Abstract
Dendritic cells efficiently internalize exogenous protein antigens by fluid-phase uptake and receptor-mediated endocytosis. Such antigens contribute to cross-presentation by being translocated into the cytosol for proteasomal degradation, which liberates immunogenic peptides that can bind to major histocompatibility complex (MHC) class I molecules after being transported into the endoplasmic reticulum (ER). MHC class I–peptide complexes are then expressed on the cell surface and presented to CD8+ T cells. Here we show that internalized proteins can have an alternative fate. After internalization, proteins are first unfolded to allow translocation into the cytosol using a pathway related to ER-associated degradation (ERAD). Subsequently the unfolded proteins can undergo cytosolic refolding assisted by the chaperone Hsp90. These observations not only clarify the cellular processes regulating cytosolic access following endocytosis, but also demonstrate that functional proteins can potentially regain their activity in the cytosol of dendritic cells.
Keywords: cross-presentation, dendritic cells, ERAD, refolding, retrotranslocation
Introduction
Major histocompatibility complex (MHC) class I molecules were initially believed to present peptides derived solely from endogenous proteins, that is, proteins synthesized by the cell itself, including viral proteins produced upon infection. Subsequently, it was discovered that exogenous antigens could be presented by MHC class I molecules and stimulate CD8+ T cells. This process is called cross-presentation and is critical for the initiation of T-cell immunity to viral infections (Rock and Shen, 2005). Dendritic cells (DCs) are the major cell types that mediate cross-presentation, and they can generate functional MHC class I–peptide complexes from exogenous proteins internalized via various endocytic mechanisms such as phagocytosis, macropinocytosis, or receptor-mediated endocytosis. They can cross-present exogenous antigens in the form of soluble proteins, immune complexes, or apoptotic bodies derived from virally infected cells (Rodriguez et al, 1999; Mellman and Steinman, 2001).
We previously showed that efficient internalization of exogenous antigens by DCs is followed by their translocation into the cytosol via an endoplasmic reticulum (ER)-associated degradation (ERAD)-related mechanism (Ackerman et al, 2006; Rock, 2006). In the ERAD pathway, misfolded or unassembled proteins in the ER are targeted for degradation through the cytoplasmic ubiquitin–proteasome system (Meusser et al, 2005). Misfolded substrates are first selected and recognized by lumenal chaperones (e.g., BiP, GRP94) and then targeted for dislocation into the cytosol (retrotranslocation; Matlack et al, 1999; Nishikawa et al, 2005). Retrotranslocation probably uses the Sec61 channel or a related channel involving Derlin-1, and it is coordinated by the Cdc48p–Npl4p–Ufd1p complex, which constitutes a transport cascade for substrate delivery from the site of ubiquitination to the proteasome (Ye et al, 2001, 2003; Lilley and Ploegh, 2004; Wahlman et al, 2007). Whether derived from ER proteins or from exogenous proteins internalized by DCs, peptides generated by the proteasome may then be translocated into the ER by the transporter associated with antigen processing (TAP) and loaded onto MHC class I molecules using the classical TAP-dependent MHC class I assembly pathway (Cresswell et al, 2005). MHC class I–peptide complexes are then transported via the Golgi apparatus to the cell surface and surveyed by CD8+ T cells.
Here we show that in DCs, in addition to dislocation followed by immunogenic peptide generation, exogenous proteins can use the same mechanism for cytosolic access and be refolded in the cytosol rather than being degraded. At least partial unfolding appears to precede retrotranslocation. It has previously been proposed that cytosolic chaperones such as Hsp90 cooperate with the ERAD pathway to determine the fate of misfolded proteins (Wang et al, 2006), and here we show a similar requirement for the functional refolding of exogenous proteins in the cytosol of DCs.
Results
Exogenous proteins access the cytosol of the DC-like cell line KG-1 in a folded and functional form
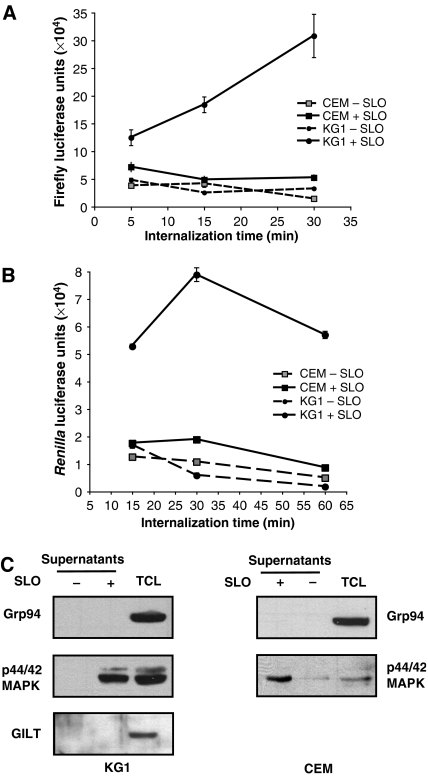
To investigate the kinetics of internalization of exogenous soluble proteins and their entry into the cytosol, we designed a new set of assays using soluble recombinant Firefly and Renilla reniformis luciferases as substrates. From previous studies we know that soluble luciferases are efficiently internalized by fluid-phase uptake by the DC-like cell line KG-1 (Ackerman et al, 2006). To measure exogenous protein delivery to the cytosol, KG-1 cells that had internalized soluble luciferases were permeabilized with streptolysin-O (SLO). SLO permeabilization allowed the measurement of cytosolic luciferase activity in the supernatants of the cells following centrifugation. We established that in KG-1 cells luciferase activity was present in the cytosol after 5 min of internalization. No luciferase activity could be detected in the cytosol of CEM cells, a human T lymphoblastoid cell line with poor macropinocytotic capacity (Figure 1A and B). To ensure that the SLO permeabilization procedure was specifically releasing cytosolic components without ER or lysosomal leakage, supernatants from untreated or SLO-treated cells were subjected to SDS–PAGE and immunoblotted for p44/42 MAP kinase (MAPK) as a cytosolic marker, GRP94 as an ER marker, and gamma-interferon-inducible lysosomal thiolreductase (GILT; Arunachalam et al, 2000) as a lysosomal marker. Figure 1C clearly shows that supernatants from SLO-permeabilized cells contained the cytosolic marker p42/44 MAPK, whereas GRP94 and GILT were undetectable. We also showed that endosomal integrity was maintained upon SLO permeabilization: a 10-kDa fluorescein-conjugated Dextran internalized by KG-1 cells was undetectable in supernatants from SLO-treated cells, but was readily detectable in the residual cell pellet following permeabilization (Supplementary Figure 1).
Figure 1.
Exogenous luciferase enters the cytosol of KG-1 cells following internalization. (A, B) Firefly luciferase (A) or R. reniformis luciferase (B) were added to KG-1 cells (•) or CEM cells (▓ and ▪) for the indicated times. Cells were then permeabilized with SLO in the presence of DTT (+ SLO, solid lines) or resuspended in DPBS with DTT (− SLO, dashed lines) as controls. After centrifugation, supernatants from SLO-permeabilized cells or control cells were analyzed for luciferase activity with a luminometer. All graphs show the mean value (±s.d.) of triplicate samples and they are representative of at least three independent experiments. (C) To ensure specific cytosolic isolation from SLO-permeabilized cells, supernatants from KG-1 cells (left panel) or CEM cells (right panel) treated with or without SLO were analyzed by western blotting for the ER lumenal marker GRP94, the cytosolic marker p44/42 MAPK, and the lysosomal marker GILT.
Cytosolic access is inhibited by cytochalasin D pretreatment
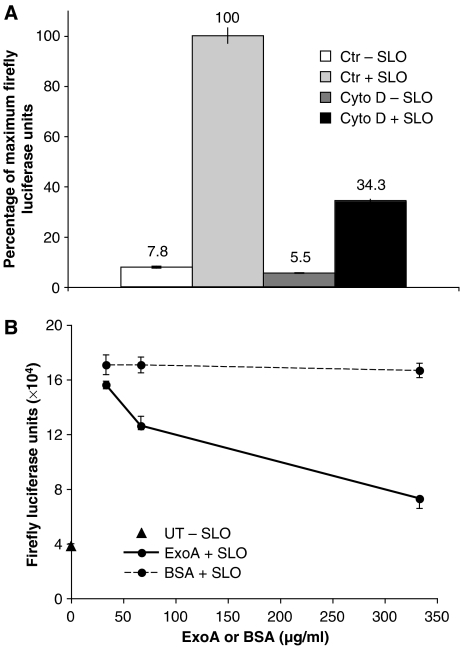
We wanted to ensure that the luciferase activity present in the cytosol of KG-1 cells was dependent on active internalization. Macropinocytosis, the predominant mechanism of fluid-phase uptake by DCs, is an actin-dependent process (Sallusto et al, 1995; Amyere et al, 2002). KG-1 cells were therefore pretreated with the actin polymerization inhibitor cytochalasin D for 30 min before addition of luciferase for an additional 30 min. Exposure to cytochalasin D strongly reduced the luciferase activity liberated into the supernatants of SLO-permeabilized cells (Figure 2A, black bar) compared with control cells treated with vehicle (DMSO) alone (Figure 2A, light gray bar). No significant luciferase activity was detected in the supernatants of non-permeabilized cells (Figure 2A, white and dark gray bars). Cytochalasin D treatment also decreased significantly the total amount of Firefly luciferase internalized by KG-1 cells, as assessed by enzymatic activity measured after detergent lysis (Supplementary Figure 2). These data indicate that internalization of exogenous luciferase is actin-dependent. We interpret this to suggest that luciferase is internalized by macropinocytosis followed by its translocation into the cytosol of KG-1 cells.
Figure 2.
Cytosolic access is inhibited by cytochalasin D and by Pseudomonas ExoA. (A) KG-1 cells, pretreated for 30 min with cytochalasin D (10 μg/ml), added in DMSO (Cyto D, ▒ and ▪) or with DMSO added as control (Ctr,  and ▓), were incubated with Firefly luciferase for 30 min. Cells were then permeabilized with SLO in the presence of DTT (+ SLO, ▓ and ▪) or resuspended in DPBS with DTT (− SLO,
and ▓), were incubated with Firefly luciferase for 30 min. Cells were then permeabilized with SLO in the presence of DTT (+ SLO, ▓ and ▪) or resuspended in DPBS with DTT (− SLO,  and ▒) as a control. After centrifugation, supernatants were analyzed for luciferase activity. The values are presented as percentages of those obtained with DMSO alone. (B) ExoA (solid lines) or BSA (dashed lines) was added at the indicated concentrations together with Firefly luciferase for 15 min at 37°C to allow co-internalization. Cells were then permeabilized with SLO in the presence of DTT (+ SLO, •) or resuspended in DPBS with DTT (− SLO, ▴). After centrifugation, supernatants from SLO-permeabilized cells or control cells were analyzed for luciferase activity. All graphs show the mean value (±s.d.) of triplicate samples and the results are representative of at least three independent experiments.
and ▒) as a control. After centrifugation, supernatants were analyzed for luciferase activity. The values are presented as percentages of those obtained with DMSO alone. (B) ExoA (solid lines) or BSA (dashed lines) was added at the indicated concentrations together with Firefly luciferase for 15 min at 37°C to allow co-internalization. Cells were then permeabilized with SLO in the presence of DTT (+ SLO, •) or resuspended in DPBS with DTT (− SLO, ▴). After centrifugation, supernatants from SLO-permeabilized cells or control cells were analyzed for luciferase activity. All graphs show the mean value (±s.d.) of triplicate samples and the results are representative of at least three independent experiments.
Access of luciferase to the cytosol is inhibited by Exotoxin A
To investigate the mechanism by which exogenous luciferase enters the cytosol of KG-1 cells, we used Exotoxin A (ExoA), a bacterial toxin derived from Pseudomonas aeruginosa. ExoA reversibly inhibits retrotranslocation from the ER into the cytosol, a process putatively mediated by the Sec61 channel (Koopmann et al, 2000). We have recently shown that ExoA is efficiently internalized by KG-1 cells expressing the mouse MHC class I allele H2-Kb, and that it can block cross-presentation of the soluble antigen ovalbumin (OVA) to a Kb-restricted T-cell hybridoma (Ackerman et al, 2006). This finding was used in part to argue that ERAD-like mechanisms govern cross-presentation. To determine if luciferase accesses the cytosol using the same ExoA-inhibitable pathway, we mixed soluble ExoA, or BSA as a control, at increasing concentrations with a fixed concentration of Firefly luciferase and added them to KG-1 cells. The addition of ExoA significantly reduced the luciferase activity detectable in supernatants of SLO-permeabilized cells (Figure 2B, solid lines) compared with the BSA control (Figure 2B, dashed lines). No significant luciferase activity was detected in the supernatant of non-permeabilized cells (Figure 2B, black triangle). The effect was clearly dose-dependent, with reduced luciferase activity present at higher concentrations of ExoA. ExoA did not affect luciferase uptake or the capacity of the cytosol to mediate refolding (Supplementary Figure 3). These data strongly suggest that luciferase is dislocated into the cytosol by the same mechanism used by exogenous antigens during cross-presentation by DCs.
Firefly and Renilla luciferases can refold in vitro
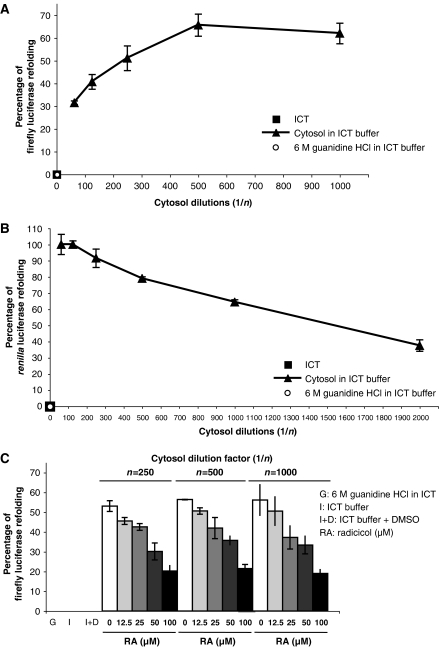
The assay we have developed is based on the ability to detect luciferase activity in cytosolic fractions derived from KG-1 cells. This implies that both Firefly and Renilla luciferases can access the cytosol in a folded and functionally active form. To investigate whether the luciferase activity detected in supernatants of SLO-permeabilized cells might depend on cytosolic refolding, we performed preliminary in vitro refolding experiments. Firefly or Renilla luciferases were chemically unfolded in 6 M guanidine–HCl in intracellular transport (ICT) buffer for 30 min at room temperature. This procedure completely abrogated the activities of both enzymes (Figure 3A and B, white circles). To determine if cytosolic components could mediate refolding, unfolded luciferases were added to serial dilutions of cytosol isolated from KG-1 cells that was dialyzed into ICT buffer. Both Firefly and Renilla luciferases were efficiently refolded in vitro after 30 min incubation at room temperature in the presence of KG-1 cytosol (Figure 3A and B, black triangles). No significant refolding was observed in the presence of ICT buffer alone (Figure 3A and B, black squares). Cytosol similarly isolated from CEM cells was also capable of refolding luciferases in vitro, indicating that no specific property of KG-1 cytosol was responsible (data not shown). Extending refolding reactions up to 1 h and adding protease inhibitors or lactacystin to the refolding reactions did not increase refolding efficiency significantly (data not shown). Taken together, these data suggest that after being internalized and retrotranslocated into the cytosol, Firefly and Renilla luciferases could potentially undergo refolding in the DC-like cell line KG-1.
Figure 3.
KG-1 cytosol-mediated refolding in vitro is inhibited by radicicol. (A, B) Firefly luciferase (A) or R. reniformis luciferase (B) were chemically unfolded in 6 M guanidine–HCl in ICT buffer (•). Refolding reactions were performed in ICT buffer alone (▪) or in serial dilutions of KG-1 cytosol in ICT buffer (▴). (C) Chemically unfolded Firefly luciferase was refolded in ICT buffer alone or in serial dilutions of KG-1 cytosol in ICT buffer with increasing radicicol concentrations (gray bars) or with DMSO as control (white bars). For all in vitro refolding experiments, refolding efficiency was expressed as percentage of luciferase input in the refolding reaction. All graphs show the mean value (±s.d.) of triplicate samples and they are representative of at least three independent experiments.
Hsp90 in the cytosol seemed a likely candidate for mediating the in vitro luciferase refolding. We therefore examined the effect of the potent Hsp90 inhibitor radicicol (Schulte et al, 1998; Sharma et al, 1998) on the refolding reaction. After chemical unfolding, we observed a dose-dependent inhibition of Firefly luciferase refolding by radicicol in the presence of KG-1 cytosol (Figure 3C, gray bars). No inhibition occurred in cytosol containing DMSO as control (Figure 3C, white bars), and no significant refolding was observed in 6 M guanidine–HCl or in ICT buffer alone (Figure 3C, first three columns). These data suggest that in vitro luciferase refolding is mediated by the cytosolic chaperone Hsp90.
Exogenous Firefly and Renilla luciferases are refolded in the cytosol by Hsp90
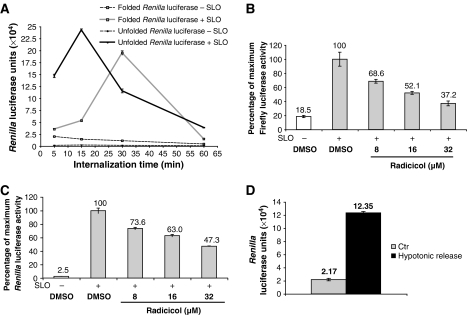
We wished to determine if the recovery of active luciferase from the cytosol of KG-1 cells depended upon refolding. For this purpose, Renilla luciferase was denatured in 6 M guanidine–HCl in ICT buffer and dialyzed overnight against PBS. After dialysis against PBS, chemically unfolded Renilla luciferase, but not Firefly luciferase, remained soluble but enzymatically inactive (Supplementary Figure 4). However, when the inactive enzyme was added to KG-1 cells, we could detect significant luciferase activity in the cytosol released by SLO permeabilization (Figure 4A, solid black lines). The activity recovered after addition of 20 μg of unfolded Renilla luciferase was comparable to that recovered after adding 0.2 μg of native enzyme to the same number of KG-1 cells (Figure 4A, solid gray lines). No significant luciferase activity was detected in the supernatants from non-permeabilized cells incubated with either folded enzyme (Figure 4A, gray square symbols with dashed lines) or unfolded enzyme (Figure 4A, black circles with dashed lines), and no spontaneous refolding was observed in the cell culture medium (data not shown). These data strongly favor the hypothesis that cytosolic refolding occurs after internalization by KG-1 cells.
Figure 4.
Refolding is required for the detection of cytosolic luciferase activity. (A) Native (▓) or chemically unfolded (•) R. reniformis luciferase was added to KG-1 cells for the indicated times. Cells were then permeabilized with SLO in the presence of DTT (+ SLO, solid lines) or resuspended in DPBS with DTT (− SLO, dashed lines) as a control. After centrifugation, supernatants from SLO-permeabilized cells or control cells were analyzed for luciferase activity. The graph shows the mean value (±s.d.) of triplicate samples and it is representative of at least three independent experiments. (B, C) KG-1 cells were pretreated at the indicated concentrations with radicicol or with DMSO as control (grey columns) for 1 h. After extensive washes in serum-free medium, Firefly luciferase (B) or chemically unfolded R. reniformis luciferase in PBS (C) were added for 15 min to allow internalization. Cells were then permeabilized with SLO in the presence of DTT (+ SLO, grey columns) or resuspended in DPBS with DTT (− SLO, white columns) as a control. After centrifugation, supernatants were analyzed for luciferase activity. (D) Chemically unfolded R. reniformis luciferase was added for 15 min to primary human DCs. Cells were then resuspended in PBS as control (Ctr, grey column) or subjected to hypotonic lysis with 10 mM Tris, pH 7.4 (black column). After centrifugation, supernatants were analyzed for luciferase activity with a luminometer. All graphs show the mean value (±s.d.) of triplicate samples and they are representative of at least three independent experiments.
The in vitro refolding experiments suggested that Hsp90 was the critical chaperone. To determine whether this was the case for in vivo refolding, we again used the Hsp90 inhibitor, radicicol. KG-1 cells were pretreated with radicicol for 1 h before 15-min incubation with native Firefly or unfolded Renilla luciferase, followed by washing and SLO permeabilization. Radicicol pretreatment strongly reduced the cytosolic activity recovered for both enzymes (Figure 4B and C, gray bars). No significant luciferase activity was detected in supernatants of non-permeabilized cells (Figure 4B and C, white bars). Similar results were obtained with overnight radicicol treatment at lower concentrations (Supplementary Figure 5), arguing against nonspecific effects, which could have resulted from the relatively high radicicol concentration used in Figure 4B and C, chosen to allow short incubation times. Radicicol treatment did not affect cell macropinocytotic activity, as assessed by fluid-phase Lucifer Yellow uptake (Supplementary Figure 6), and no toxic effects of radicicol on KG-1 cells were observed, assessed both by cell recovery and viability (data not shown). Taken together, these data implicate the Hsp90 chaperone in the cytosolic refolding of exogenous luciferases internalized by KG-1 cells.
Primary human DCs can mediate refolding of Renilla luciferase
The fragility of primary DCs prevented us from using SLO permeabilization to evaluate cytosolic access by luciferase in these cells; significant ER and lysosomal leakage was observed upon SLO treatment (data not shown). We therefore used hypotonic lysis to release the total cellular contents rather than SLO permeabilization to analyze luciferase refolding in primary DCs. After 15-min incubation with inactive, denatured Renilla luciferase, DCs were washed in ice-cold PBS, resuspended in ice-cold 10 mM Tris-chloride, pH 7.4, for 5 min, and centrifuged at 2000 g for 10 min. Significant luciferase activity could be detected in the supernatants derived from hypotonically lysed cells compared with those from an equivalent number of non-lysed cells resuspended in PBS (Figure 4D). The data suggest that Renilla luciferase is highly likely to refold in the cytosol of primary human DCs.
Inhibitory shRNA for Hsp90 β significantly decreases cytosolic refolding
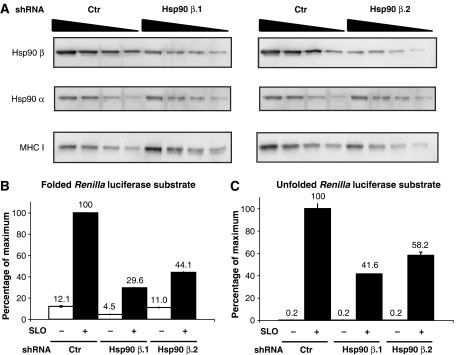
To further confirm the role of Hsp90 in cytosolic refolding, we attempted to independently ‘knock down' the Hsp90 α and β isoforms using small-hairpin RNA (shRNA) constructs. We were unable to significantly reduce expression of the Hsp90 α isoform because KG-1 cells transduced with a specific shRNA were impaired in their ability to grow, and we could not recover enough cells to perform the experiment (data not shown). Decreased growth of cells upon Hsp90 α knockdown has been previously reported (Teng et al, 2004; Kunisawa and Shastri, 2006). Hsp90 β, however, was more tractable. KG-1 cells were transduced with an shRNA construct specific for the Hsp90 β isoform. A GFP-silencing sequence was used as control. After selection in puromycin, we recovered two KG-1 cell clones (Hsp90 β.1 and Hsp90 β.2) with stable reduction of Hsp90 β. Hsp90 α and β expression was quantitated by SDS–PAGE followed by quantitative western blotting on serially diluted cell extracts. Approximately 65% reduction of Hsp90 β was obtained for both Hsp90 β.1 and Hsp90 β.2 clones compared with GFP–shRNA control cells (Figure 3A, top panels). No significant decrease in Hsp90 α protein was observed (Figure 5A, middle panels); MHC class I heavy-chain blots served as a loading control and normalizer (Figure 5A, bottom panels).
Figure 5.
Knockdown of Hsp90 β expression inhibits cytosolic refolding. (A) Serial dilutions of cell extracts from shRNA control (Ctr) or shRNA Hsp90 β (Hsp90 β.1 and Hsp90 β.2) KG-1 cells were subjected to SDS–PAGE and blotted for Hsp90 β (top panels) and α (middle panels) isoform-specific antibodies. Membranes were also blotted for MHC I rat mAb as loading control (bottom panels). (B, C) Native (B) or chemically unfolded (C) R. reniformis luciferase was added to shRNA control (Ctr) or shRNA Hsp90 β (Hsp90 β.1 and Hsp90 β.2) KG-1 cells for 15 min. Cells were then washed and permeabilized with SLO in the presence of DTT (+ SLO, ▪) or resuspended in DPBS with DTT (− SLO,  ) as a control. After centrifugation, supernatants from SLO-permeabilized cells or control cells were analyzed for luciferase activity with a luminometer. Values are indicated as percent of those obtained with shRNA control cells. All graphs show the mean value (±s.d.) of triplicate samples and they are representative of at least three independent experiments.
) as a control. After centrifugation, supernatants from SLO-permeabilized cells or control cells were analyzed for luciferase activity with a luminometer. Values are indicated as percent of those obtained with shRNA control cells. All graphs show the mean value (±s.d.) of triplicate samples and they are representative of at least three independent experiments.
To assess the effect of Hsp90 β reduction on refolding, the shRNA-expressing cells were incubated for 15 min with native or unfolded Renilla luciferase, followed by washing and SLO permeabilization. Significantly decreased cytosolic luciferase activity was recovered from KG-1 cells with reduced levels of Hsp90 β compared with GFP–shRNA control cells (Figure 5B and C, black bars). No significant luciferase activity was detected in supernatants of non-permeabilized cells (Figure 5B and C, white bars). No differences were observed in the macropinocytotic activity of the Hsp90 β shRNA-expressing cells compared with the shRNA control cells, as assessed by fluid-phase Lucifer Yellow uptake (Supplementary Figure 7). Together with the data showing inhibition by radicicol, these experiments strongly support a key role for the Hsp90 chaperone in the cytosolic refolding of exogenous luciferases internalized by KG-1 cells.
Discussion
We previously showed that for cross-presentation in DCs, exogenous antigens are translocated into the cytosol using mechanisms related to ERAD (Ackerman et al, 2006). For part of this work we purified phagosomes from KG-1 cells containing internalized Firefly luciferase and showed that they could transport the enzyme into the topological equivalent of the cytosol. The translocation process was ATP-dependent and required the active form of the AAA-ATPase, p97 (Ye et al, 2001; Ackerman et al, 2006). Perhaps the most surprising aspect of this finding was that the translocated luciferase was enzymatically active. In the present work we have extended our previous findings to luciferases taken up by fluid-phase mechanisms. We showed that the appearance of active enzyme in the cytosol could be inhibited by radicicol and by reducing the levels of Hsp90 β. This indicates that cytosolic refolding is mediated by Hsp90, and implies that an unfolding step occurs before translocation (see model in Figure 6).
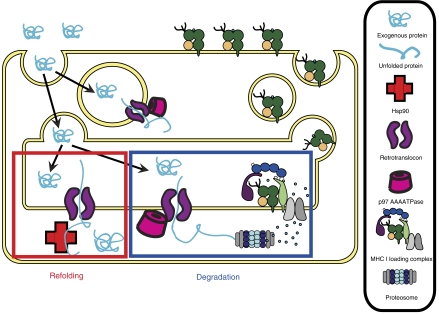
Figure 6.
Model for ERAD retrotranslocation and Hsp90-mediated cytosolic refolding in DCs. In DCs exogenous proteins are efficiently internalized and retrotranslocated from the endosomal compartment or from the ER into the cytosol. At least partial unfolding of the exogenous protein may be required for efficient retrotranslocation to occur. Once in the cytosol, the internalized protein can be degraded by the proteasome to generate immunogenic peptides, or it can regain its activity by Hsp90 chaperone-mediated refolding.
The SLO permeabilization approach provides a useful strategy for determining the rate at which soluble proteins internalized by the DC-like KG-1 cell line enter the cytosol. Problems with cell fragility prevented us from using the same technique with monocyte-derived DCs. However, hypotonic lysis of DCs after internalization of inactive Renilla luciferase released active enzyme, suggesting that DCs were similarly capable of cytosolic refolding of internalized proteins (Figure 4D). Previous work investigating cytosolic access of exogenous proteins used imaging techniques such as immunofluorescence and electron microscopy, and focused on single-cell analysis (Rodriguez et al, 1999; Houde et al, 2003). The SLO-permeabilization/luciferase release assay allowed us to estimate retrotranslocation efficiency in whole-cell populations over time, avoiding potential bias in imaging selection. We were careful to exclude that lysosomal or ER lumenal components were released by SLO (Figure 1C), and we determined that SLO did not cause endosomal permeabilization in KG-1 cells by using internalized fluorescein-conjugated dextran as an endocytic marker (Supplementary Figure 1). Pores formed by SLO are 30-nm diameter (Walev et al, 2001), large enough to accommodate a dextran of 10 kDa. Overall, these data confirm that the luciferase activity detected in supernatants from SLO-permeabilized cells is genuinely derived from the cytosol.
It is unclear from which structures retrotranslocation into the cytosol occurs. Initial entry into the cells is likely to involve macropinocytosis, an actin-dependent process allowing internalization of large volumes of extracellular fluid. The ability of cytochalasin D to inhibit cytosolic luciferase accumulation in KG-1 cells (Figure 2A; Supplementary Figure 2), as well as the inability of the non-macropinocytotic CEM cell line to mediate the process (Figure 1), are consistent with this idea. After macropinocytosis, translocation into the cytosol could occur from macropinosomes directly or it could occur after exogenous protein entry into the ER. We have provided some evidence based on immunofluorescence analysis for the appearance of ER proteins in macropinosomes (Ackerman et al, 2003), and also have shown that internalized proteins can access the ER (Ackerman et al, 2005), so both mechanisms seem possible. However, luciferase entry into the cytosol of KG-1 cells was rapid, with enzymatic activity detectable as early as 5 min after initiation of internalization (Figures 1A, 2A, and 4A). The speed of the retrotranslocation and refolding suggests that recruitment of the ER retrotranslocation machinery to macropinosomes is perhaps more likely to be involved than transport of the enzyme to the ER. Whether other cell types capable of macropinocytosis, such as neutrophils or endothelial cells (Catizone et al, 1993; Botelho et al, 2002), can perform this function remains to be investigated.
The identity of the retrotranslocation channel for ERAD is still subject to debate (Tsai et al, 2002; Ahner and Brodsky, 2004; Meusser et al, 2005), and the same debate applies to the work we describe here. There is evidence, particularly from work with yeast, that the Sec61 channel is responsible (Plemper and Wolf, 1999; Plemper et al, 1999), but evidence from both yeast and mammalian cells suggests that the multiple membrane-spanning protein Derlin-1 may be involved (Lilley and Ploegh, 2004; Ye et al, 2004; Wahlman et al, 2007). ExoA has been reported to block the Sec61 channel and inhibit the retrotranslocation of peptides from ER vesicles (Koopmann et al, 2000), and here we have shown that it inhibits the cytosolic recovery of luciferase (Figure 2B), but it is conceivable that other channels could be implicated in this process. However, regardless of the channel involved, the luciferase we find in the cytosol is active and therefore presumably intact; thus, the channel must be capable of translocating a protein of at least 62 kDa. This is the Mr for Firefly luciferase, which is folded into two compact domains (Conti et al, 1996). The data showing that both radicicol and Hsp90 β knockdown inhibit refolding, and the observation that denatured Renilla luciferase can be reactivated after internalization, argue that unfolding is a pre-requisite for translocation and that Hsp90 then mediates cytosolic refolding. Thus, the diameter of the retrotranslocation channel need not be unusually large. However, glycans remain attached during retrotranslocation for ERAD (Wiertz et al, 1996a, 1996b), and there are data arguing that folded domains, including EGFP (25 kDa) (Fiebiger et al, 2002) and a folded dihydrofolate reductase domain (42 kDa) (Tirosh et al, 2003), can be retrotranslocated. There is no reason to suppose that this would not also be true for retrotranslocation for cross-presentation. However, denatured Renilla luciferase appears in the cytosol more rapidly than the native form (Figure 4A), indicating that prior in vitro unfolding may significantly speed up the retrotranslocation process. Perhaps certain checkpoints involved in substrate detection, recognition, and unfolding are bypassed, allowing faster retrotranslocation to occur. However, the validity of this conclusion is tempered by the requirement for higher concentrations of the unfolded luciferase to achieve a usable signal. Conceivably this could be responsible for the more rapid appearance of the non-native form in the cytosol.
At least two ERAD pathways exist: the ERAD-L and the ERAD-C pathway (Vashist and Ng, 2004; Carvalho et al, 2006). During protein synthesis, the ERAD-C pathway monitors the folding status of cytosolic domains of membrane proteins and rapidly clears misfolded variants from the ER. This occurs without regard to the state of the lumenal domain. If the conformation of the cytosolic domain passes the ERAD-C checkpoint, the ERAD-L pathway monitors the state of the lumenal domain. If a lesion is detected, the protein is processed for ERAD using a distinct set of factors not required for the ERAD-C pathway. In DCs, exogenous proteins can be directly delivered to the ER lumen for retrotranslocation or can be retrotranslocated from ER-containing phagosomes through the ERAD pathway. In both cases, we might speculate that extracellular proteins delivered to ER lumenal compartments would preferentially use ERAD-L. Previous work in yeast showed the importance of Hsp90 in maintaining the stability of the ERAD-L substrate CFTR (Youker et al, 2004). This would be in agreement with the key role of Hsp90 indicated by our luciferase cytosolic refolding data.
Firefly luciferase readily refolds in vitro in the presence of purified Hsp90, Hsp70, and the PA28 ATPase proteasomal subunit (Minami et al, 2000, 2001). The use of radicicol as an inhibitor provides evidence for Hsp90 as the key cytosolic chaperone that mediates refolding after retrotranslocation. Radicicol did not affect the efficiency of luciferase internalization, indicating that decreased enzymatic activity in the cytosol was specifically due to Hsp90 inhibition (Supplementary Figure 6). The effect of radicicol is also unlikely to be mediated by the ER Hsp90 homologue GRP94, because even lower concentrations than those used in the primary experiments gave similar results (Supplementary Figure 5). It has been reported that the radicicol affinity for Hsp90 is fivefold higher than its affinity for GRP94 (Schulte et al, 1999). This argues against the possibility that the decreased cytosolic luciferase activity seen upon radicicol treatment is due to decreased retrotranslocation caused by GRP94 inhibition.
Unfortunately, we were unable to ‘knock down' both the Hsp90 α and β isoforms using either siRNA oligonucleotides or shRNA approaches in the KG-1 cell line. Our attempts to obtain significant and stable silencing for Hsp90 α in KG-1 cells were unsuccessful. The reduced rate of cell division previously reported (Teng et al, 2004; Kunisawa and Shastri, 2006) combined with the high number of cells required for our experiments rendered the Hsp90 α shRNA strategy unusable. We were able to establish ∼65% stable knock down for the Hsp90 β isoform in KG-1 cells (Figure 5A). We observed no differences in Lucifer Yellow uptake between shRNA control and shRNA Hsp90 β cells (Supplementary Figure 7), and no effect on cell growth rate or survival was observed (data not shown). Reduced levels of Hsp90 β caused a significant decrease in enzymatic activities recovered in the cytosolic fraction using native or unfolded Renilla luciferase as exogenous protein substrates (Figure 5B and C), confirming the role of Hsp90 suggested by the potent inhibitor radicicol (Figure 4).
Workers using OVA as a probe for cytosolic access in DCs found only a 30-kDa fragment of the protein in the cytosol, indicating that proteolysis had occurred, but there is no evidence that proteolysis is required for cytosolic access (Rodriguez et al, 1999). The reduced yield of active Renilla luciferase when denatured enzyme was internalized, compared with the activity recovered when native enzyme was used, might indicate that proteolysis is more likely when the retrotranslocated substrate is totally denatured. No significant differences in the luciferase activity recovered were observed in cells pretreated with concanamycin B or leupeptin to inhibit lysosomal proteolysis (data not shown). However, proteolysis of acquired proteins could still occur in endocytic vesicles as well as in the cytosol after translocation. Lactacystin pretreatment of KG-1 cells followed by SLO treatment caused some ER lumenal contamination of the released cytosol, as assessed by western blotting for GRP94 (data not shown), so we were unable to determine if proteasome inhibition resulted in an increase in cytosolic luciferase activity. However, upon prolonged incubation, cytosolic luciferase activity did decrease (Figure 4A), which suggests that the refolded enzyme is ultimately degraded by cytosolic proteolysis.
An estimate of retrotranslocation efficiency can be extrapolated by comparing the luciferase activity recovered in SLO-permeabilized samples (Figure 1A) to the total amount of internalized enzyme measured by cell lysis (Supplementary Figure 2). Thirty minutes after internalization was initiated, at least 60% of the internal luciferase could be recovered in the cytosol, suggesting that the level of proteolysis, either pre- or post-retrotranslocation, is relatively low for luciferase. For other internalized proteins, degradation may well be the predominant fate. This is the fate that gives rise to the peptides that are transported into the ER by TAP, and therefore the key pathway involved in cross-presentation. However, the unfolding/refolding mechanism we have identified that allows exogenous proteins to regain their activity in the cytosol could potentially be exploited by DCs for some aspects of their regulation or function.
There are a few examples in the literature where cytosolic delivery of growth factors and signaling molecules can mediate biological effects. Fibroblast growth factor (FGF)-1 and FGF-2 are internalized through the FGF receptor and then dislocated into the cytosol. Subsequently FGF traffics to the nucleus and binds to specific DNA sequences, causing distinct cellular effects; cytosolic FGF triggers a signaling cascade temporally and physiologically different from stimulation via FGF receptor tyrosine kinase (Wesche et al, 2005, 2006). A similar phenomenon has also been reported for epidermal growth factor (EGF). EGF binding to the cell-surface EGF receptor (EGFR) triggers EGF–EGFR trafficking to the ER. EGF is then retrotranslocated into the cytosol, apparently through Sec61, signaling to the nucleus and inducing cyclin D gene expression (Lin et al, 2001; Liao and Carpenter, 2007). It is tempting to speculate that similar principles may apply to exogenous molecules internalized by DCs. For example, proteins secreted by neighboring cells or contained in apoptotic bodies could be internalized by DCs, penetrate the cytosol, and modulate their ability to migrate or function as APCs. To date, however, there are no data supporting this notion.
Materials and methods
Antibodies and reagents
Rat anti-GRP94mAb and rabbit anti-MAPK pAb were from Stressgen Biotechnologies and Cell Signaling, respectively. QuantiLum recombinant Firefly luciferase (14.9 mg/ml) and luciferin were purchased from Promega. R. reniformis and native coloenterazine (nCTZ) were purchased from Prolume, AZ. R. reniformis luciferase was resuspended in PBS; nCTZ (1 mg/ml stock in ethanol) was diluted 1:1000 in PBS for luciferase assays. SLO was from Aalto, Ireland. Cytochalasin D and radicicol were from Sigma and AG Scientific Inc., CA, respectively. ExoA was from EMD.
Cells and cultures
The human DC-like KG1 and human T lymphoblastoid cell line CEM were previously described (Howell et al, 1985; Ackerman and Cresswell, 2003). Primary human DCs were generated (Sallusto and Lanzavecchia, 1994) from human mononuclear cells isolated using lymphocyte separation medium (LSM; Cellgro) according to the manufacturer's protocol.
SLO cell permeabilization
A total of 6 × 106 KG-1 or CEM cells in 1% FBS IMDM were incubated with soluble recombinant Firefly or R. reniformis luciferases for the indicated time points at the final concentrations of 49 and 0.13 μg/ml, respectively. After extensive washes in serum-free medium, cells were permeabilized with SLO as previously described (Androlewicz et al, 1993). Briefly, cells were resuspended in 200 μl serum-free medium and incubated for 15 min on ice in the presence of 0.3 mM DTT with or without SLO at 22 μg/ml. Cells were then washed three times with Dulbecco's phosphate-buffered saline without calcium and magnesium (DPBS; Gibco) and resuspended in 100 μl DPBS. Cells were then incubated at 37°C for 5 min to allow permeabilization. After centrifuging at 14 000 g for 5 min at 4°C, 20 μl of supernatants from SLO-permeabilized cells were analyzed for luciferase activity with 100 μl of either nCTZ or luciferin (Promega) using a luminometer (GE).
Firefly and R. reniformis luciferase chemical unfolding and in vitro refolding
In vitro refolding assays were performed as previously described (Minami et al, 2000). Briefly, chemical unfolding was performed in 6 M guanidine–HCl in ICT buffer (50 mM HEPES, 78 mM KCl, 4 mM MgCl2, 8.37 mM CaCl2, and 10 mM EGTA) for 30 min at room temperature. Refolding reactions were performed for 30 min at room temperature with serial dilutions of KG-1 cytosol, isolated as previously described (Ackerman et al, 2006), in ICT buffer in the presence or absence of radicicol. Refolding efficiency was calculated as regained luciferase activity, measured by luminometer, and expressed as percentage of luciferase input in the refolding reaction.
Unfolded R. reniformis luciferase refolding in KG-1 cells and in primary human DCs
R. reniformis luciferase was chemically unfolded in 6 M guanidine–HCl in ICT buffer for 2 h and then dialyzed overnight against PBS, retaining its unfolded form. For experiments with KG-1 cells, 0.3 μg of native or 40 μg of chemically unfolded R. reniformis luciferases were added to KG-1 cells at the final concentration of 0.13 or 20 μg/ml, respectively. Cells were then washed three times with DPBS and resuspended in 100 μl DPBS before permeabilization with SLO as described above. After centrifuging at 14 000 g for 5 min at 4°C, 20 μl of supernatants from SLO-permeabilized cells were analyzed for luciferase activity. For experiments with human DCs, 30 μg of chemically unfolded R. reniformis luciferase in PBS were added to 2 × 106 primary human DCs seeded in Ultra Low Cluster Plates (Costar). After 15-min incubation at 37°C to allow internalization, DCs were washed with cold PBS and resuspended in 100 μl of PBS or subjected to hypotonic lysis with 100 μl of 10 mM Tris, pH 7.4, both in the presence of protease inhibitors (EDTA-free; Roche). After centrifuging at 2000 g for 10 min at 4°C, 20 μl of supernatants were analyzed for luciferase activity.
Western blotting
Supernatants from SLO-permeabilized cells or total cell lysates were separated by reducing SDS–PAGE (10% (w/v) acrylamide) and electrophoretically transferred to Immobilon-P membrane (Millipore). The membrane was blocked in TBS with 0.05% Tween 20 with 5% dehydrated milk and probed with rabbit anti-human GILT serum (1/10 000), rat anti-GRP94mAb, or anti MAPK rabbit Ab. Membranes were then washed, incubated with HRP-conjugated goat anti-rabbit or anti-rat IgG (1/5000; Jackson ImmunoResearch Laboratories) and ECL substrate (SuperSignal West Pico; Pierce), and exposed to film.
Hsp90 β shRNA in KG-1 cells
shRNA-expressing cells were generated as previously described (Barton and Medzhitov, 2002) using the pSUPER.retro. puro vector (OligoEngine). The siRNA sequence specific for the Hsp90 β isoform has been previously described (Kunisawa and Shastri, 2006); the shRNA construct targeting GFP used as control was a kind gift from Dr Hidde Ploegh (Lilley and Ploegh, 2005). Clonal selection was performed in 20 μg/ml puromycin. Control and Hsp90 β shRNA KG-1 cells were maintained in 20 μg/ml puromycin to maintain selection pressure. Knockdown was assessed by reducing SDS–PAGE followed by western blotting with Hsp90 β isoform-specific rabbit Ab (1/1000; Lab Vision Corporation). To ensure specificity, membranes were stripped for 4 min in 0.2 M NaOH and reprobed with Hsp90 α isoform-specific rabbit Ab (1/2000; Stressgen). Membranes were also probed with anti-MHC-I rat mAb 3B10.7 as a loading control and normalizer. Alkaline phosphatase-conjugated goat anti-rabbit or anti-rat IgG (1/5000; Jackson ImmunoResearch Laboratories) and ECF substrate (GE) were used for signal detection with a FluorImager (GE).
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure Legends
Acknowledgments
We thank N Dometios for helpful assistance with the manuscript. This work was supported by the Howard Hughes Medical Institute and NIH grant R37-AI23081 (PC).
References
- Ackerman AL, Cresswell P (2003) Regulation of MHC class I transport in human dendritic cells the dendritic-like cell line KG-1. J Immunol 170: 4178–4188 [DOI] [PubMed] [Google Scholar]
- Ackerman AL, Giodini A, Cresswell P (2006) A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity 25: 607–617 [DOI] [PubMed] [Google Scholar]
- Ackerman AL, Kyritsis C, Tampe R, Cresswell P (2003) Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci USA 100: 12889–12894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman AL, Kyritsis C, Tampe R, Cresswell P (2005) Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nat Immunol 6: 107–113 [DOI] [PubMed] [Google Scholar]
- Ahner A, Brodsky JL (2004) Checkpoints in ER-associated degradation: excuse me, which way to the proteasome? Trends Cell Biol 14: 474–478 [DOI] [PubMed] [Google Scholar]
- Amyere M, Mettlen M, Van Der Smissen P, Platek A, Payrastre B, Veithen A, Courtoy PJ (2002) Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int J Med Microbiol 291: 487–494 [DOI] [PubMed] [Google Scholar]
- Androlewicz MJ, Anderson KS, Cresswell P (1993) Evidence that transporters associated with antigen processing translocate a major histocompatibility complex class I-binding peptide into the endoplasmic reticulum in an ATP-dependent manner. Proc Natl Acad Sci USA 90: 9130–9134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam B, Phan UT, Geuze HJ, Cresswell P (2000) Enzymatic reduction of disulfide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT). Proc Natl Acad Sci USA 97: 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton GM, Medzhitov R (2002) Retroviral delivery of small interfering RNA into primary cells. Proc Natl Acad Sci USA 99: 14943–14945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho RJ, Tapper H, Furuya W, Mojdami D, Grinstein S (2002) Fc gamma R-mediated phagocytosis stimulates localized pinocytosis in human neutrophils. J Immunol 169: 4423–4429 [DOI] [PubMed] [Google Scholar]
- Carvalho P, Goder V, Rapoport TA (2006) Distinct ubiquitin–ligase complexes define convergent pathways for the degradation of ER proteins. Cell 126: 361–373 [DOI] [PubMed] [Google Scholar]
- Catizone A, Medolago Albani L, Reola F, Alescio T (1993) A quantitative assessment of non specific pinocytosis by human endothelial cells surviving in vitro. Cell Mol Biol (Noisy-le-grand) 39: 155–169 [PubMed] [Google Scholar]
- Conti E, Franks NP, Brick P (1996) Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure 4: 287–298 [DOI] [PubMed] [Google Scholar]
- Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA (2005) Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev 207: 145–157 [DOI] [PubMed] [Google Scholar]
- Fiebiger E, Story C, Ploegh HL, Tortorella D (2002) Visualization of the ER-to-cytosol dislocation reaction of a type I membrane protein. EMBO J 21: 1041–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, Laplante A, Princiotta MF, Thibault P, Sacks D, Desjardins M (2003) Phagosomes are competent organelles for antigen cross-presentation. Nature 425: 402–406 [DOI] [PubMed] [Google Scholar]
- Howell DN, Andreotti PE, Dawson JR, Cresswell P (1985) Natural killing target antigens as inducers of interferon: studies with an immunoselected, natural killing-resistant human T lymphoblastoid cell line. J Immunol 134: 971–976 [PubMed] [Google Scholar]
- Koopmann JO, Albring J, Huter E, Bulbuc N, Spee P, Neefjes J, Hammerling GJ, Momburg F (2000) Export of antigenic peptides from the endoplasmic reticulum intersects with retrograde protein translocation through the Sec61p channel. Immunity 13: 117–127 [DOI] [PubMed] [Google Scholar]
- Kunisawa J, Shastri N (2006) Hsp90alpha chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity 24: 523–534 [DOI] [PubMed] [Google Scholar]
- Liao HJ, Carpenter G (2007) Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell 18: 1064–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley BN, Ploegh HL (2004) A membrane protein required for dislocation of misfolded proteins from the ER. Nature 429: 834–840 [DOI] [PubMed] [Google Scholar]
- Lilley BN, Ploegh HL (2005) Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane. Proc Natl Acad Sci USA 102: 14296–14301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC (2001) Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol 3: 802–808 [DOI] [PubMed] [Google Scholar]
- Matlack KE, Misselwitz B, Plath K, Rapoport TA (1999) BiP acts as a molecular ratchet during posttranslational transport of prepro-alpha factor across the ER membrane. Cell 97: 553–564 [DOI] [PubMed] [Google Scholar]
- Mellman I, Steinman RM (2001) Dendritic cells: specialized and regulated antigen processing machines. Cell 106: 255–258 [DOI] [PubMed] [Google Scholar]
- Meusser B, Hirsch C, Jarosch E, Sommer T (2005) ERAD: the long road to destruction. Nat Cell Biol 7: 766–772 [DOI] [PubMed] [Google Scholar]
- Minami M, Nakamura M, Emori Y, Minami Y (2001) Both the N- and C-terminal chaperone sites of Hsp90 participate in protein refolding. Eur J Biochem 268: 2520–2524 [DOI] [PubMed] [Google Scholar]
- Minami Y, Kawasaki H, Minami M, Tanahashi N, Tanaka K, Yahara I (2000) A critical role for the proteasome activator PA28 in the Hsp90-dependent protein refolding. J Biol Chem 275: 9055–9061 [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Brodsky JL, Nakatsukasa K (2005) Roles of molecular chaperones in endoplasmic reticulum (ER) quality control and ER-associated degradation (ERAD). J Biochem (Tokyo) 137: 551–555 [DOI] [PubMed] [Google Scholar]
- Plemper RK, Bordallo J, Deak PM, Taxis C, Hitt R, Wolf DH (1999) Genetic interactions of Hrd3p and Der3p/Hrd1p with Sec61p suggest a retro-translocation complex mediating protein transport for ER degradation. J Cell Sci 112 (Part 22): 4123–4134 [DOI] [PubMed] [Google Scholar]
- Plemper RK, Wolf DH (1999) Endoplasmic reticulum degradation. Reverse protein transport and its end in the proteasome. Mol Biol Rep 26: 125–130 [DOI] [PubMed] [Google Scholar]
- Rock KL (2006) Exiting the outside world for cross-presentation. Immunity 25: 523–525 [DOI] [PubMed] [Google Scholar]
- Rock KL, Shen L (2005) Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev 207: 166–183 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S (1999) Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol 1: 362–368 [DOI] [PubMed] [Google Scholar]
- Sallusto F, Cella M, Danieli C, Lanzavecchia A (1995) Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med 182: 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A (1994) Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179: 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte TW, Akinaga S, Murakata T, Agatsuma T, Sugimoto S, Nakano H, Lee YS, Simen BB, Argon Y, Felts S, Toft DO, Neckers LM, Sharma SV (1999) Interaction of radicicol with members of the heat shock protein 90 family of molecular chaperones. Mol Endocrinol 13: 1435–1448 [DOI] [PubMed] [Google Scholar]
- Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, Neckers LM (1998) Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones 3: 100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SV, Agatsuma T, Nakano H (1998) Targeting of the protein chaperone, HSP90, by the transformation suppressing agent, radicicol. Oncogene 16: 2639–2645 [DOI] [PubMed] [Google Scholar]
- Teng SC, Chen YY, Su YN, Chou PC, Chiang YC, Tseng SF, Wu KJ (2004) Direct activation of HSP90A transcription by c-Myc contributes to c-Myc-induced transformation. J Biol Chem 279: 14649–14655 [DOI] [PubMed] [Google Scholar]
- Tirosh B, Furman MH, Tortorella D, Ploegh HL (2003) Protein unfolding is not a prerequisite for endoplasmic reticulum-to-cytosol dislocation. J Biol Chem 278: 6664–6672 [DOI] [PubMed] [Google Scholar]
- Tsai B, Ye Y, Rapoport TA (2002) Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat Rev Mol Cell Biol 3: 246–255 [DOI] [PubMed] [Google Scholar]
- Vashist S, Ng DT (2004) Misfolded proteins are sorted by a sequential checkpoint mechanism of ER quality control. J Cell Biol 165: 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlman J, DeMartino GN, Skach WR, Bulleid NJ, Brodsky JL, Johnson AE (2007) Real-time fluorescence detection of ERAD substrate retrotranslocation in a mammalian in vitro system. Cell 129: 943–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walev I, Bhakdi SC, Hofmann F, Djonder N, Valeva A, Aktories K, Bhakdi S (2001) Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc Natl Acad Sci USA 98: 3185–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H, Riordan JR, Kelly JW, Yates JR III, Balch WE (2006) Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell 127: 803–815 [DOI] [PubMed] [Google Scholar]
- Wesche J, Malecki J, Wiedlocha A, Ehsani M, Marcinkowska E, Nilsen T, Olsnes S (2005) Two nuclear localization signals required for transport from the cytosol to the nucleus of externally added FGF-1 translocated into cells. Biochemistry 44: 6071–6080 [DOI] [PubMed] [Google Scholar]
- Wesche J, Malecki J, Wiedlocha A, Skjerpen CS, Claus P, Olsnes S (2006) FGF-1 and FGF-2 require the cytosolic chaperone Hsp90 for translocation into the cytosol and the cell nucleus. J Biol Chem 281: 11405–11412 [DOI] [PubMed] [Google Scholar]
- Wiertz EJ, Jones TR, Sun L, Bogyo M, Geuze HJ, Ploegh HL (1996a) The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84: 769–779 [DOI] [PubMed] [Google Scholar]
- Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL (1996b) Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384: 432–438 [DOI] [PubMed] [Google Scholar]
- Ye Y, Meyer HH, Rapoport TA (2001) The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414: 652–656 [DOI] [PubMed] [Google Scholar]
- Ye Y, Meyer HH, Rapoport TA (2003) Function of the p97–Ufd1–Npl4 complex in retrotranslocation from the ER to the cytosol: dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J Cell Biol 162: 71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA (2004) A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 429: 841–847 [DOI] [PubMed] [Google Scholar]
- Youker RT, Walsh P, Beilharz T, Lithgow T, Brodsky JL (2004) Distinct roles for the Hsp40 and Hsp90 molecular chaperones during cystic fibrosis transmembrane conductance regulator degradation in yeast. Mol Biol Cell 15: 4787–4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure Legends