion of intradermal injection technique problems as well as elimination of errors in reading and interpretation.
In addition to the whole blood assay, a rapid ex vivo enzyme linked immunospot assay (ELISPOT) has also been developed that counts individual antigen specific T cells. In this assay, each T cell that secretes IFN‐γ in response to the TB specific antigens ESAT‐6 and CFP‐10 leaves a footprint that can be enumerated using either an ELISPOT reader or a magnifying lens.12 Studies in adults using the whole blood13,14 and the ELISPOT15,16 IFN‐γ assays and, more recently, the ELISPOT assay in children17,18 have shown that IFN‐γ based assays have high sensitivity and specificity for TB disease, but none have focused specifically on the use of whole blood IFN‐γ assays in the diagnosis of latent infection in children.
The aim of this study was to compare the performance of the whole blood IFN‐γ assay with TST in children referred for the evaluation of latent TB infection and TB disease.
Methods
Patients
Children (less than 18 years) with a high risk of latent TB infection or TB disease referred to the Royal Children's Hospital Melbourne, Australia between April 2004 and April 2005 were eligible for inclusion. High risk was defined as children with siblings or parents recently diagnosed with TB disease, clinical suspicion of TB disease, and those recently (within 5 years) immigrated from countries with a high prevalence of TB. Patients were recruited from the hospital wards (inpatients admitted for suspicion of clinical TB) and two hospital based outpatient clinics (a TB clinic and a refugee health clinic). Demographic details recorded included age, country of birth, history of BCG vaccination (documented or verbally from parents), and the presence or absence of a typical BCG scar in the deltoid region, thigh, or proximal forearm.
Tests
All patients underwent clinical evaluation, TST, and whole blood IFN‐γ assay. Chest radiography was performed when clinically indicated or in those with a positive TST. TST was performed by intradermal instillation of 10 international units of tuberculin (Cellestis Tuberculin PPD 100 IU/ml, Melbourne, Australia) by trained staff, and read after 48–72 hours, measuring the transverse diameter of induration. A positive TST was defined as induration of >15 mm in individuals with evidence of prior BCG vaccination, >5 mm in those who were known TB contacts (irrespective of BCG), and >10 mm for all others.7 The whole blood IFN‐γ assay was performed in a certified clinical reference laboratory according to the manufacturer's recommendations as described elsewhere.9 In summary, aliquots (5 ml) of undiluted heparinised whole blood were incubated overnight with ESAT‐6 and CFP‐10, a negative control, and the mitogen phytohaemagglutinin as a positive control. Following 16–24 hours of incubation, plasma samples were harvested for IFN‐γ quantification by a single step sandwich‐type ELISA. A concentration of >0.35 IU/ml IFN‐γ in whole blood in response to exposure with either ESAT‐6 or CFP‐10 constituted a positive test.13 The assay was deemed inconclusive if there was a high negative control (response with no antigen) or a negative mitogen control (indicating possible T cell anergy). In these instances the test was deemed to have “failed” and was not repeated. The whole blood IFN‐γ assay was performed as part of routine tests and the study was approved as a clinical audit by the hospital research ethics committee.
Definitions
In the absence of a recognised gold standard and in agreement with published studies, latent TB infection was defined as an asymptomatic child with a positive TST as defined above and a normal chest radiograph.16,19,20 TB disease was defined as a child with a positive TST who was symptomatic and/or had an abnormal chest radiograph consistent with TB and/or who clinically responded to antituberculous medication or a child with acid‐fast bacilli detected, or MTB cultured or detected by molecular methods (PCR) from clinical specimens. Uninfected was defined as an asymptomatic child with a negative TST.
The agreement between the whole blood IFN‐γ assay and the TST for the diagnosis of latent TB infection was investigated.
Analysis of data
Data were analysed using Stata Version 8.2 (StataCorp, Texas, USA). The median age of the children in the latent TB group with a positive whole blood IFN‐γ assay was compared with those with a negative assay using a Wilcoxon rank sum test. The measure of agreement between TST and whole blood IFN‐γ assay for diagnosing latent TB infection was tested using the kappa statistic. The correlation between mitogen control response and age was assessed using the Spearman's correlation coefficient.
Results
The clinical and demographic details are summarised in table 1. Of 106 children (61 boys) enrolled in the study, five (5%) failed to return for TST reading and could not therefore be assigned a diagnosis. Of the remaining 101, the majority (91%) originated from the Horn of Africa (Sudan, Somalia, Kenya and Ethiopia), reflecting recent migration demographics to Australia. On the basis of the TST, 42 children (41%) were identified with latent TB infection, nine (9%) with TB disease, and 50 (50%) were uninfected (table 1). The median (range) age of the latent TB, TB disease, and uninfected group was 9.2 (0.6–17.9) years, 3.9 (1.2–17.1) years, and 6.8 (0.4–16.9) years, respectively. Forty nine children (49%) had an identifiable BCG vaccination scar. Four children (9%) with a history of BCG vaccination had no scar. Only one child had written documentation of vaccination status including BCG.
Table 1 Demographic and clinical details of study subjects.
| Diagnosis | |||
|---|---|---|---|
| Latent TB (n = 42) | TB disease (n = 9) | Uninfected (n = 50) | |
| Demographic data | |||
| Median age in years (range) | 9.2 (0.6–17.9) | 3.9 (1.2–17.1) | 6.8 (0.4–16.9) |
| Male | 23 (55%) | 6 (67%) | 32 (64%) |
| Born in high TB prevalence area | 37 (88%) | 7 (78%) | 48 (96%) |
| TB contact | |||
| Household† | 24 (60%) | 5 (56%) | 6 (12%) |
| Non‐household | 0 (0%) | 0 (0%) | 1 (2%) |
| Unknown | 7 (14%) | 1 (11%) | 8 (16%) |
| None | 11 (26%) | 3 (33%) | 35 (70%) |
| Clinical | |||
| BCG | |||
| Scar present | 19 (45%) | 3 (33%) | 27 (54%) |
| History but no scar | 2 (5%) | 0 | 2 (4%) |
| No evidence of prior BCG | 21 (50%) | 6 (67%) | 21 (42%) |
| Fevers | 1 (2%) | 7 (78%) | 0 |
| Night sweats | 0 (0%) | 6 (66%) | 0 |
| Cough >2 weeks | 0 (0%) | 6 (66%) | 0 |
| Tuberculin skin test | |||
| 0–5 mm | 0 (0%) | 37 (74%) | |
| >5–10 mm | 9 (22%) | 2 (22%)* | 9 (18%) |
| >10–15 mm | 11 (26%) | 1 (11%) | 4 (8%) |
| >15 mm | 22 (52%) | 3 (33%) | 0 |
| Chest radiograph | |||
| Normal | 38 (90%) | 3 (33%) | 12 (24%) |
| Abnormal | 0 (0%) | 6 (66%) | 0 |
| Not done/unavailable | 4 (10%) | 38 (76%) | |
*Three children with TB disease did not have TST.
†34 with pulmonary TB; 1 with lymph node TB.
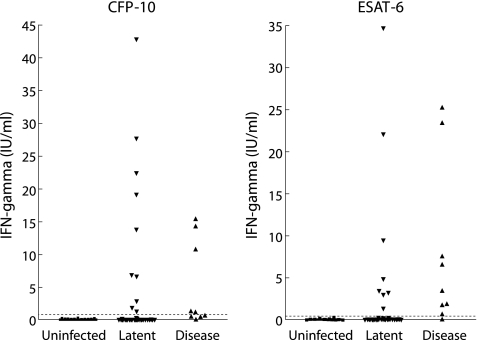
The levels of response to CFP‐10 and ESAT‐6 from the whole blood IFN‐γ assays are shown in fig 1. Of the 101 children, 98 (97%) had both TST and a whole blood IFN‐γ assay performed (table 2). Seventeen (17%) of the whole blood IFN‐γ assays “failed” either because of a high negative control response (n = 12) or an inadequate mitogen control response (n = 5). Three patients diagnosed with TB disease did not have a TST performed due to concerns regarding potential ulceration.
Figure 1 Results of whole blood IFN‐γ assay for CFP‐10 and ESAT‐6. The dotted line depicts the test cut off value for a positive result (0.35 IU/ml).
Table 2 Results of whole blood IFN‐γ assay by diagnostic group.
| Diagnosis (based on TST) | Whole blood IFN‐γ assay result | Total | ||
|---|---|---|---|---|
| Negative | Positive | Failed | ||
| Uninfected | 38 (76%) | 0 | 12 (24%)* | 50 (100%) |
| Latent TB | 26 (62%) | 11 (26%) | 5 (12%)† | 42 (100%) |
| TB disease | 0 | 9 (100%)‡ | 0 | 9 (100%) |
| Total | 64 | 20 | 17 | 101 |
*Nine high negative control; three inadequate mitogen control.
†Three high negative control; two inadequate mitogen control.
‡Three patients did not have a TST.
There was poor correlation between the whole blood IFN‐γ assay and the TST (kappa statistic 0.30, table 2). Of 42 children with TST defined latent TB infection, 37 had a successful whole blood IFN‐γ assay and, of these, 26 (70%) were negative. Of these 37 children, 24 (65%) had a known TB household contact. There were no instances of a positive whole blood IFN‐γ assay with a negative TST. The whole blood IFN‐γ assay was positive in all nine (100%) patients diagnosed with TB disease. TB disease was diagnosed in three children by PCR analysis of clinical samples. One further patient was diagnosed on the basis of clinical symptoms and granulomatous inflammation detected following biopsy of a paravertebral mass. The remaining children were diagnosed based on a combination of suggestive clinical features, radiographic abnormalities, and response to antituberculous treatment.
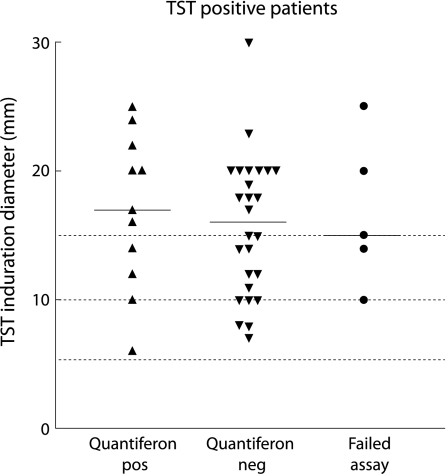
Of the 26 children with TST defined latent TB and a negative whole blood IFN‐γ assay, 13 (50%) had a TST induration of >15 mm and, of all the children with TST induration >15 mm, only 7/22 (31.8%) had a positive whole blood IFN‐γ assay (fig 2).
Figure 2 Relationship between whole blood IFN‐γ assay results and TST induration diameter in the 42 patients with TST defined latent TB infection. The bars depict the median value for each group.
Of the 42 children with TST defined latent TB, 21 (50%) had no evidence of prior BCG vaccination, and BCG status was similar in those with positive and negative whole blood IFN‐γ assay results (11/26 negative assays compared with 7/11 positive assays had received BCG, χ2 = 0.68, p = 0.41). Of the 21 BCG unvaccinated children (no scar or history of immunisation), 19 (90.5%) were from countries with a high prevalence of TB and the remaining two lived in families originally from a high prevalence country. Nine of the 21 (42.8%) reported exposure to a TB household contact. In this subgroup of 21 children with a very high likelihood of latent TB infection, 16 (76.2%) whole blood IFN‐γ assays yielded determinate results of which only four (25%) were positive.
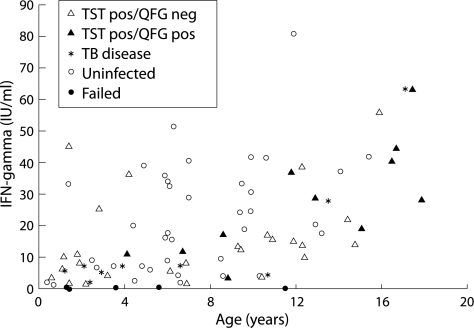
In the whole blood IFN‐γ assay, the quantitative mitogen “positive” control response was significantly correlated with age (Spearman's coefficient = 0.53, p<0.001, fig 3). In those with TST diagnosed latent TB infection, a positive whole blood assay was also associated with increased age (median 12.9 v 6.92 years, p = 0.0065). In addition, children with positive whole blood IFN‐γ assays tended to have higher mitogen control values (mean 27.73 IU/ml (95% CI 17.38 to 38.08)) than those with negative assays (15.47 IU/ml (95% CI 12.71 to 18.23), p = 0.03), probably reflecting that the former were older.
Figure 3 Age related mitogen (positive) control response for different diagnostic categories. TST, tuberculin skin test; QFG, QuantiFERON‐TB Gold whole blood IFN‐γ assay.
Discussion
Identifying and treating infectious cases is critical to TB control. In developed countries, where resources are more readily available and secondary prevention of reactivation disease feasible, considerable emphasis is also placed on identifying individuals latently infected with M tuberculosis.12 The burden of TB in developed countries is substantially borne by recent immigrants from high prevalence areas.21,22
To our knowledge, this is the first study to investigate the performance of the whole blood IFN‐γ assay in diagnosing latent TB infection in children. We have shown that, in predominantly well children at high risk of latent TB infection, there is poor correlation between the TST and the whole blood IFN‐γ assay, with 70% of children identified by TST to have latent TB infection having a negative whole blood IFN‐γ assay result. This suggests that the whole blood IFN‐γ has lower sensitivity than the TST or, less likely, that it is more specific, revealing “false positive” TST results. The former hypothesis is strongly supported by the finding that, in 24 children deemed latently infected by TST and with documented household contact, the whole blood IFN‐γ assay was positive in only eight (33%). Furthermore, out of a subgroup of 16 children with a very high likelihood of latent TB infection (all BCG unvaccinated, from families from high TB prevalence areas, and TST induration 8–30 mm), only one quarter had a positive whole blood IFN‐γ assay. Eight of these children had reported exposure to a TB household contact, of which only two had a positive whole blood IFN‐γ assay.
Children with a positive whole blood IFN‐γ assay were older and tended to have a higher mitogen control response. This association may have been due to cumulative probability of TB exposure or an increased ability to produce IFN‐γ.
Of particular concern in our study was the relatively high “failure” rate of the whole blood IFN‐γ assay. Studies in adults using the whole blood IFN‐γ assay in a cohort of predominantly immunocompetent individuals have reported indeterminate results in 0.1–11% of assays.23 However, a recent hospital based study using a whole blood IFN‐γ assay in routine clinical practice reported indeterminate results in 21.4% of 318 patients. These were nearly all attributable to an inadequate mitogen control response. Indeterminate results were more frequent in patients with a negative TST and in patients receiving immunosuppressive medications.24 In our study 17% of the assays performed yielded inconclusive results, suggesting that widespread application of the whole blood assay in children is questionable. The reason for these “failed” tests is not apparent in our cohort of predominantly well children. In contrast to most previously published studies of whole blood IFN‐γ assays for TB, we used routine testing facilities under normal clinical conditions, with no special provision for handling, transport, or processing of samples. However, the state laboratory that performs the whole blood IFN‐γ assays is a reference laboratory with over 2 years' experience in the use of this assay, and performs in excess of 3000 assays per year. The 17% failure rate in our study therefore represents the “real world” use of this assay in paediatric clinical practice.
High IFN‐γ levels in the negative control have not been reported in children previously. Under most circumstances the negative control will not generate IFN‐γ above the allowed threshold of 1.0 IU/ml. Results below this maximum threshold are subtracted from the result of the test specimen. A smaller number failed due to an inadequate mitogen control response. Decreased IFN‐γ production to various stimuli has been well documented in infants, potentially increasing the chance of false negative assays or requiring re‐evaluation of lower threshold values for positive responses.25 In agreement with published adult data, a threshold of 0.35 IU/ml IFN‐γ was used to indicate a positive result. Using a lower cut off of 0.1 IU/ml would yield only three more individuals with positive IFN‐γ assays in the TB infected group. However, other studies have shown that IFN‐γ production increases rapidly during infancy to reach adult levels.26,27 Of the five children with TST defined latent TB infection and a negative mitogen control, none were infants. Furthermore, the youngest child in our study, who was 4 months of age, had a mitogen control response within the normal range.
Being unaffected by prior BCG vaccination, the new generation of IFN‐γ assays holds much promise in reliably identifying patients requiring antituberculous therapy. These ex vivo assays currently exist in two forms—an ELISA assay that directly measures IFN‐γ production and an ELISPOT assay that counts individual antigen specific T cells. These assays have been evaluated in adults in multiple settings described elsewhere.28 In a study of South African children with TB disease with a high prevalence of HIV infection and malnutrition, Liebeschuetz et al found that the ELISPOT assay was more sensitive than the TST (83% v 63%).17 The sensitivity of the ELISPOT assay was unaffected by age, HIV co‐infection, or malnutrition, all known to influence the TST. Combining both tests increased the sensitivity to 91%. Also, using an ELISPOT assay, Nicol et al18 reported a positive response in 83% of children with confirmed TB. Of the nine children diagnosed with TB in our study, all had positive whole blood IFN‐γ assays.
The sensitivity of IFN‐γ based assays among groups with a high likelihood of latent TB infection has also been reported.19,29,30 In two school outbreaks the ELISPOT assay was unaffected by BCG vaccination status and showed significantly better correlation with MTB exposure than TST in 11–15 year old students,29 while the whole blood IFN‐γ assay correlated well with TST results in Danish teenagers and was also unaffected by BCG status.30 Recent studies in young infants and children using the ELISPOT assay have suggested higher sensitivity and specificity than the TST.31,32 In the absence of a gold standard test for latent TB, a number of investigators have chosen to report the agreement between TST and IFN‐γ assays. Although variable, most studies in adults suggest an agreement between both whole blood IFN‐γ or ELISPOT assays and TST of 60–80%.28 A prospective comparison of the performance of the whole blood IFN‐γ and ELISPOT assays in children with latent TB infection and disease would be valuable.
The effect of prior BCG vaccination on TST has been the subject of many reviews. A recent meta‐analysis by Wang et al33 suggested that an induration of >15 mm was more likely to be due to TB infection than to prior BCG. In our study BCG status did not appear to be associated with the whole blood IFN‐γ assay result in those with TST diagnosed latent TB infection. Those with positive and negative whole blood IFN‐γ assay results were equally likely to have received BCG in the past.
Our study has highlighted the considerable disagreement between the TST and the whole blood IFN‐γ assay in children at high risk for latent TB infection. Further studies are required to clarify the negative predictive value of this assay, particularly in children with possible latent TB infection.34
Acknowledgements
The authors thank the participating staff and families involved in the study.
Abbreviations
BCG - Bacille Calmette Geurin
ELISPOT - enzyme linked immunospot assay
ESAT‐6 - early secretory antigenic target 6
CFP‐10 - culture filtrate protein 10
IFN‐γ - interferon gamma
MTB - Mycobacterium tuberculosis
TB - tuberculosis
TST - tuberculin skin test
Footnotes
*TGC and NC are joint first authors.
This study received funding from the John Burge Trust, Victoria, Australia. TGC is the recipient of a European Society of Paediatric Infectious Diseases (ESPID)/Wyeth fellowship award.
Competing interests: none.
References
- 1.Dolin P J, Raviglione M C, Kochi A. Global tuberculosis incidence and mortality during 1990–2000. Bull WHO 199472213–220. [PMC free article] [PubMed] [Google Scholar]
- 2.Donald P R. Childhood tuberculosis: out of control? Curr Opin Pulm Med 20028178–182. [DOI] [PubMed] [Google Scholar]
- 3.Tufariello J M, Chan J, Flynn J L. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis 20033578–590. [DOI] [PubMed] [Google Scholar]
- 4.Newport M J, Huxley C M, Huston S.et al A mutation in the interferon‐gamma‐receptor gene and susceptibility to mycobacterial infection. N Engl J Med 19963351941–1949. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Lin Y, Iyer D V.et al T‐cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun 1995633231–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jasmer R M, Nahid P, Hopewell P C. Clinical practice. Latent tuberculosis infection. N Engl J Med 20023471860–1866. [DOI] [PubMed] [Google Scholar]
- 7.Shingadia D, Novelli V. Diagnosis and treatment of tuberculosis in children. Lancet Infect Dis 20033624–632. [DOI] [PubMed] [Google Scholar]
- 8.Lee E, Holzman R S. Evolution and current use of the tuberculin test. Clin Infect Dis 200234365–370. [DOI] [PubMed] [Google Scholar]
- 9.Andersen P, Munk M E, Pollock J M.et al Specific immune‐based diagnosis of tuberculosis. Lancet 20003561099–1104. [DOI] [PubMed] [Google Scholar]
- 10.Carter E R, Lee C M. Interpretation of the tuberculin skin test reaction by pediatric providers. Pediatr Infect Dis J 200221200–203. [DOI] [PubMed] [Google Scholar]
- 11.Mazurek G H, Villarino M E. Guidelines for using the QuantiFERON‐TB test for diagnosing latent Mycobacterium tuberculosis infection. Centers for Disease Control and Prevention. MMWR Recomm Rep 200352(RR‐2)15–18. [PubMed] [Google Scholar]
- 12.Lalvani A. Spotting latent infection: the path to better tuberculosis control. Thorax 200358916–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori T, Sakatani M, Yamagishi F.et al Specific detection of tuberculosis infection: an interferon‐gamma‐based assay using new antigens. Am J Respir Crit Care Med 200417059–64. [DOI] [PubMed] [Google Scholar]
- 14.Scholvinck E, Wilkinson K A, Whelan A O.et al Gamma interferon‐based immunodiagnosis of tuberculosis: comparison between whole‐blood and enzyme‐linked immunospot methods. J Clin Microbiol 200442829–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman A L, Munkanta M, Wilkinson K A.et al Rapid detection of active and latent tuberculosis infection in HIV‐positive individuals by enumeration of Mycobacterium tuberculosis‐specific T cells. AIDS 2002162285–2293. [DOI] [PubMed] [Google Scholar]
- 16.Lalvani A, Pathan A A, McShane H.et al Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen‐specific T cells. Am J Respir Crit Care Med 2001163824–828. [DOI] [PubMed] [Google Scholar]
- 17.Liebeschuetz S, Bamber S, Ewer K.et al Diagnosis of tuberculosis in South African children with a T‐cell‐based assay: a prospective cohort study. Lancet 20043642196–2203. [DOI] [PubMed] [Google Scholar]
- 18.Nicol M P, Pienaar D, Wood K.et al Enzyme‐linked immunospot assay responses to early secretory antigenic target 6, culture filtrate protein 10, and purified protein derivative among children with tuberculosis: implications for diagnosis and monitoring of therapy. Clin Infect Dis 2005401301–1308. [DOI] [PubMed] [Google Scholar]
- 19.Lalvani A, Pathan A A, Durkan H.et al Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen‐specific T cells. Lancet 20013572017–2021. [DOI] [PubMed] [Google Scholar]
- 20.Vekemans J, Lienhardt C, Sillah J S.et al Tuberculosis contacts but not patients have higher gamma interferon responses to ESAT‐6 than do community controls in The Gambia. Infect Immun 2001696554–6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broekmans J F, Migliori G B, Rieder H L.et al European framework for tuberculosis control and elimination in countries with a low incidence. Recommendations of the World Health Organization (WHO), International Union Against Tuberculosis and Lung Disease (IUATLD) and Royal Netherlands Tuberculosis Association (KNCV) Working Group. Eur Respir J 200219765–775. [DOI] [PubMed] [Google Scholar]
- 22.Zuber P L, McKenna M T, Binkin N J.et al Long‐term risk of tuberculosis among foreign‐born persons in the United States. JAMA 1997278304–307. [PubMed] [Google Scholar]
- 23.Pai M, Lewinsohn D M. Interferon‐γ assays for tuberculosis: is anergy the Achilles' heel? Am J Respir Crit Care Med 2005172519–521. [DOI] [PubMed] [Google Scholar]
- 24.Ferrara G, Losi M, Meacci M.et al Routine hospital use of a new commercial whole blood interferon‐γ assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med 2005172631–635. [DOI] [PubMed] [Google Scholar]
- 25.Smart J M, Kemp A S. Ontogeny of T‐helper 1 and T‐helper 2 cytokine production in childhood. Pediatr Allergy Immunol 200112181–187. [DOI] [PubMed] [Google Scholar]
- 26.Frenkel L, Bryson Y J. Ontogeny of phytohemagglutinin‐induced gamma interferon by leukocytes of healthy infants and children: evidence for decreased production in infants younger than 2 months of age. J Pediatr 198711197–100. [DOI] [PubMed] [Google Scholar]
- 27.Miyawaki T, Seki H, Taga K.et al Dissociated production of interleukin‐2 and immune (gamma) interferon by phytohaemagglutinin stimulated lymphocytes in healthy infants. Clin Exp Immunol 198559505–511. [PMC free article] [PubMed] [Google Scholar]
- 28.Pai M, Riley L W, Colford J M., Jr Interferon‐gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 20044761–776. [DOI] [PubMed] [Google Scholar]
- 29.Ewer K, Deeks K, Alvarez L.et al Comparison of T‐cell‐based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet 20033611168–1173. [DOI] [PubMed] [Google Scholar]
- 30.Brock I, Weldingh K, Lillebaek T.et al Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med 200417065–69. [DOI] [PubMed] [Google Scholar]
- 31.Richeldi L, Ewer K, Losi M.et al T cell‐based tracking of multidrug resistant tuberculosis infection after brief exposure. Am J Respir Crit Care Med 2004170288–295. [DOI] [PubMed] [Google Scholar]
- 32.Soysal A, Millington K A, Bakir M.et al Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community‐based study. Lancet 20053661443–1451. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Turner M O, Elwood R K.et al A meta‐analysis of the effect of Bacille Calmette Guerin vaccination on tuberculin skin test measurements. Thorax 200257804–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazurek G H, Jereb J, LoBue P.et al Guidelines for using the QuantiFERON‐TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 200554(RR‐15)49–55. [PubMed] [Google Scholar]