Abstract
Targeted inactivation of genes in the tumor necrosis factor (TNF)/lymphotoxin (LT) ligand and receptor system has recently revealed essential roles for these molecules in lymphoid tissue development and organization. Lymphotoxin-αβ (LTαβ)/lymphotoxin-β receptor (LTβ-R) signaling is critical for the organogenesis of lymph nodes and Peyer’s patches and for the structural compartmentalization of the splenic white pulp into distinct B and T cell areas and marginal zones. Moreover, an essential role has been demonstrated for TNF/p55 tumor necrosis factor receptor (p55TNF-R) signaling in the formation of splenic B lymphocyte follicles, follicular dendritic cell networks, and germinal centers. In contrast to a previously described essential role for the p55TNF-R in Peyer’s patch organogenesis, we show in this report that Peyer’s patches are present in both TNF and p55TNF-R knockout mice, demonstrating that these molecules are not essential for the organogenesis of this lymphoid organ. Furthermore, we show that in the absence of TNF/p55TNF-R signaling, lymphocytes segregate normally into T and B cell areas and a normal content and localization of dendritic cells is observed in both lymph nodes and Peyer’s patches. However, although B cells are found to home normally within Peyer’s patches and in the outer cortex area of lymph nodes, organized follicular structures and follicular dendritic cell networks fail to form. These results show that in contrast to LTαβ signaling, TNF signaling through the p55TNF-R is not essential for lymphoid organogenesis but rather for interactions that determine the cellular and structural organization of B cell follicles in all secondary lymphoid tissues.
In addition to their well-characterized proinflammatory activities (1, 2), tumor necrosis factor (TNF) and lymphotoxin-α (LTα) have also emerged as key mediators of immune responses, by delivering critical signals for cellular trafficking, differentiation, proliferation, and death. Both cytokines exist in soluble homotrimeric forms, which signal through two distinct cell surface receptors designated the p55 and p75 TNF receptors (TNF-R) (3). In addition to its soluble form, TNF exists also as a transmembrane protein that cooperates with the same receptors to transduce biological signals (4). LTα may also accumulate on the cellular membrane when complexed in heterotrimeric forms with lymphotoxin-β (LTβ), another TNF-like type II transmembrane protein (5). LTα2β1 trimers may still engage the TNF receptors, while LTα1β2 trimers signal exclusively through the lymphotoxin-β receptor (LTβ-R) (6).
Gene targeting in the LT/TNF and TNF-R loci has recently demonstrated critical roles for either cytokine in the development and organization of peripheral lymphoid tissue. LTα knockout mice lack lymph nodes and Peyer’s patches and show a severely disturbed splenic white pulp architecture with the absence of distinct B and T cell areas, germinal centers, and follicular dendritic cell (FDC) networks (7, 8). Exposure of mice to a soluble LTβ-R-immunoglobulin fusion protein led to the disruption of lymph node and Peyer’s patch development, indicating that the organogenetic activities of LTα are mediated by heteromeric lymphotoxin-αβ (LTαβ) complexes (9), most probably by signaling through the LTβ-R. Further support for a critical role of surface LTαβ ligand in normal lymphoid organ development was provided by the demonstration of anatomic abnormalities in spleens and Peyer’s patches of transgenic mice expressing at a postnatal stage a soluble LTβ-R-Fc fusion protein (10). In addition to these activities, it has recently been postulated that LTα could selectively engage the p55 tumor necrosis factor receptor (p55TNF-R) to signal organogenesis of Peyer’s patches, since no such tissue could be detected in p55TNF-R knockout mice (11). Notably, exposure of mice to a soluble p55TNF-R-Fc fusion protein did not interfere with Peyer’s patch organogenesis, although it could disrupt splenic MAdCAM-1 expression (9). Furthermore, in TNF knockout mice lymphoid organogenesis remains intact (12), yet, as in p55TNF-R knockout mice (13), follicular architecture of the spleen is impaired, suggesting that TNF-mediated p55TNF-R signaling does not mediate lymphoid organogenesis, but rather that this specific ligand-receptor pair is crucially involved in the structural organization of lymphoid tissue.
It has been previously demonstrated that correct formation of splenic B cell follicles (12, 13) and FDC networks (12–14) requires TNF-mediated p55TNF-R signaling. To assess whether the requirement of this ligand-receptor interaction is specific for lymphoid structures in the spleen or whether it extends to additional secondary lymphoid organs, we have examined Peyer’s patch and lymph node structure in TNF and p55TNF-R knockout mice. Surprisingly, in contrast to a previous report where Peyer’s patches could not be identified in p55TNF-R knockout mice (11), we show here that in both TNF and p55TNF-R knockout mice, Peyer’s patches containing distinct B and T cell areas are present, albeit at reduced numbers and with a flatter appearance in comparison to normal controls. In this study, we also show that in lymph nodes and Peyer’s patches of either TNF or p55TNF-R knockout mice, B cell follicles and FDC networks fail to form, yet no defect in the development and localization of dendritic cells (DC) and B/T cells is observed. Taken together, our data show that the structural lymphoid abnormalities observed in TNF and p55TNF-R knockout mice are not associated with defects in lymphoid organogenesis, but rather that this ligand/receptor pair is required for the correct formation of follicular structures in all secondary lymphoid organs.
MATERIALS AND METHODS
Mice.
Wild-type, TNFα knockout (12), and p55TNF-R knockout mice (15) were maintained on a mixed 129Sv × C57BL/6 genetic background in the animal facilities of the Hellenic Pasteur Institute under specific pathogen-free conditions.
Antibodies.
Primary antibodies used for immunohistology included a peroxidase-conjugated goat-polyclonal antibody to murine IgM (Sigma), rat anti-mouse monoclonal antibodies: anti-CD45R/B220 (clone RA3–6B2, PharMingen), anti-IgD (Southern Biotechnology Associates), anti-FDC [clones FDC-M1; ref. 16 and FDC-M2; provided by Marie Kosco-Vilbois (Geneva Biomedical Research Institute)], anti-CD3 [clone KT3; ref. 17, provided by Stephen Cobbold (Sir William Dunn School of Pathology, Oxford)], and anti-DC [NLDC-145; ref. 18, provided by Georg Kraal (Department of Cell Biology and Immunology, Vrije Universiteit, Amsterdam)]. The hamster anti-mouse CD11c mAb N418 (19) was provided by Ralph Steinman (Laboratory of Cellular Physiology and Immunology, The Rockefeller University, New York). Secondary antibodies used were peroxidase-conjugated goat anti-rat IgG (Southern Biotechnology Associates) and biotinylated goat anti-hamster IgG (Vector Laboratories).
Immunohistochemistry and Fluorescence-Activated Cell Sorter (FACS) Analysis.
Freshly dissected tissue samples were embedded in OCT compound (BDH) and were frozen in liquid nitrogen. Cryostat sections were cut at 6 μm, thaw-mounted on gelatinized slides, air dried, and stored desiccated at −20°C. Immediately before use sections were fixed for 10 min in acetone containing 0.03% H2O2 to block endogenous peroxidase activity. Sections were rehydrated in PBS, blocked with 3% goat serum and 2% mouse serum in PBS containing 0.1% BSA for 30 min, and incubated with the primary antibodies diluted in PBS/0.1% BSA for 1 hr at room temperature. Subsequently, sections were washed in PBS and incubated with either peroxidase-conjugated goat anti-rat IgG or with biotinylated goat anti-hamster IgG followed by streptavidin–peroxidase. Bound peroxidase activity was visualized by incubating sections for 10 min in 0.05% (wt/vol) 3,3-diaminobenzidine (Sigma) in 0.05 M Tris⋅HCl (pH 7.5) containing 0.03% H2O2. Sections were counterstained with hematoxylin or methyl green. Photographs were taken using a Nikon Diaphot 300 microscope. FACS analysis was performed by standard procedures on a FACStar plus (Becton Dickinson) using fluorescein isothiocyanate- or phycoerythrin-conjugated antibodies against CD3, CD4, CD8 (Sigma), or CD45R/B220 (PharMingen).
RESULTS AND DISCUSSION
The cellular content and the structural organization of peripheral lymphoid organs was investigated in mice deficient for either TNF or the p55TNF-R. FACS analysis of CD4+ and CD8+ T cell subsets in thymus, spleen, and lymph nodes from TNF knockout mice showed no significant changes in comparison to wild-type controls (not shown). Similar results have been reported for mice carrying a targeted inactivation of the p55TNF-R gene (15, 20). Moreover, a normal content of B220+ B cells was observed in spleens and lymph nodes of TNF knockout mice. Thus, expression of TNF and the p55TNF-R should not be essential for the development of B and T lymphocytes. In addition, normal lymphocyte counts could be measured in peripheral blood of TNF knockout mice as assessed by flow cytometry using antibodies to CD3, CD4, CD8, and B220 (not shown) indicating that lymphocyte homing to peripheral lymphoid organs is not affected.
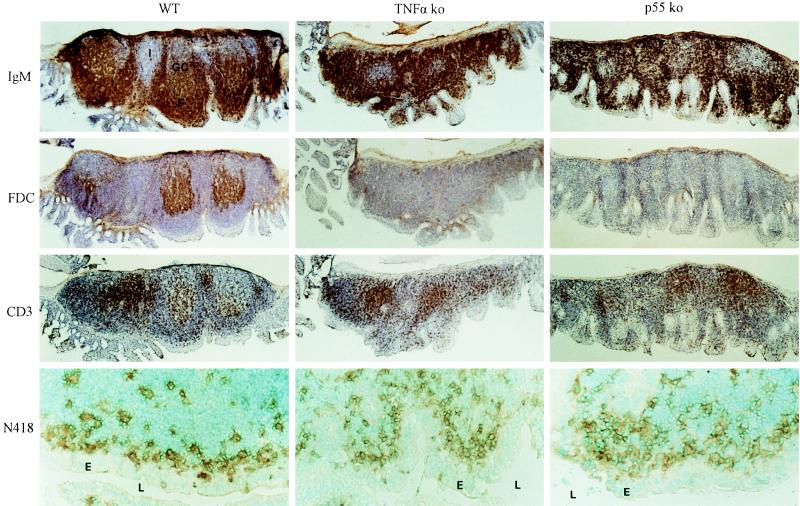
We have previously reported that Peyer’s patch organogenesis is not disrupted in TNF knockout mice (12). Macroscopic and low power microscopic examination of the intestine of p55TNF-R knockout mice also revealed the presence of Peyer’s patches. In either TNF or p55TNF-R knockout mice Peyer’s patches are usually small and flat in appearance and their number is reduced to an average of 2–4 per mouse, compared with an average of 6–8 patches found in wild-type controls (n = 8–12 mice for each group). To assess the structural integrity of Peyer’s patches forming in TNF and p55TNF-R knockout mice we have performed immunocytochemical analysis using antibodies specific for B cells, T cells, DC, and FDC. Staining with anti-IgM (Fig. 1), anti-CD45R/B220, and anti-IgD antibodies (not shown) revealed a complete lack of organized B lymphocyte follicles in both mutant mouse strains, yet large numbers of B cells were found to home within the patch area suggesting that B cell homing remains unaffected. Furthermore, a complete absence of organized FDC networks could be demonstrated by staining with the FDC-specific mAbs FDC-M2 (Fig. 1) and FDC-M1 (not shown). CD3+ T cell rich areas clearly segregate from B cell areas (Fig. 1) and contain DC that stain with the N418 and the NLDC-145 mAbs (not shown). In addition, similar to wild-type mice, N418+ DC (Fig. 1), which do not stain with NLDC-145 (not shown) and CD4+ T cells (not shown), were found in the subepithelial regions of the Peyer’s patches from TNF and p55TNF-R knockout mice. These results demonstrate that although TNF and the p55TNF-R are not essential for the organogenesis of Peyer’s patches, they play a dominant role in the formation of structured B cell follicles and FDC networks in this tissue. Our findings are in contrast to a previous report on p55TNF-R knockout mice (11) in which only a single patch of lymphoid tissue could be identified in the intestine of 6 of 18 animals examined, and the absence of organized follicles, germinal centers, and dome areas from this tissue was interpreted as defective Peyer’s patch organogenesis, suggesting a critical role for the p55TNF-R in this phenomenon. Peyer’s patches in mice develop postnatally and are known to reach their fully developed size and appearance only after stimulation by gut antigens that initiate a chronic germinal center reaction (21, 22) within a structurally dominating follicular B cell compartment. This and previous reports on TNF/TNF-R knockout mice (12–14) have clearly demonstrated an essential role for TNF and the p55TNF-R in the formation of B cell follicles and germinal centers. The apparent anatomic abnormalities of Peyer’s patches in TNF and p55TNF-R knockout mice may therefore be explained by the inability of these Peyer’s patches to develop structured B cell follicles and germinal centers and to undergo an expansion in their B cell compartment. Moreover, subepithelial domes of Peyer’s patches are characterized by the presence of a network of N418+/NLDC-145− DC, which are thought to be important for the uptake, processing, and presentation of luminal antigens to adjacently positioned CD4+ T cells (23). Typical subepithelial dome areas are not clearly forming in Peyer’s patches from TNF and p55TNF-R-deficient mice, again most probably due to the impaired follicular architecture. However, the presence of N418+ DC (Fig. 1) and CD4+ T cells (not shown) in the subepithelial area of these Peyer’s patches suggests that its functional role is maintained.
Figure 1.
Defective follicular organization in Peyer’s patches of TNF and p55TNF-R knockout mice. Immunocytochemical analysis was performed on cryostat sections of Peyer’s patches from TNF and p55TNF-R knockout and wild-type control mice. Bound antibodies were visualized with diaminobenzidine and sections were counterstained with hematoxylin or methyl green. Peyer’s patches from wild-type mice stained with an antibody to IgM are found to contain organized B cell follicles (F) with visible germinal centers (GC). Large numbers of B cells are present in Peyer’s patches from TNF or p55TNF-R-deficient mice but they fail to form organized follicular structures and no germinal centers can be observed. Staining with the FDC-M2 mAb reveals the presence of FDC networks within follicles in Peyer’s patches from wild-type but not TNF or p55TNF-R-deficient mice. T cells stained here with an anti-CD3 mAb are found to form distinct T cell-rich areas. DC stained with the N418 anti-CD11c mAb are observed in the subepithelial region of Peyer’s patches from both TNF and p55TNF-R knockout mice. F, B cell follicles; GC, germinal centers; I, interfollicular regions; E, epithelium; L, lumen. (×25; ×125 for N418 staining).
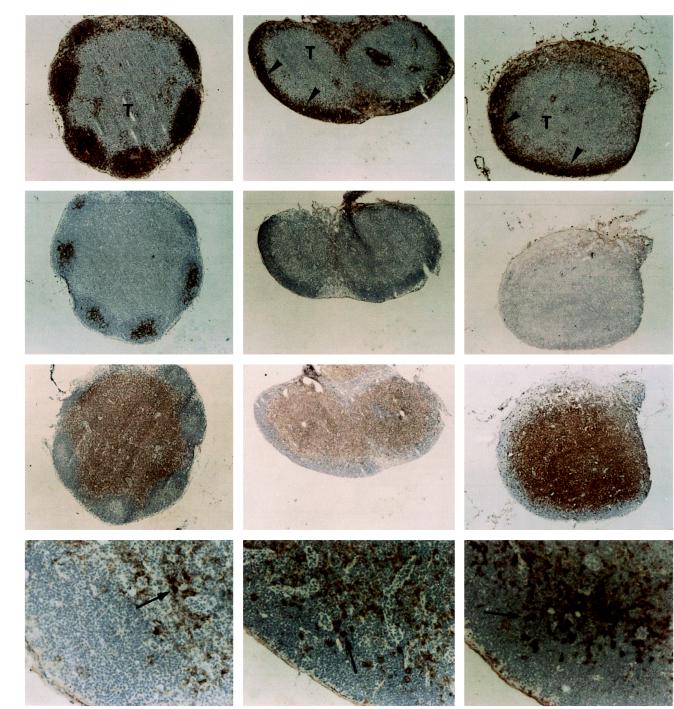
Deficient B lymphocyte follicle and FDC network formation could also be demonstrated in mesenteric (not shown) and peripheral lymph nodes (Fig. 2) of either TNF or p55TNF-R knockout mice. However, B cells could readily be observed to home in the outer cortex area of lymph nodes (Fig. 2), indicating that the deficiency in B cell follicle formation is not associated with B cell homing defects. Furthermore, the absence of TNF or the p55TNF-R did not interfere with normal segregation of B and T cell areas in the lymph nodes, as documented by staining with anti-CD3 antibodies (Fig. 2). In addition, normal patterns of DC could be observed in T cell areas, as demonstrated by staining with N418 (Fig. 2) and NLDC-145 antibodies (not shown).
Figure 2.
Normal B cell homing, yet impaired formation of organized follicles in lymph nodes of mice lacking either TNF or the p55TNF-R. Cryostat sections of inguinal lymph nodes from wild-type, TNF knockout, and p55TNF-R knockout mice were analyzed by immunoperoxidase staining, developed with diaminobenzidine, and counterstained with hematoxylin. Staining with antibody to IgM shows a normal localization of B cells in the outer cortex of lymph nodes from TNF or p55TNF-R knockout mice (arrowheads); however, in contrast to wild-type mice, they are not organized into follicles but form a thin layer covering the cortex area. A similar distribution of B cells was observed by staining with anti-B220 or anti-IgD antibodies (not shown). FDC networks identified here with the FDC-M2 mAb are observed to form within follicles in wild-type but not in TNF- or p55-deficient lymph nodes. Additional staining with FDC-M1 failed to detect any FDC networks in lymph nodes from TNF or p55 knockout mice (not shown). In contrast, T cells visualized here with anti-CD3 mAb and N418+ DC (arrows) appear to develop normally in lymph nodes from these mice. Similar results were observed in mesenteric lymph nodes. F, B cell follicles; T, T cell-rich areas. (×20; ×100 for N418 staining).
Absence of organized FDC networks in severe combined immunodeficient (24) or B cell-depleted mice (25) is thought to be a consequence of the absence of B cells in these systems. Similarly, the observed impairment of FDC network formation in lymphoid organs of either TNF or p55TNF-R knockout mice could be attributed to deficient B cell migration and homing. However, in this study it is shown that although B cells localize normally in Peyer’s patches and in the cortex of lymph nodes of either TNF or p55TNF-R knockout mice, B cell follicles and FDC networks fail to form, suggesting that the lack of follicular organization is not due to the absence of follicular B cells. It is therefore suggested that the primary defect explaining the lack of follicular organization in all secondary lymphoid organs from TNF and p55TNF-R-deficient mice, lies in a defective migration and/or differentiation of FDC. Further experiments should address the specific TNF/p55TNF-R-mediated cellular interactions that allow for the physiological structural organization of B cell follicles in lymphoid organs.
The lymphoid organogenetic and T/B cell organizational activities of LTα were recently shown to be mediated by surface LTαβ complexes (9, 10), which are known to signal through the LTβ-R (6, 26). With the available experimental evidence and the findings reported in this study, the activities of the LTαβ/LTβ-R seem to segregate from those of the TNF/p55TNF-R, in that the latter are not necessary for lymph node or Peyer’s patch organogenesis nor are they required for the compartmentalization of T and B cell areas in lymphoid organs. The localization of the LTβ-R on stromal cells in the red pulp of human fetal spleen (27) and of LTαβ complexes on lymphocytes (26), suggests that stromal–lymphocyte interactions mediated by the LTαβ/LTβ-R pair may be pivotal in the initiation of morphogenetic events and architectural positioning of T and B cells in lymphoid organs. Once this level of lymphoid development and organization is reached, TNF/LTα signaling through the p55TNF-R should then be essential for the formation of primary B cell follicles, FDC networks, and germinal centers.
In conclusion, the reduced number and the apparent structural abnormalities of the Peyer’s patches in TNF and p55TNF-R knockout mice do not reflect a specific organogenetic role for this ligand/receptor pair, but they are rather a consequence of the B cell follicle morphogenetic role played by these molecules in all secondary lymphoid organs. In addition, the data presented in this report indicate that these lymphoid deficiencies may be associated with the observed impaired formation of FDC networks and not with B cell homing and trafficking defects.
Acknowledgments
We would like to thank the many friends and colleagues who generously provided antibodies for immunocytochemistry (see Materials and Methods) and Reina Mebius and Brigitte Neumann for discussions. This work was supported in part by the Hellenic Secretariat for Research and Technology and European Union Grants BIO-CT94-2092, BIO-CT96-0174, and BIO-CT96-0077.
ABBREVIATIONS
- TNF
tumor necrosis factor
- TNF-R
TNF receptor
- DC
dendritic cells
- FDC
follicular dendritic cells
- LTα
lymphotoxin-α
- LTαβ
lymphotoxin-αβ
- LTβ
lymphotoxin-β
- LTβ-R
lymphotoxin-β receptor
- p55TNF-R
p55 tumor necrosis factor receptor
- FACS
fluorescence-activated cell sorter
References
- 1.Vassalli P. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 2.Paul N L, Ruddle N H. Annu Rev Immunol. 1988;6:407–438. doi: 10.1146/annurev.iy.06.040188.002203. [DOI] [PubMed] [Google Scholar]
- 3.Vandenabeele P, Declercq W, Beyaert R, Fiers W. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 4.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 5.Browning J L, Ngam ek A, Lawton P, DeMarinis J, Tizard R, Chow E P, Hession C, O’Brine-Greco B, Foley S F, Ware C F. Cell. 1993;72:847–856. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- 6.Crowe P D, VanArsdale T L, Walter B N, Ware C F, Hession C, Ehrenfels B, Browning J L, Din W S, Goodwin R G, Smith C A. Science. 1994;264:707–710. [PubMed] [Google Scholar]
- 7.De Togni P, Goellner J, Ruddle N H, Streeter P R, Fick A, Mariathasan S, Smith S C, Carlson R, Shornick L P, Strauss-Schoenberger J, Russell J H, Karr R, Chaplin D D. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto M, Mariathasan S, Nahm M H, Baranyay F, Peschon J J, Chaplin D D. Science. 1996;271:1289–1291. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 9.Rennert P D, Browning J L, Mebius R, Mackay F, Hochman P. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ettinger R, Browning J L, Michie S A, van Ewijk W, McDevit H O. Proc Natl Acad Sci USA. 1996;93:13102–13107. doi: 10.1073/pnas.93.23.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann B, Luz A, Pfeffer K, Holtzmann B. J Exp Med. 1996;184:259–264. doi: 10.1084/jem.184.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. J Exp Med. 1996;184:1397–1412. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasparakis M, Alexopoulou L, Douni E, Kollias G. Cytokine Growth Factor Rev. 1996;7:223–229. doi: 10.1016/s1359-6101(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 14.Le Hir M, Bluethmann H, Kosco-Vilbois M H, Muller M, di Padova F, Moore M, Ryffel B, Eugster H P. J Exp Med. 1996;183:2367–2372. doi: 10.1084/jem.183.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Nature (London) 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 16.Kosco M H, Pflugfelder E, Gray D. J Immunol. 1992;148:2331–2339. [PubMed] [Google Scholar]
- 17.Tomonari K. Immunogenetics. 1988;28:455–458. doi: 10.1007/BF00355379. [DOI] [PubMed] [Google Scholar]
- 18.Kraal G, Breel M, Janse M, Bruin G. J Exp Med. 1986;163:981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metlay J P, Witmer-Pack M D, Agger R, Crowley M T, Lawless D, Steinman R M. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeffer K, Matsuyama T, Kundig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Kronke M, Mak T W. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 21.Griebel P J, Hein W R. Immunol Today. 1996;17:30–39. doi: 10.1016/0167-5699(96)80566-4. [DOI] [PubMed] [Google Scholar]
- 22.Pollard M, Sharon N. Infect Immun. 1970;2:96–100. doi: 10.1128/iai.2.1.96-100.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelsall B L, Strober W. J Exp Med. 1996;183:237–247. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapasi Z F, Burton G F, Shultz L D, Tew J G, Szakal A K. J Immunol. 1993;150:2648–2658. [PubMed] [Google Scholar]
- 25.Cerny A, Zinkernagel R M, Groscurth P. Cell Tissue Res. 1988;254:449–454. doi: 10.1007/BF00225818. [DOI] [PubMed] [Google Scholar]
- 26.Ware C F, VanArsdale T L, Crowe P D, Browning J L. Curr Top Microbiol Immunol. 1995;198:175–218. doi: 10.1007/978-3-642-79414-8_11. [DOI] [PubMed] [Google Scholar]
- 27.Murphy, M., Pike-Nobile, L., Browning, J. L., Ware, C. F. & Epstein, L. B. (1995) Proc. Ninth Int. Cong. Immunol., 770 (abstr.).