Abstract
Species of the ascomycete genus Mycosphaerella are regarded as some of the most destructive leaf pathogens of a large number of economically important crop plants. Amongst these, approximately 60 Mycosphaerella spp. have been identified from various Eucalyptus spp. where they cause leaf diseases collectively known as Mycosphaerella Leaf Disease (MLD). Species concepts for this group of fungi remain confused, and hence their species identification is notoriously difficult. Thus, the introduction of DNA sequence comparisons has become the definitive characteristic used to distinguish species of Mycosphaerella. Sequences of the Internal Transcribed Spacer (ITS) region of the ribosomal RNA operon have most commonly been used to consider species boundaries in Mycosphaerella. However, sequences for this gene region do not always provide sufficient resolution for cryptic taxa. The aim of this study was, therefore, to use DNA sequences for three loci, ITS, Elongation factor 1-alpha (EF-1α) and Actin (ACT) to reconsider species boundaries for Mycosphaerella spp. from Eucalyptus. A further aim was to study the anamorph concepts and resolve the deeper nodes of Mycosphaerella, for which part of the Large Subunit (LSU) of the nuclear rRNA operon was sequenced. The ITS and EF-1α gene regions were found to be useful, but the ACT gene region did not provide species-level resolution in Mycosphaerella. A phylogeny of the combined DNA datasets showed that species of Mycosphaerella from Eucalyptus cluster in two distinct groups, which might ultimately represent discrete genera.
Keywords: Actin, Ascomycetes, Translation Elongation factor 1-alpha, Multi-gene phylogeny, Mycosphaerella, Mycosphaerella Leaf Disease, ribosomal RNA operon
INTRODUCTION
Species of Eucalyptus are native to Australia with isolated pockets of native Eucalyptus forests also occurring in Papua New Guinea and the Philippines (Turnbull 2000). Many Eucalyptus spp. have been removed from these centres of origin to new environments where they are typically propagated in plantations for the production of paper, pulp and other wood products (Wingfield 1999, Turnbull 2000, Wingfield et al. 2001). In these non-native environments, Eucalyptus trees are susceptible to many pests and diseases including those known in their areas of origin and others that have undergone host shifts (Wingfield 2003, Slippers et al. 2005). These pests and diseases cause significant annual losses to Eucalyptus plantations resulting in decreased revenue for commercial forestry companies.
Mycosphaerella Johanson is one of the largest genera of the ascomycetes, accommodating more than 2000 species. Approximately 60 Mycosphaerella spp. have been associated with leaf diseases of many Eucalyptus spp., and these are collectively referred to as Mycosphaerella Leaf Disease (MLD) (Crous 1998, Maxwell et al. 2003, Crous et al. 2004a). The disease is particularly prevalent on the juvenile leaves and shoots of Eucalyptus trees, where infection results in premature defoliation, twig cankers and stunting of tree growth (Lundquist & Purnell 1987, Crous 1998, Park et al. 2000). However, several Mycosphaerella spp. can also infect adult Eucalyptus foliage, and this has been attributed to their ability to produce a proto-appressorium that enables direct cuticle penetration (Ganapathi 1979, Park & Keane 1982b). In some situations, trees may thus be subjected to infection by a suite of different Mycosphaerella spp.
Identification of Mycosphaerella spp. based on morphology is known to be difficult. This is because these fungi tend to produce very small fruiting structures with highly conserved morphology, and they are host-specific pathogens that grow poorly in culture. Traditionally, morphological characters of the teleomorph and anamorph have been used in species delimitation (Crous 1998). Park & Keane (1982a) introduced ascospore germination patterns as an additional characteristic to identify Mycosphaerella spp., and Crous (1998) subsequently identified 14 different ascospore germination patterns for the Mycosphaerella spp. occurring on Eucalyptus. Crous (1998) and Crous et al. (2000) also introduced features of these fungi growing in culture and especially anamorph morphology as important and useful characteristics on which to base species delimitation. DNA-based methods such as RAPDs and species-specific primers have also been employed to distinguish between Mycosphaerella species occurring on Eucalyptus (Carnegie et al. 2001, Maxwell et al. 2005).
Comparisons of DNA sequence data have emerged as the most reliable technique to identify Mycosphaerella spp. The majority of studies employing DNA sequence data for species identification have relied on sequence data from the Internal Transcribed Spacer (ITS) region of the ribosomal RNA operon (Crous et al. 1999, 2001, 2004a, b, Hunter et al. 2004a, b). Although comparisons of gene sequences for this region have been useful, the resolution provided by this region is not uniformly adequate to discriminate between individuals of a species complex or to effectively detect cryptic species (Crous et al. 2004b). Thus, recent studies have shown the importance of employing Multi-Locus Sequence Typing (MLST) to effectively identify cryptic fungal species and to study species concepts (Taylor & Fischer 2003).
A single morphological species does not always reflect a single phylogenetic unit (Taylor et al. 2000). Within Mycosphaerella, teleomorph morphology is conserved and the anamorph morphology provides additional characteristics to discriminate between taxa (Crous et al. 2000). Yet the collective teleomorph and anamorph morphology is often not congruent with phylogenetic data. Thus, recent phylogenetic studies have led to the recognition of several species complexes within Mycosphaerella (Crous et al. 2001, 2004b, Braun et al. 2003). Most of these studies have been based on comparisons of sequences for the ITS regions of the ribosomal DNA operon. Given the important data that have emerged from them, it is well recognised that greater phylogenetic resolution will be required for future taxonomic studies on Mycosphaerella species.
The aim of this study was to use MLST to consider species and anamorph concepts in Mycosphaerella spp. occurring on Eucalyptus. This was achieved by sequencing four nuclear gene regions, namely part of the Large Subunit (D1–D3 of LSU) and ITS region of the nuclear rRNA operon, and a portion of the Actin (ACT) and Elongation Factor 1-alpha (EF-1α) gene regions.
MATERIALS AND METHODS
Mycosphaerella isolates
For this study, an attempt was made to obtain cultures of as many Mycosphaerella spp. known to infect Eucalyptus leaves as possible. All cultures used in the investigation are housed in culture collections of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, South Africa and the Centraalbureau voor Schimmelcultures (CBS), Utrecht, The Netherlands (Table 1). All cultures were grown on 2 % (w/v) malt extract agar (MEA) (Biolab, South Africa), at 25 °C for approximately 2–3 mo to obtain sufficient mycelial growth for DNA extraction.
Table 1.
Isolates of Mycosphaerella used in this study for DNA sequencing and phylogenetic analysis.
| Teleomorph | Anamorph |
Isolate
No.a
|
Host | Country | Collector |
GenBank Accession No.
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CMW | CBS | STEU | LSU | ITS | ACT | EF-1a | |||||
| M. africana | Unknown | 3026 | CBS 116155 | 795 | E. viminalis | South Africa | P.W. Crous | DQ246258 | DQ267577 | DQ147608 | DQ235098 |
| 4945 | CBS 116154 | 794 | E. viminalis | South Africa | P.W. Crous | DQ246257 | AF309602 | DQ147609 | DQ235099 | ||
| M. ambiphylla | Phaeophleospora sp. | 14180 | CBS 110499 | N/A | E. globulus | Australia | A. Maxwell | DQ246219 | AY725530 | DQ147669 | DQ235103 |
| M. aurantia | Unknown | 14460 | CBS 110500 | N/A | E. globulus | Australia | A. Maxwell | DQ246256 | AY725531 | DQ147610 | DQ235097 |
| M. colombiensis | Pseudocercospora colombiensis | 4944 | CBS 110969 | 1106 | E. urophylla | Colombia | M.J. Wingfield | DQ204744 | AY752149 | DQ147639 | DQ211660 |
| 11255 | CBS 110967 | 1104 | E. urophylla | Colombia | M.J. Wingfield | DQ204745 | AY752147 | DQ147640 | DQ211661 | ||
| M. communis | Dissoconium commune | 14672 | CBS 114238 | 10440 | E. globulus | Spain | J.P. Mansilla | DQ246262 | AY725541 | DQ147655 | DQ235141 |
| 14673 | CBS 110976 | 849 | E. cladocalyx | South Africa | P.W. Crous | DQ246261 | AY725537 | DQ147654 | DQ235140 | ||
| M. cryptica | Colletogloeopsis nubilosum | 3279 | CBS 110975 | 936 | E. globulus | Australia | A.J. Carnegie | DQ246222 | AF309623 | DQ147674 | DQ235119 |
| 2732 | N/A | 355 | Eucalyptus sp. | Chile | M.J. Wingfield | N/A | AF309622 | N/A | N/A | ||
| M. crystallina | Pseudocercospora crystallina | 3042 | N/A | 800 | E. bicostata | South Africa | M.J. Wingfield | DQ204746 | DQ267578 | DQ147637 | DQ211662 |
| 3033 | CBS 681.95 | 802 | E. bicostata | South Africa | M.J. Wingfield | DQ204747 | AY490757 | DQ147636 | DQ211663 | ||
| M. ellipsoidea | Uwebraunia ellipsoidea | 4934 | N/A | 1224 | Eucalyptus sp. | South Africa | Unknown | DQ246253 | AF309592 | DQ147647 | DQ235129 |
| 5166 | N/A | 1225 | Eucalyptus sp. | South Africa | Unknown | DQ246254 | AF309593 | DQ147648 | DQ235127 | ||
| M. endophytica | Pseudocercosporella endophytica | 14912 | CBS 111519 | 1191 | Eucalyptus sp. | South Africa | P.W. Crous | DQ246255 | DQ267579 | DQ147646 | DQ235131 |
| 5225 | N/A | 1192 | Eucalyptus sp. | South Africa | P.W. Crous | DQ246252 | DQ267580 | DQ147649 | DQ235128 | ||
| M. flexuosa | Unknown | 5224 | CBS 111012 | 1109 | E. globulus | Colombia | M.J. Wingfield | DQ246232 | AF309603 | DQ147653 | DQ235126 |
| M. fori | Pseudocercospora sp. | 9095 | N/A | N/A | E. grandis | South Africa | G.C. Hunter | DQ204748 | AF468869 | DQ147618 | DQ211664 |
| 9096 | N/A | N/A | E. grandis | South Africa | G.C. Hunter | DQ204749 | DQ267581 | DQ147619 | DQ211665 | ||
| M. gracilis | Pseudocercospora gracilis | 14455 | CBS 243.94 | 730 | E. urophylla | Indonesia | A.C. Alfenas | DQ204750 | DQ267582 | DQ147616 | DQ211666 |
| M. grandis | Unknown | 8557 | N/A | N/A | E. globulus | Chile | A.Rotella | DQ246241 | DQ267583 | DQ147644 | DQ235108 |
| 8554 | N/A | N/A | E. globulus | Chile | M.J. Wingfield | DQ246240 | DQ267584 | DQ147643 | DQ235107 | ||
| M. gregaria | Unknown | 14462 | CBS 110501 | N/A | E. globulus | Australia | A. Maxwell | DQ246251 | DQ267585 | DQ147650 | DQ235130 |
| M. heimii | Pseudocercospora heimii | 4942 | CBS 110682 | 760 | Eucalyptus sp. | Madagascar | P.W. Crous | DQ204751 | AF309606 | DQ147638 | DQ211667 |
| M. heimioides | Pseudocercospora heimioides | 14776 | CBS 111364 | N/A | Eucalyptus sp. | Indonesia | M.J. Wingfield | DQ204752 | DQ267586 | DQ147632 | DQ211668 |
| 3046 | CBS 111190 | 1312 | Eucalyptus sp. | Indonesia | M.J. Wingfield | DQ204753 | AF309609 | DQ147633 | DQ211669 | ||
| M. intermedia | Unknown | 7163 | CBS 114356 | 10902 | E. saligna | New Zealand | K. Dobbie | DQ246247 | AY725546 | N/A | N/A |
| 7164 | CBS 114415 | 10922 | E. saligna | New Zealand | K. Dobbie | DQ246248 | AY725547 | DQ147627 | DQ235132 | ||
| M. irregulariramosa | Pseudocercospora irregulariramosa | 4943 | CBS 114774 | 1360 | E. saligna | South Africa | M.J. Wingfield | DQ204754 | AF309607 | DQ147634 | DQ211670 |
| 5223 | N/A | 1362 | E. saligna | South Africa | M.J. Wingfield | DQ204755 | AF309608 | DQ147635 | DQ211671 | ||
| M. ohnowa | Unknown | 4937 | CBS 112896 | 1004 | E. grandis | South Africa | M.J. Wingfield | N/A | AF309604 | DQ147662 | DQ235125 |
| 4936 | CBS 112973 | 1005 | E. grandis | South Africa | M.J. Wingfield | DQ246231 | AF309605 | DQ147661 | DQ235124 | ||
| M. keniensis | Unknown | 5147 | CBS 111001 | 1084 | E. grandis | Kenya | T. Coutinho | DQ246259 | AF309601 | DQ147611 | DQ235100 |
| M. lateralis | Dissoconium dekkeri | 14906 | CBS 110748 | 825 | E. grandis × E. saligna | South Africa | G. Kemp | DQ204768 | AF173315 | DQ147651 | DQ211684 |
| 5164 | CBS 111169 | 1232 | E. globulus | Zambia | T. Coutinho | DQ246260 | AY25550 | DQ147652 | DQ235139 | ||
| M. madeirae | Pseudocercospora sp. | 14458 | CBS 112895 | 3745 | E. globulus | Madeira | S. Denman | DQ204756 | AY725553 | DQ147641 | DQ211672 |
| M. marksii | Unknown | 14781 | CBS 682.95 | 842 | E. grandis | South Africa | G. Kemp | DQ246249 | DQ267587 | DQ147624 | DQ235133 |
| 5150 | CBS 110920 | 935 | E. botryoides | Australia | A.J. Carnegie | DQ246250 | AF309588 | DQ147625 | DQ235134 | ||
| 5230 | N/A | 782 | E. botryoides | Australia | A.J. Carnegie | DQ246246 | DQ267588 | DQ147626 | DQ235135 | ||
| M. mexicana | Unknown | 14461 | CBS 110502 | N/A | E. globulus | Australia | A. Maxwell | DQ246237 | AY725558 | DQ147660 | DQ235123 |
| M. readeriellophora | Readeriella readeriellophora | 14233 | CBS 114240 | 10375 | E. globulus | Spain | J.P. Mansilla | DQ246238 | AY725577 | DQ147658 | DQ235117 |
| M. molleriana | Colletogloeopsis molleriana | 4940 | CBS 111164 | 1214 | E. globulus | Portugal | S. McCrae | DQ246220 | AF309620 | DQ147671 | DQ235104 |
| 2734 | CBS 111132 | 784 | E. globulus | U. S. A. | M.J. Wingfield | DQ246223 | AF309619 | DQ147670 | DQ235105 | ||
| M. nubilosa | Uwebraunia juvenis | 3282 | CBS 116005 | 937 | E. globulus | Australia | A.J. Carnegie | DQ246228 | AF309618 | DQ147666 | DQ235111 |
| 9003 | CBS 114708 | N/A | E. nitens | South Africa | G.C. Hunter | DQ246229 | AF449099 | DQ147667 | DQ235112 | ||
| M. parkii | Stenella parkii | 14775 | CBS 387.92 | 353 | E. grandis | Brazil | M.J. Wingfield | DQ246245 | AY626979 | DQ147612 | DQ235137 |
| M. parva | Unknown | 14459 | CBS 110503 | N/A | E. globulus | Australia | A. Maxwell | DQ246243 | AY626980 | DQ147645 | DQ235110 |
| 14917 | CBS 116289 | 10935 | Eucalyptus sp. | South Africa | P.W. Crous | DQ246242 | AY725576 | DQ147642 | DQ235109 | ||
| M. suberosa | Unknown | 5226 | CBS 436.92 | 515 | E. dunnii | Brazil | M.J. Wingfield | DQ246235 | AY626985 | DQ147656 | DQ235101 |
| 7165 | N/A | N/A | E. muelleriana | New Zealand | Unknown | DQ246236 | DQ267589 | DQ147657 | DQ235102 | ||
| M. suttonii | Phaeophleospora epicoccoides | 5348 | N/A | 1346 | Eucalyptus sp. | Indonesia | M.J. Wingfield | DQ246227 | AF309621 | DQ147673 | DQ235116 |
| M. vespa | Colletogloeopsis sp. | 11558 | CBS 117924 | N/A | E. globulus | Tasmania | Unknown | DQ246221 | DQ267590 | DQ147668 | DQ235106 |
| M. tasmaniensis | Passalora tasmaniensis | 14780 | CBS 111687 | 1555 | E. nitens | Tasmania | M.J. Wingfield | DQ246233 | DQ267591 | DQ147676 | DQ235121 |
| 14663 | CBS 114556 | N/A | E. nitens | Tasmania | M.J. Wingfield | DQ246234 | DQ267592 | DQ147677 | DQ235122 | ||
| M. toledana | Phaeophleospora toledana | 14457 | CBS 113313 | N/A | Eucalyptus sp. | Spain | P.W. Crous | DQ246230 | AY725580 | DQ147672 | DQ235120 |
| M. walkerii | Sonderhenia eucalypticola | 20333 | N/A | N/A | E. globulus | Chile | M.J. Wingfield | DQ267574 | DQ267593 | DQ147630 | DQ235095 |
| 20334 | N/A | N/A | E. globulus | Chile | M.J. Wingfield | DQ267575 | DQ267594 | DQ147631 | DQ235096 | ||
| Unknown | Passalora eucalypti | 14907 | CBS 111306 | 1457 | E. saligna | Brazil | P.W. Crous | DQ246244 | AF309617 | DQ147678 | DQ235138 |
| Unknown | Passalora zambiae | 14782 | CBS 112971 | 1227 | E. globulus | Zambia | T. Coutinho | DQ246264 | AF725523 | DQ147675 | DQ235136 |
| Unknown | Pseudocercospora epispermogonia | 14778 | CBS 110750 | 822 | E. grandis × E. saligna | South Africa | G. Kemp | DQ204757 | DQ267596 | DQ147629 | DQ211673 |
| 14786 | CBS 110693 | 823 | E. grandis × E. saligna | South Africa | G. Kemp | DQ204758 | DQ267597 | DQ147628 | DQ211674 | ||
| Unknown | Phaeophleospora eucalypti | 11687 | CBS 113992 | N/A | E. nitens | New Zealand | M. Dick | DQ246225 | DQ267598 | DQ147664 | DQ235115 |
| 14910 | CBS 111692 | 1582 | Eucalyptus sp. | New Zealand | M.J. Wingfield | DQ246224 | DQ267599 | DQ147663 | DQ235114 | ||
| Unknown | Pseudocercospora basitruncata | 14914 | CBS 114664 | 1202 | E. grandis | Colombia | M.J. Wingfield | DQ204759 | DQ267600 | DQ147622 | DQ211675 |
| 14785 | CBS 111280 | 1203 | E. grandis | Colombia | M.J. Wingfield | DQ204760 | DQ267601 | DQ147621 | DQ211676 | ||
| Unknown | Pseudocercospora basiramifera | 5148 | N/A | N/A | E. pellita | Thailand | M.J. Wingfield | DQ204761 | AF309595 | DQ147607 | DQ211677 |
| Unknown | Pseudocercospora eucalyptorum | 5228 | CBS 110777 | 16 | E. nitens | South Africa | P.W. Crous | DQ204762 | AF309598 | DQ147614 | DQ211678 |
| Unknown | Pseudocercospora natalensis | 14777 | CBS 111069 | 1263 | E. nitens | South Africa | T. Coutinho | DQ267576 | N/A | DQ147620 | N/A |
| 14784 | CBS 111070 | 1264 | E. nitens | South Africa | T. Coutinho | DQ204763 | AF309594 | DQ147623 | DQ211679 | ||
| Unknown | Pseudocercospora paraguayensis | 14779 | CBS 111286 | 1459 | E. nitens | Brazil | P.W. Crous | DQ204764 | DQ267602 | DQ147606 | DQ211680 |
| Unknown | Pseudocercospora pseudoeucalyptorum | 14908 | CBS 114242 | 10390 | E. globulus | Spain | J.P. Mansilla | DQ204765 | AY725526 | DQ147613 | DQ211681 |
| 14911 | CBS 114243 | 10500 | E. nitens | New Zealand | W. Gams | DQ204766 | AY725527 | DQ147615 | DQ211682 | ||
| Unknown | Pseudocercospora robusta | 5151 | CBS 111175 | 1269 | E. robusta | Malaysia | M.J. Wingfield | DQ204767 | AF309597 | DQ147617 | DQ211683 |
| Unknown | Readeriella novaezelandiae | 14913 | CBS 114357 | 10895 | E. botryoides | New Zealand | M.A. Dick | DQ246239 | DQ267603 | DQ147659 | DQ235118 |
| Botryosphaeria ribis | Fusicoccum ribis | 7773 | N/A | N/A | Ribus sp. | U. S. A. | G. Hudler. | DQ246263 | DQ267604 | DQ267605 | DQ235142 |
CMW: Culture collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, South Africa.
CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
STEU: Culture collection of Stellenbosch University, South Africa. Isolate numbers from Crous (1998).
N/A: Not available
DNA isolation
Mycelium from actively growing cultures was scraped from the surface of cultures, freeze-dried for 24 h and then ground to a fine powder using liquid nitrogen. DNA was isolated using the phenol: chloroform (1: 1) extraction protocol as described in Hunter et al. (2004a, b). DNA was precipitated by the addition of absolute ethanol (100 % EtOH). Isolated DNA was cleaned by washing with 70 % Ethanol (70 % EtOH) and dried under vacuum. SABAX water was used to resuspend the isolated DNA. RNaseA (10 μg/μL) was added to the resuspended DNA and incubated at 37 °C for approximately 2 h to digest any residual RNA. Isolated DNA was visualised in a 1 % agarose gel (w/v) (Roche Diagnostics, Mannheim), stained with ethidium bromide and visualised under ultra-violet light.
PCR amplification and purification
DNA (ca. 20 ng) isolated from the Mycosphaerella spp. used in this study was used as a template for amplification using the Polymerase Chain Reaction (PCR). All PCR reactions were mixed in a total volume of 25 μL containing 10× PCR Buffer (5 mM Tris-HCl, 0.75 mM MgCl2, 25 mM KCl, pH 8.3) (Roche Diagnostics, South Africa), 2.5 mM of each dNTP (dATP, dTTP, dCTP, dGTP) (Roche Diagnostics, South Africa), 0.2 μM of forward and reverse primers (Inqaba Biotech, South Africa) and 1.25 U Taq DNA Polymerase (Roche Diagnostics, South Africa) and DNA (20 ng/μL). Sterilised distilled water was added to obtain a final volume of 25 μL.
The ITS-1, ITS-2 and the 5.8 S gene regions of the ITS region of the rRNA operon were amplified using primers ITS-1 (5′– TCC GTA GGT GAA CCT GCG G –3′) and LR-1 (5′- GGT TGG TTT CTT TTC CT – 3′) (Vilgalys & Hester 1990, White et al. 1990). Reaction conditions for the ITS gene regions followed those of Crous et al. (2004a) and Hunter et al. (2004a, b).
A portion of the LSU (including domains D1–D3) of the rRNA operon was amplified using primers LR0R (5'-ACC CGC TGA ACT TAA GC-3') (Moncalvo et al. 1995) and LR7 (5'-TAC TAC CAC CAA GAT CT-3') (Vilgalys & Hester 1990). PCR cycling conditions were as follows: an initial denaturation step of 96 °C for 2 min, followed by 35 cycles of denaturation at 94 °C for 30 s, primer annealing at 62 °C for 30 s, primer extension at 72°C for 1 min and a final elongation step at 72 °C for 7 min.
A portion of the EF-1α was amplified using the primers EF1-728F (5'-CAT CGA GAA GTT CGA GAA GG-3') and EF1-986R (5'-TAC TTG AAG GAA CCC TTA CC-3') (Carbone & Kohn 1999). Reaction conditions were: an initial denaturation step of 96 °C for 2 min, followed by 35 cycles of denaturation at 94 °C for 30 s, primer annealing at 56 °C for 30 s and primer extension at 72 °C for 30 s. The reaction was completed with a final extension at 72 °C for 7 min.
A portion of the ACT gene was amplified using the primers ACT-512F (5'-ATG TGC AAG GCC GGT TTC GC-3') and ACT-783R (5'-TAC GAG TCC TTC TGG CCC AT-3') (Carbone & Kohn 1999). PCR reaction conditions were: an initial denaturation step at 96 °C for 2 min, followed by 10 cycles of denaturation at 94 °C for 30 s, primer annealing at 61 °C for 45 s and extension at 72 °C for 45 s. This was followed by 25 cycles of denaturation at 94 °C for 30 s, primer annealing at 61 °C and elongation at 72 °C for 45 s with an increase of 5 s per cycle. The reaction was completed with a final elongation step at 72 °C for 7 min.
All PCR products were visualised in 1.5 % agarose gels (wt/v) stained with ethidium bromide and viewed under ultra-violet light. Sizes of PCR amplicons were estimated by comparison against a 100 bp molecular weight marker (O' RangeRuler™ 100 bp DNA ladder) (Fermentas Life Sciences, U.S.A.). Prior to DNA sequencing, PCR products were purified through Centrisep spin columns (Princeton Separations, Adelphia, NJ) containing Sephadex G-50 (Sigma Aldrich, St. Louis, MO) as outlined by the manufacturer.
DNA sequencing and phylogenetic analysis
Purified PCR products were used as template DNA for sequencing reactions on an ABI PRISM™ 3100 Automated DNA sequencer (Applied Biosystems, Foster City, CA). The ABI Prism Big Dye Terminator Cycle sequencing reaction kit v. 3.1 (Applied Biosystems, Foster City, CA) was used for sequencing reactions following the manufacturer's instructions. Most sequencing reactions were performed with the same primers used for PCR reactions. Exceptions were in the case of the ITS region where two internal primers ITS-2 (5'-GCT GCG TTC TTC ATC GAT GC-3') and ITS-3 (5'-GCA TCG ATG AAG AAC GCA GC-3') (White et al. 1990) were included for the sequencing reactions. Similarly, for the LSU region two internal primers LR3R (5'-GTC TTG AAA CAC GGA CC-3') and LR-16 (5'-TTC CAC CCA AAC ACT CG-3') were used for the sequencing reactions.
All resulting sequences were analysed with Sequence Navigator v. 1.0.1 (Applied Biosystems, Foster City, CA). Sequence alignments were done using MAFFT (Multiple alignment program for amino acid or nucleotide sequences) v. 5.667 (Katoh et al. 2005) and manually adjusted where necessary. Phylogenetic analyses and most parsimonious trees were generated in PAUP v. 4.0b10 (Swofford 2002) by heuristic searches with starting trees obtained through stepwise addition with simple addition sequence and with the MULPAR function enabled. Tree Bisection Reconnection (TBR) was employed as the swapping algorithm. All gaps were coded as missing data and characters were assigned equal weight. Branch support for nodes was obtained by performing 1000 bootstrap replicates of the aligned sequences. For parsimony analyses, measures that were calculated include tree length (TL), retention index (RI), consistency index (CI), rescaled consistency index (RC) and homoplasy index (HI). Botryosphaeria ribis Grossenb. & Duggar was used as the outgroup to root all trees.
A Partition Homogeneity Test (Farris et al. 1994), of all possible combinations, consisting of 1000 replicates on all informative characters was conducted in PAUP to determine if the LSU, ITS and EF-1α data sets were combinable. All sequences of Mycosphaerella spp. used in this study have been deposited in GenBank (Table. 1). Sequence alignments and trees of the LSU, ITS, EF-1α and ACT have been deposited in TreeBASE (accession numbers: LSU = SN2535, ITS = SN2534, EF-1α = SN2536, ACT = SN2537).
Parsimony and distance analyses of combined DNA sequence alignments were conducted in PAUP. Parsimony analyses of all DNA sequence alignments were identical to those described earlier. For distance analyses, Modeltest v. 3.04 (Posada & Crandall 1998) was used to determine the best evolutionary model to fit the combined DNA sequence alignment. A neighbour-joining analysis with an evolutionary model was conducted in PAUP. Here, the distance measure was a general time-reversible (GTR) and the proportion of sites assumed to be invariable (I) was 0.4919, identical sites were removed proportionally to base frequencies estimated from all sites, rates of variable sites assumed to follow a gamma distribution (G) with shape parameter of 0.6198. Ties (if encountered) were broken randomly.
RESULTS
DNA sequencing and phylogenetic analysis
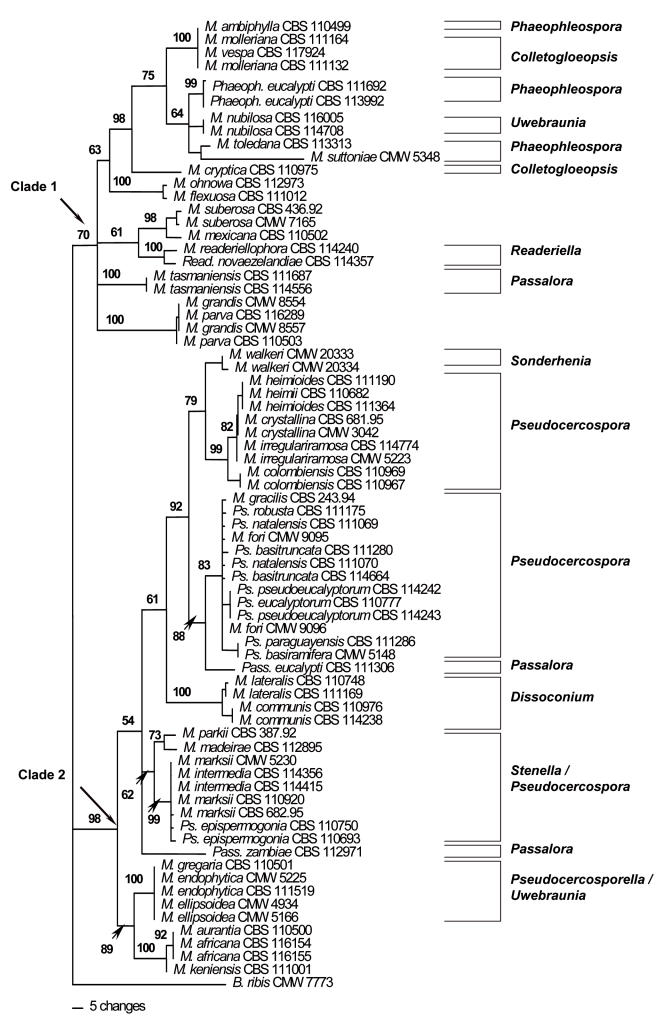
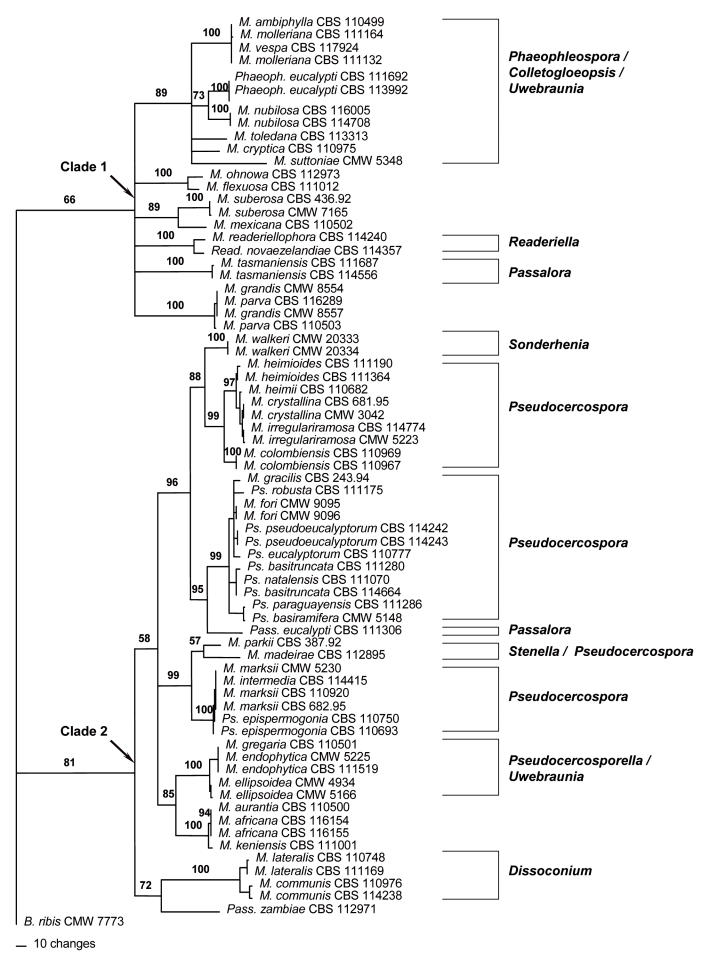
Large Subunit (LSU) phylogeny: The LSU alignment had a total length of 1714 characters. An indel of 383 bp present in M. ohnowa Crous & M.J. Wingf. (CBS 112973) and Mycosphaerella mexicana Crous (CBS 110502) was excluded from the analyses. In the LSU data set, 1075 characters were constant while 77 characters were parsimony-uninformative and 179 characters were parsimony-informative. Parsimony analysis of the LSU data set resulted in the retention of thirty most parsimonious trees (TL = 663, CI = 0.519, RI = 0.878, RC = 0.456). One of these trees (Fig. 1) could be resolved into two clades (Clades 1–2). Clade 1, supported with a bootstrap value of 70 %, included Mycosphaerella isolates characterised by Phaeophleospora Rangel (M. ambiphylla A. Maxwell, M. suttoniae Crous & M.J. Wingf.), Colletogloeopsis Crous & M.J. Wingf. [M. molleriana (Thüm.) Lindau, M. vespa Carnegie & Keane, M. cryptica (Cooke) Hansf.], Uwebraunia Crous & M.J. Wingf. [M. nubilosa (Cooke) Hansf.], M. ohnowa, Readeriella Syd. & P. Syd. (M. readeriellophora Crous & J.P. Mansilla), and Passalora Fr. (M. tasmaniensis Crous & M.J. Wingf.) anamorphs.
Fig. 1.
Phylogram obtained from the Large Subunit (LSU) rDNA sequence alignment of Mycosphaerella spp. occurring on Eucalyptus leaves showing two well-supported main clades (Clades 1–2). Tree length = 663, CI = 0.519, RI = 0.878, RC = 0.456. Bootstrap values based on 1000 replicates are indicated above branches. Anamorph affinities are indicated next to the vertical lines.
The second major clade (Clade 2) resolved in the LSU tree was well-supported with a bootstrap value of 98 %. Mycosphaerella species in this clade also grouped strongly following their anamorph associations. Here Mycosphaerella isolates could be resolved into several sub-clades also characterised by their anamorph associations. These were Sonderhenia (M. walkeri R.F. Park & Keane.), Pseudocercospora Speg. [M. heimioides Crous & M.J. Wingf., M. heimii Crous, M. crystallina Crous & M.J. Wingf., M. irregulariramosa Crous & M.J. Wingf., M. colombiensis Crous & M.J. Wingf., M. gracilis Crous & Alfenas, Pseudocercospora robusta Crous & M.J. Wingf., Ps. natalensis Crous & T. Coutinho, M. fori G.C. Hunter, Crous & M.J. Wingf., Ps. basitruncata Crous, Ps. pseudoeucalyptorum Crous, Ps. eucalyptorum Crous, M.J. Wingf., Marasas & B. Sutton., Ps. paraguayensis (Koboyashi) Crous, Ps. basiramifera Crous] Passalora [Pass. eucalypti (Crous & Alfenas) Crous & U. Braun, Pass. zambiae Crous & T. Coutinho], and Dissoconium (M. lateralis Crous & M.J. Wingf., M. communis Crous & J.P. Mansilla).
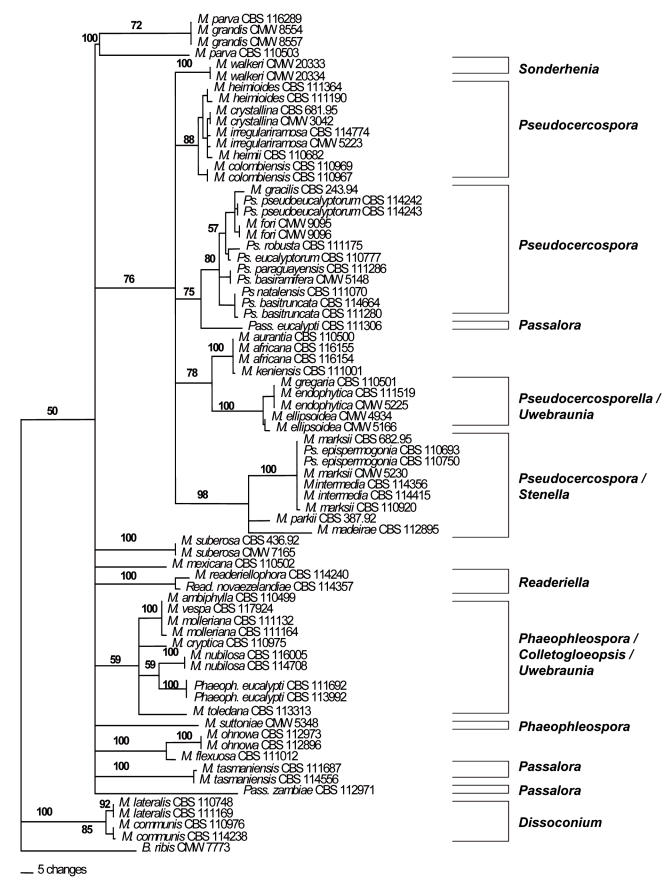
Internal Transcribed Spacer Region (ITS) phylogeny: The ITS sequence alignment consisted of a total of 793 characters. Of these 499 characters were constant, 62 characters were variable and parsimony-uninformative and 232 characters were parsimony-informative. A 185 bp indel was observed in isolates of M. gregaria Carnegie & Keane (CBS 110501), M. endophytica Crous & H. Smith (CBS 111519) and M. endophytica (CMW 5225) and was excluded in the phylogenetic analysis.
A heuristic search of the ITS data set resulted in the retention of four most parsimonious trees (TL = 871, RI = 0.782, CI = 0.358, RC = 0.280). One of these phylogenetic trees (Fig. 2) generated by parsimony analysis of the ITS alignment could be resolved into two monophyletic clades (Clades 1–2). Clade 1 was only weakly supported with a bootstrap value of 50 % after 1000 bootstrap replicates. Clade 1 could be further resolved into several smaller sub-clades where isolates grouped strongly based on their anamorph affiliations. These included Sonderhenia, Pseudocercospora, Passalora, Uwebraunia/Pseudocercosporella, Stenella, Readeriella, Phaeophleospora and Colletogloeopsis. The second monophyletic clade (Clade 2) grouped sister to the first larger monophyletic clade and contained isolates of M. lateralis and M. communis (Dissoconium anamorphs). This clade was well-supported with a bootstrap value of 100 % after 1000 bootstrap replicates.
Fig. 2.
Phylogram obtained from the Internal Transcribed Spacer (ITS) DNA sequence alignment of Mycosphaerella spp. occurring on Eucalyptus leaves indicating two monophyletic clades (Clades 1–2). Tree length = 871, CI = 0.358, RI = 0.782, RC = 0.280.
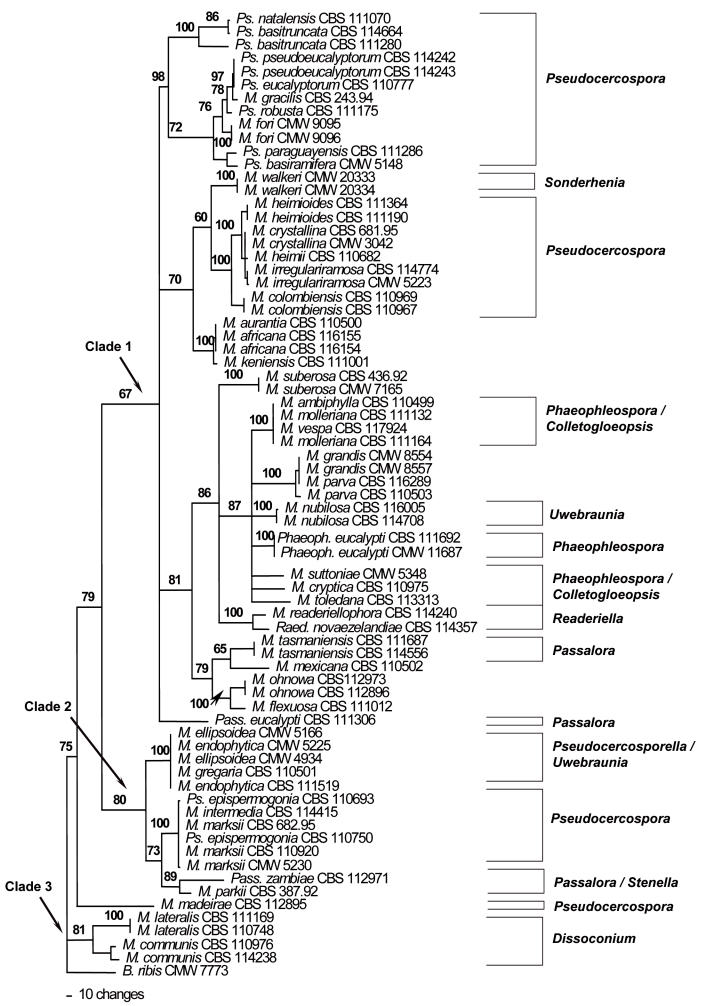
Translation Elongation factor 1-alpha (EF -1α) phylogeny: The EF-1α alignment contained 373 characters. Of these, 41 characters were constant, 23 characters were variable and parsimony-uninformative and 309 characters were parsimony-informative. Heuristic searches resulted in the retention of six most parsimonious trees (TL = 3194, RI = 0.777, CI = 0.345, RC = 0.268), one of which is shown (Fig. 3). Species of Mycosphaerella could be resolved into three clades (Clades 1–3).
Fig. 3.
Phylogram obtained from the Elongation factor 1-alpha (EF-1α) DNA sequence alignment of Mycosphaerella spp. occurring on Eucalyptus leaves showing three main clades. Tree length = 3194, CI = 0.345, RI = 0.777, RC = 0.268.
Clade 1 was weakly supported with a bootstrap value of 67 %. This clade contained Mycosphaerella isolates represented by Pseudocercospora, Sonderhenia, Phaeophleospora, Colletogloeopsis, Uwebraunia, Readeriella and Passalora anamorphs. Clade 2 was sister to Clade 1 and had a higher bootstrap support of 80 %. Within this clade, Mycosphaerella isolates could be separated into three sub-clades that were well-supported. These three sub-clades contained species of Mycosphaerella that produced Pseudocercosporella, Uwebraunia, Pseudocercospora, Passalora and Stenella anamorphs. Clade 3 with bootstrap support of 80 % included isolates of M. lateralis and M. communis and was basal to Clades 1 and 2.
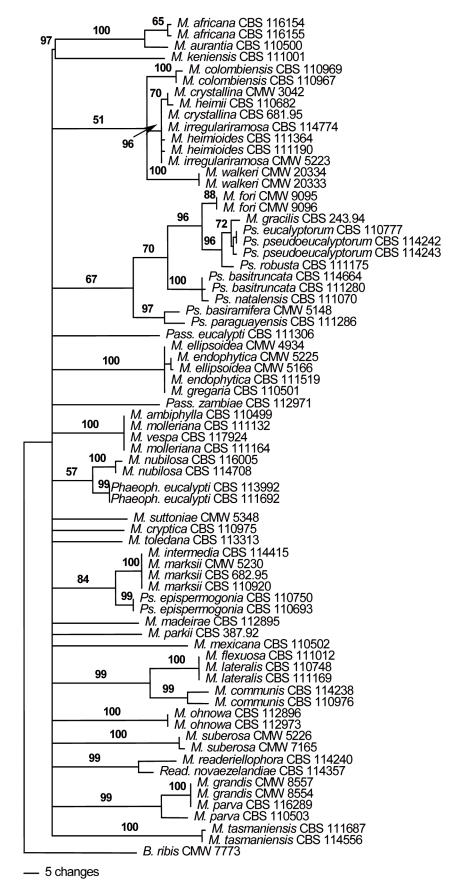
Actin (ACT) phylogeny: The aligned ACT sequence dataset contained a total of 294 characters. Of these, 135 characters were constant, 30 characters were variable and parsimony-uninformative and 129 characters were parsimony-informative. Heuristic searches of the aligned ACT dataset resulted in the retention of six most parsimonious trees (TL = 1007, RI = 0.682, CI = 0.235, RC = 0.160). One of these trees, shown in Fig. 4, was very poorly resolved and all deeper nodes were present in a basal polytomy. However, certain smaller clades were resolved and these included a clade including M. fori, M. gracilis, Ps. eucalyptorum, Ps. pseudoeucalyptorum, Ps. robusta, Ps. basitruncata, Ps. natalensis, Ps. basiramifera and Ps. paraguayensis. This clade was supported with a bootstrap value of only 67 %. Another clade supported with a bootstrap value of 100 % contained isolates of M. ellipsoidea Crous & M.J. Wingf., M. endophytica and M. gregaria. Isolates of M. ambiphylla, M. molleriana and M. vespa also clustered together with 100 % bootstrap support. Isolates of M. intermedia M.A. Dick & Dobbie, M. marksii Carnegie & Keane and Pseudocercospora epispermogonia Crous & M. J. Wingf. grouped together in a clade that was supported with a bootstrap value of 84 %. Isolates of M. flexuosa Crous & M.J. Wingf., M. lateralis and M. communis were also accommodated in a well-supported clade with a bootstrap value of 99 %. Isolates of M. grandis Carnegie & Keane and M. parva R.F. Park & Keane were also resolved into a clade with a bootstrap value of 99 %.
Fig. 4.
Phylogram obtained from the Actin (ACT) DNA sequence alignment of Mycosphaerella spp. occurring on Eucalyptus leaves. Tree length = 1007, CI = 0.235, RI = 0.682, RC = 0.160.
Phylogeny of combined data set: A partition homogeneity test of the combined LSU, ITS and EF-1α alignment conducted in PAUP resulted in a P-value of 0.001 for all possible combinations of the LSU, ITS and EF-1α DNA alignments. This value is less than the conventionally accepted P-value of P > 0.05 required to combine data. However, several studies have accepted a P-value of 0.001 or greater and have further stated that the conventional P-value of 0.005 is inordinately conservative (Cunningham 1997, Darlu & Lecointre 2002, Dettman et al. 2003). Thus, the LSU, ITS and EF-1α DNA sequence data sets were combined. The ACT dataset was omitted from the combined data set due to the lack of resolution among species of Mycosphaerella. Therefore, the combined LSU, ITS and EF-1α data set had a total length of 2880 characters. Of these, 1459 were constant, 150 were variable and parsimony-uninformative and 701 characters were parsimony-informative. An indel of 382 bp was excluded for M. ohnowa CBS 112973 and M. mexicana CBS 110502 and another indel of 186 bp was excluded for M. gregaria CBS 110501 and M. endophytica CMW 5225 and CBS 111519. A parsimony analysis resulted in the retention of ten most parsimonious trees (TL = 1677, CI = 0.384, RI = 0.817, RC = 0.314, HI = 0.616). One of these trees (Fig. 5) exhibited a similar topology to that obtained from the LSU alignment. From the analysis of the combined data set, isolates of Mycosphaerella could again be resolved into two clades (Clades 1–2) (Fig. 5). Clade 1 was poorly supported with a bootstrap value of only 66 % and the same isolates were contained in this clade as in the LSU Clade 1 (Fig. 1). Clade 2 of the combined phylogenetic tree was well-supported with a bootstrap value of 81 %. This clade could be further resolved into several smaller well-supported sub-clades containing Mycosphaerella isolates that grouped according to their anamorph associations (Fig. 5). Neighbour-joining analysis yielded a phylogenetic tree with the same topology as the most parsimonious trees (data not shown). Here, all Mycosphaerella spp. could be resolved into two main clades (Clade 1–2), similar to those of the parsimony analysis (Fig. 5). Mycosphaerella spp. could be further sub-divided into several sub-clades corresponding to their anamorph associations, similar to those observed for the parsimony analysis.
Fig. 5.
Phylogram obtained from the combined LSU, ITS and EF-1α DNA sequence alignment of Mycosphaerella spp. occurring on Eucalyptus leaves showing two main clades. Tree length = 1677, CI = 0.384, RI = 0.817, RC = 0.314.
DISCUSSION
Results of this study represent the first attempt to employ DNA sequence data from a relatively large number of nuclear gene regions in order to consider the phylogenetic relationships for Mycosphaerella spp. occurring on Eucalyptus leaves. Other similar studies have relied entirely on sequence data of the ITS region (Crous et al. 1999, 2001, 2004a, and 2006 – this volume, Hunter et al. 2004b). Although the ITS region offers sufficient resolution to distinguish most taxa, it has not been adequate to separate cryptic taxa in Mycosphaerella (Crous et al. 2004b). Results of the present study showed that combined DNA sequence data from the LSU, ITS, EF-1α gene regions offer increased genetic resolution to study species concepts in Mycosphaerella. However, genes such as the ACT, did not support data emerging from the other loci sequenced, and indicated variation within some clades that were well supported by sequences of other loci and morphological characteristics. These observations led us to exclude ACT data from the final analyses. A similar finding has also emerged from other studies including greater numbers of Mycosphaerella species (Crous & Groenewald, unpubl. data).
Mycosphaerella ambiphylla, M. molleriana and M. vespa grouped together in a well-supported clade in the phylogeny emerging from the combined alignment. This was also true for the ITS, EF-1α and ACT phylogenies where these isolates grouped in a distinct clade with a 100 % bootstrap support. Mycosphaerella molleriana and M. vespa both have Colletogloeopsis anamorphs, however, M. ambiphylla produces a Phaeophleospora anamorph (Crous & Wingfield 1997a, Maxwell et al. 2003). Interestingly, the Phaeophleospora anamorph of M. ambiphylla was differentiated from Colletogloeopsis only by the fact that conidia are produced in a pycnidium as opposed to an acervulus (Maxwell et al. 2003). Application of conidiomatal structure to differentiate anamorphs of Mycosphaerella has previously been viewed with circumspection especially because Mycosphaerella anamorphs can produce different conidiomatal forms under differing environmental conditions (Crous et al. 2000, Cortinas et al. 2006 – this volume). Therefore, the placement of the M. ambiphylla anamorph in Phaeophleospora is questioned and it should be re-evaluated in terms of its morphological similarities to Colletogloeopsis.
Ascospore germination patterns of M. ambiphylla, M. molleriana and M. vespa are all similar, with germ tubes that grow parallel to the long axis of the spore and ascospores with a slight constriction at the median septum, typical of a type C ascospore germination pattern (Crous 1998, Carnegie & Keane 1998, Maxwell et al. 2003). Furthermore, overlap is seen in ascospore dimensions of the three species where those of M. molleriana are (11–)12–14(–17) × (2.5–)3.5–4(–4.5) μm, those of M. ambiphylla are (12–)14–15(–22) ×(3.5–)4.5–5(–6) μm and those of M. vespa 9.5–16.5 × 2.5–4 μm (Crous 1998, Carnegie & Keane 1998, Maxwell et al. 2003). Leaf lesions of the three species are also similar, pale brown to dark red-brown with lesions of M. vespa and M. ambiphylla often producing a red margin that was, however, not observed in M. molleriana (Crous 1998, Carnegie & Keane 1998, Maxwell et al. 2003). Morphological features of M. ambiphylla, M. molleriana and M. vespa are also very similar. This is supported in the DNA phylogeny of the present study where these three species appear to represent a single taxon and therefore suggest that M. ambiphylla, M. molleriana and M. vespa should be synonomised under M. molleriana, which is the oldest epithet. We therefore reduce M. ambiphylla and M. vespa to synonymy with M. molleriana as follows:
Mycosphaerella molleriana (Thüm.) Lindau, Natürliche Pfanzenfamilie, 1: 424. 1897.
≡ Sphaerella molleriana Thüm., Revista Inst. Sci. Lit. Coimbra 28: 31. 1881.
= Mycosphaerella vespa Carnegie & Keane, Mycol. Res. 102: 1275. 1998.
= Mycosphaerella ambiphylla A. Maxwell, Mycol. Res. 107: 354. 2003.
Anamorph: Colletogloeopsis molleriana Crous & M.J. Wingf., Canad. J. Bot. 75: 670. 1997.
Mycosphaerella flexuosa has no known anamorph (Crous 1998). An isolate of this fungus included in the present study grouped together with isolates of M. ohnowa in the LSU, ITS, EF-1α and combined data set with high bootstrap support. This similarity was also observed in a recent study of Mycosphaerella spp. on Eucalyptus based on ITS sequence data (Crous et al. 2004a). Mycosphaerella ohnowa is also not known to produce an anamorph (Crous et al. 2004a). Although these two species are phylogenetically similar, they can be distinguished from one another based on different ascus and ascospore dimensions, ascospore germination patterns and cultural characteristics (Crous 1998, Crous et al. 2004a). Although morphologically distinct, it is interesting that these two taxa are phylogenetically so closely related and might suggest a recent speciation event.
Isolates of M. grandis and M. parva consistently grouped together in a separate clade in all of the DNA sequence data sets in this study. This has also been shown by Crous et al. (2004a), where isolates of these two species grouped together in a distinct clade based on ITS DNA sequences. Mycosphaerella grandis was originally described from E. grandis in Australia, and recognised as a distinct species of Mycosphaerella due to its lesion characteristics, and ascospore morphology (Carnegie & Keane 1994). However, Crous (1998) examined the type of M. grandis and M. parva and found the two species to be congeneric, and reduced them to synonymy under M. parva. Results from the present study support the synonymy.
Mycosphaerella lateralis and M. communis, both known to have Dissoconium anamorphs, showed various phylogenetic placements in this study. From the LSU phylogeny, M. lateralis and M. communis were situated within a large Mycosphaerella clade sister to a Pseudocercospora sub-clade. However, in the ITS and EF-1α phylogenies the Dissoconium clade was situated basal to the larger Mycosphaerella clade. This is consistent with findings of Crous et al. (1999, 2000) where the Dissoconium clade also resided outside the larger monophyletic Mycosphaerella clade. The LSU gene region is well-known to be conserved and to show less nucleotide differences than the ITS and EF-1α gene regions. Although the house-keeping genes investigated here lead to the conclusion that Dissoconium could be different from Mycosphaerella s. str., this proved not to be the case when LSU data were considered. Dissoconium is morphologically identical to Uwebraunia, and the separation of these two genera no longer seems tenable. Only two species, M. ellipsoidea and M. nubilosa, have Uwebraunia anamorphs (Crous et al. 2004a). However, cultures of both species produced these anamorphs only upon initial isolation, and those that are currently available are sterile. In contrast, strains with Dissoconium anamorphs readily produce those in culture, and they usually sporulate profusely. It appears that the status of Uwebraunia will only be resolved once fresh, sporulating collections of either M. ellipsoidea or M. nubilosa can be obtained.
Mycosphaerella spp. with Pseudocercospora anamorphs grouped into three clades in all of the phylogenies generated in this study. Species in the Pseudocercospora clades have short branch lengths arising from a common internode, suggesting that they have speciated relatively recently from a common ancestor (Ávila et al. 2005) and, most likely have co-evolved with their Eucalyptus hosts as suggested by Crous et al. (2000). Ávila et al. (2005) suggested that Pseudocercospora may represent a monophyletic lineage. But, results of this and other studies (Ayala-Escobar et al. 2006) have shown that Pseudocercospora is paraphyletic in Mycosphaerella and has evolved more than once in the genus. The availability of new DNA datasets for several gene regions are likely to resolve cryptic species and species complexes within Pseudocercospora, as has already been shown for the M. heimii and the P. eucalyptorum species complexes (Crous et al. 2000, 2004a).
Mycosphaerella heimioides, M. heimii, M. crystallina and M. irregulariramosa are all morphologically similar and are regarded as members of the M. heimii species complex (Crous & Wingfield 1997b, Crous et al. 2001). Previous studies based on ITS DNA sequence data have demonstrated the phylogenetic relatedness of these four species (Crous et al. 2001, Crous et al. 2004a). However, bootstrap support for their phylogenetic placement was low (Crous et al. 2004a). The phylogeny of combined DNA sequence data in this study showed that the four species in the M. heimii complex reside in a well-supported clade (bootstrap support 97 %). The short branch lengths indicate that the four species have also recently diverged from a common ancestor.
In the phylogeny based on the combined sequence data sets, M. gracilis grouped in a well-supported Pseudocercospora clade that also included isolates of Ps. robusta, M. fori, Ps. pseudoeucalyptorum, Ps. eucalyptorum, Ps. basitruncata, Ps. natalensis, Ps. paraguayensis and Ps. basiramifera. This is the first study in which DNA sequence data for M. gracilis have been incorporated into a phylogeny. In the ITS, EF-1α and ACT phylogenies, M. gracilis was phylogenetically most closely related to Ps. pseudoeucalyptorum. However, M. gracilis (anamorph: Pseudocercospora gracilis Crous & Alfenas) can be distinguished from Ps. pseudoeucalyptorum by its single conidiophores arising exclusively from secondary mycelium, which is different to Ps. pseudoeucalyptorum in which conidiophores arise from loose or dense fascicles of a stroma (Crous 1998, Crous et al. 2004a). Furthermore, conidia of Ps. gracilis are more septate, longer, and more uniformly cylindrical in shape than those of Ps. pseudoeucalyptorum (Crous 1998, Crous et al. 2004a). Results of the present study clearly emphasise the fact that species which are morphologically distinct, can be very closely related.
An interesting result emerging from the phylogenetic analyses in this study was the placement of Pseudocercospora epispermogonia in relation to Mycosphaerella marksii and Mycosphaerella intermedia. Sequences for all but the ACT gene region showed that these three taxa represent the same phylogenetic species. Although it has previously been suggested that M. marksii should have a Stenella anamorph because of its proximity to M. parkii (Crous et al. 2001), the current data suggest that this anamorph could be Ps. epispermogonia. Crous & Wingfield (1996) described Ps. epispermogonia from spermatogonia on lesions colonised by M. marksii, but failed to link the two states because single-ascospore cultures did not form an anamorph in culture. Mycosphaerella intermedia is morphologically similar to M. marksii, and probably represents the same taxon. We therefore reduce M. intermedia to synonymy with M. marksii as follows:
Mycosphaerella marksii Carnegie & Keane, Mycol. Res. 98: 413–416. 1994.
= Mycosphaerella intermedia M. A. Dick & Dobbie, New Zealand J. Bot. 39: 270. 2001.
Anamorph: Pseudocercospora epispermogonia Crous & M.J. Wingf., Mycologia 88: 456. 1996.
Mycosphaerella africana, M. aurantia and M. keniensis have no known anamorphs. Previous studies based on ITS sequence data have suggested that M. africana and M. keniensis grouped close to Mycosphaerella spp. with Passalora anamorphs. It has thus been assumed that M. africana and M. keniensis would have Passalora anamorphs if they were to be found (Crous et al. 2000). However, the phylogenies emerging from LSU, ITS and EF-1α sequences and the combined data for the three regions showed that M. africana, M. keniensis and M. aurantia consistently group separately from the Passalora anamorphs, close to a clade of isolates with Uwebraunia and Pseudocercosporella anamorphs. The association of these three taxa to Passalora is thus doubted. Furthermore, the clade containing M. africana, M. aurantia and M. keniensis is also well-supported and seems to represent a single evolving lineage.
Moreover, results of the present study show that M. aurantia and M. africana represent a single phylogenetic species. These two species consistently grouped together in all phylogenies with M. keniensis grouping as a sister. Mycosphaerella aurantia was described from leaves of E. globulus in south-western Australia and is known only from this location (Maxwell et al. 2003). Morphologically, M. aurantia produces asci and ascospores that are similar in size and morphology to M. africana. However, the ascospores of M. aurantia are not constricted at the median septum whereas those of M. africana had such constrictions, and ascospores of M. aurantia are longer (9–)11–12(–15) μm than those of M. africana (7–)8–10(–11) μm (Crous 1998, Maxwell et al. 2003). Furthermore, M. aurantia produces lateral hyaline germ tubes that grow parallel to the long axis of the ascospore and become slightly verrucose to produce lateral branches upon prolonged incubation (Maxwell et al. 2003). This is in contrast to ascospores of M. africana that germinate in an irregular fashion producing distinctly dark verrucose germ tubes from different positions of the distorted ascospore (Crous 1998). It is intriguing that these two species, which are morphologically quite distinct, would represent a single phylogenetic species. Additional isolates of these species are required to determine whether they represent two distinct taxa or are conspecific.
Mycosphaerella gregaria was described from leaves of E. grandis in Victoria, Australia (Carnegie & Keane 1997). No anamorph has been observed for this species (Carnegie & Keane 1997, Crous 1998). An isolate of M. gregaria, collected from E. globulus in Australia, consistently grouped in a clade with isolates of M. endophytica and M. ellipsoidea. Mycosphaerella endophytica and M. ellipsoidea are known to have Pseudocercosporella and Uwebraunia anamorphs, respectively (Crous 1998). Based on previous studies employing ITS sequence data, isolates of M. endophytica grouped sister to isolates of M. aurantia, M. ellipsoidea and M. africana (Crous et al. 2004a). However, based on sequence data from the four gene regions employed in this study, isolates of M. endophytica grouped in a distinct well-supported clade with M. ellipsoidea. This is interesting because M. ellipsoidea has an Uwebraunia anamorph (Crous & Wingfield 1996). Mycosphaerella endophytica and M. pseudoendophytica Crous & G. Hunter are the only Mycosphaerella spp. occurring on Eucalyptus that are known to have Pseudocercosporella anamorphs (Crous 1998, Crous et al. 2006 – this volume).
Phylogenies emerging from analyses of sequences for the four gene regions considered in this study suggest that Mycosphaerella constitutes heterogenous groups of which only a few are closely linked to certain anamorph genera. It is evident that for the larger part the evolution of the anamorph genera within Mycosphaerella has been polyphyletic, and not monophyletic as previously suggested. This can be seen by the multiple evolution of anamorph genera such as Passalora, Pseudocercospora, Phaeophleospora and Stenella within Mycosphaerella (Crous et al. 2006). It would thus not be advisable to predict anamorph relationships based on the phylogenetic position within Mycosphaerella. Not only has the same morphology evolved more than once in the group, but disjunct anamorph morphologies also frequently cluster together (Crous et al. 2000, 2004a, 2006). This makes the interpretation difficult, and predictions based on position in clades unreliable.
The production of four nucleotide sequence data sets for species of Mycosphaerella occurring on Eucalyptus leaves should serve as a framework for the more accurate taxonomic placement of these fungi in future. The importance of species complexes in Mycosphaerella has become more evident in this genus in recent years (Crous et al. 2004a, b, 2006 – this volume). To study species complexes, variable gene regions must be studied and the generation of greater numbers of data sets should allow for increased resolution at the species level. This in turn will aid in the resolution of species complexes and cryptic speciation. Studies of the deeper branches for groups in Mycosphaerella can in future utilise sequence data for the LSU region that have not previously been available. These should provide a more lucid indication and support for phenotypic characters that are phylogenetically informative.
Acknowledgments
We thank the members of the Tree Protection Co-operative Programme (TPCP), the National Research Foundation (NRF), the Mellon Foundation and the THRIP initiative of the Department of Trade and Industry, South Africa for financial support.
References
- Ayala-Escobar V, Yáñez-Morales M de Jesús, Braun U, Groenewald JZ, Crous PW (2006). Pseudocercospora opuntiae sp. nov. the causal organism of cactus leaf spot in Mexico. Fungal Diversity 21: 1–9. [Google Scholar]
- Ávila A, Groenewald JZ, Trapero A, Crous PW (2005). Characterisation and epitypification of Pseudocercospora cladosporioides, the causal organism of Cercospora leaf spot of olives. Mycological Research 109: 881–888. [DOI] [PubMed] [Google Scholar]
- Braun U, Crous PW, Dugan F, Groenewald JZ, De Hoog GS (2003) Phylogeny and taxonomy of Cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s. str. Mycological Progress 2: 3–18. [Google Scholar]
- Carbone I, Kohn LM (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Carnegie AJ, Ades PK, Ford R (2001). The use of RAPD-PCR analysis for the differentiation of Mycosphaerella species from Eucalyptus in Australia. Mycological Research 105: 1313–1320. [Google Scholar]
- Carnegie AJ, Keane PJ (1994). Further Mycosphaerella species associated with leaf diseases of Eucalyptus. Mycological Research 98: 413–418. [Google Scholar]
- Carnegie AJ, Keane PJ (1997). A revised Mycosphaerella gregaria nom. nov. for M. aggregata on Eucalyptus. Mycological Research 101: 843–844. [Google Scholar]
- Carnegie AJ, Keane PJ (1998). Mycosphaerella vespa sp. nov. from diseased Eucalyptus leaves in Australia. Mycological Research 102: 1274–1276. [Google Scholar]
- Cortinas MN, Crous PW, Wingfield BD, Wingfield MJ (2006). Multilocus gene phylogenies and phenotypic characters distinguish two fungi previously identified as Colletogloeopsis zuluensis causing Eucalyptus cankers. Studies in Mycology 55: 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW (1998). Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1–170. [Google Scholar]
- Crous PW, Aptroot A, Kang JC, Braun U, Wingfield MJ (2000). The genus Mycosphaerella and its anamorphs. Studies in Mycology 45: 107–121. [Google Scholar]
- Crous PW, Groenewald JZ, Mansilla JP, Hunter GC, Wingfield MJ. (2004a). Phylogenetic analysis of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. Studies in Mycology 50: 195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Pongpanich K, Himaman W, Arzanlou M, Wingfield MJ (2004b). Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Studies in Mycology 50: 457–469. [Google Scholar]
- Crous PW, Hong L, Wingfield BD, Wingfield, MJ (2001). ITS rDNA phylogeny of selected Mycosphaerella species and their anamorphs occurring on Myrtaceae. Mycological Research 105: 425–431. [Google Scholar]
- Crous PW, Hong L, Wingfield MJ, Wingfield BD, Kang JC (1999). Uwebraunia and Dissoconium, two morphologically similar anamorph genera with different teleomorph affinity. Sydowia 51: 155–166. [Google Scholar]
- Crous PW, Wingfield MJ (1996). Species of Mycosphaerella and their anamorphs associated with leaf blotch disease of Eucalyptus in South Africa. Mycologia 88: 441–458. [Google Scholar]
- Crous PW, Wingfield MJ (1997a). Colletogloeopsis, a new coelomycete genus to accommodate anamorphs of two species of Mycosphaerella on Eucalyptus. Canadian Journal of Botany 75: 667–674. [Google Scholar]
- Crous PW, Wingfield MJ (1997b). New species of Mycosphaerella occurring on Eucalyptus leaves in Indonesia and Africa. Canadian Journal of Botany 75: 781–790. [Google Scholar]
- Crous PW, Wingfield MJ, Mansilla JP, Alfenas AC, Groenewald JZ (2006). Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology 55: 99–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CW (1997). Can three incongruence tests predict when data should be combined? Molecular Biology and Evolution 14: 733–740. [DOI] [PubMed] [Google Scholar]
- Darlu P, Lecointre G (2002). When does the incongruence length difference test fail?. Molecular Biology and Evolution 19: 432–437. [DOI] [PubMed] [Google Scholar]
- Dettman JR, Jacobson DJ, Taylor JW (2003). A multilocus genealogical approach to phylogenetic species recognition in the model eukaryote Neurospora. Evolution 57: 2703–2720. [DOI] [PubMed] [Google Scholar]
- Farris JS, Kallersjo M, Kluge AG, Bult C (1994). Testing significance of Incongruence. Cladistics 10: 315–320. [Google Scholar]
- Ganapathi A (1979). Studies on the etiology of the leaf blotch disease of Eucalyptus spp. caused by Mycosphaerella nubilosa (Cke) Hansf. PhD. dissertation. Department of Botany, University of Auckland, New Zealand.
- Hunter GC, Crous PW, Roux J, Wingfield BD, Wingfield MJ (2004a). Identification of Mycosphaerella species associated with Eucalyptus nitens leaf defoliation in South Africa. Australasian Plant Pathology 33: 349–355. [Google Scholar]
- Hunter GC, Roux J, Wingfield BD, Crous PW, Wingfield MJ (2004b). Mycosphaerella species causing leaf disease in South African Eucalyptus plantations. Mycological Research 108: 672–681. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kuma K, Toh H, Miyata T (2005). MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acid Research 33: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist JE, Purnell RC (1987). Effects of Mycosphaerella leaf spot on growth of Eucalyptus nitens. Plant Disease 71: 1025–1029. [Google Scholar]
- Maxwell A, Dell B, Neumeister-Kemp HG, Hardy EStJ (2003). Mycosphaerella species associated with Eucalyptus in southwestern Australia: new species, new records and a key. Mycological Research 107: 351–359. [DOI] [PubMed] [Google Scholar]
- Maxwell A, Jackson SL, Dell B, Hardy EStJ (2005). PCR-identification of Mycosphaerella species associated with leaf diseases of Eucalyptus. Mycological Research 109: 992–1004. [DOI] [PubMed] [Google Scholar]
- Moncalvo JM, Wamg HH, Hseu RS (1995). Phylogenetic relationships in Ganoderma inferred from the internal transcribed spacers and 25S ribosomal DNA sequences. Mycologia 87: 223–238. [Google Scholar]
- Park RF, Keane PJ (1982a). Three Mycosphaerella species from leaf diseases of Eucalyptus. Transactions of the British Mycological Society 79: 95–100. [Google Scholar]
- Park RF, Keane PJ (1982b). Leaf diseases of Eucalyptus associated with Mycosphaerella species. Transactions of the British Mycological Society 79: 101–115. [Google Scholar]
- Park RF, Keane PJ, Wingfield MJ, Crous PW (2000). Fungal diseases of Eucalypt foliage. In Diseases and Pathogens of Eucalypts. (PJ Keane, GA Kile, FD Podger, BN Brown, eds): 153–259. CSIRO Publishing, Collingwood, Australia.
- Posada D, Crandall KA (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- Slippers B, Stenlid J, Wingfield MJ (2005). Emerging pathogens: fungal host jumps following anthropogenic introduction. Trends in Ecology and Evolution 20: 420–421. [DOI] [PubMed] [Google Scholar]
- Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Sinauer Associates, Sunderland, MA.
- Taylor JW, Fischer MC (2003). Fungal multilocus sequence typing–it's not just for bacteria. Current Opinion in Microbiology 6: 351–356. [DOI] [PubMed] [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbet DS, Fischer MC (2000). Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31: 21–32. [DOI] [PubMed] [Google Scholar]
- Turnbull JW (2000). Economic and social importance of Eucalypts. In: Diseases and pathogens of eucalypts. (Keane PJ, Kile GA, Podger FD, Brown BN, eds) CSIRO Publishing, Australia: 1–7.
- Vilgalys R, Hester M (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR protocols: a guide to methods and applications. (Innis MA, Gelfand DH, Snisky JJ, White TJ, eds) Academic Press, U.S.A.: 282–287.
- Wingfield MJ (1999). Pathogens in exotic plantation forestry. International Forestry Review 1: 163–168. [Google Scholar]
- Wingfield MJ (2003). Increasing threat of diseases to exotic plantation forests in the Southern Hemisphere: lessons from Cryphonectria canker. Australasian Plant Pathology 32: 133–139. [Google Scholar]
- Wingfield MJ, Slippers B, Roux J, Wingfield BD (2001) Worldwide movement of exotic forest fungi, especially in the tropics and the Southern Hemisphere. Bioscience 51: 134–140. [Google Scholar]