Abstract
Grey leaf spot is a serious yield-reducing disease of maize (Zea mays) in many parts of the world where this crop is cultivated. The causal organism associated with the disease is Cercospora zeae-maydis. Two potential sibling species have been recognized as Groups I and II. The DNA sequences for the internal transcribed spacers (ITS1 & ITS2), the 5.8S rRNA gene, elongation factor 1-α, histone H3, actin and calmodulin gene regions suggest that Groups I and II are two distinct species. Furthermore, Cercospora zeae-maydis (Group I) can be distinguished from C. zeina sp. nov. (Group II) by its faster growth rate on artificial media, the ability to produce cercosporin, longer conidiophores, and broadly fusiform conidia. A PCR-based test that distinguishes the two species was developed using species-specific primers designed from the histone H3 gene.
Keywords: Ascomycetes, Cercospora zeae-maydis, Cercospora zeina, grey leaf spot, maize, Mycosphaerella, systematics
INTRODUCTION
Grey leaf spot of maize is a serious foliar disease of Zea mays in many countries where it is cultivated, especially in the eastern U.S.A. and Africa (Ward et al. 1999, Crous & Braun 2003). Since it was recognized as a “disease on the move” by Latterell & Rossi (1983), grey leaf spot has become increasingly important and is currently seen as one of the most serious yield-limiting diseases of maize (Nutter & Jenco 1992, Ward & Nowell 1998). The causal agent of grey leaf spot is generally regarded as Cercospora zeae-maydis Tehon & E.Y. Daniels, though C. sorghi Ellis & Everh. has also been reported from maize (Crous & Braun 2003). Chupp (1954) referred to a C. sorghi var. maydis Ellis & Everh., which is morphologically similar to C. sorghi, but suspected to represent a distinct species due to its lack of pathogenicity to sorghum. In recent years, it has become accepted that more than one species of Cercospora is associated with grey leaf spot of maize, namely C. zeae-maydis Group I, which is dominant in the U.S.A. and occurs elsewhere in the world, and C. zeae-maydis Group II, which is genetically and phenotypically distinct and occurs in the U.S.A., Africa and possibly elsewhere (Wang et al. 1998, Dunkle & Levy 2000, Goodwin et al. 2001).
The aim of the current study was to characterise the Cercospora species associated with grey leaf spot symptoms occurring on maize in South Africa. To achieve this goal isolates were subjected to DNA sequence analysis of several loci, namely the internal transcribed spacers (ITS1 & ITS2), the 5.8S rRNA gene, the elongation factor 1-α, histone 3, actin and calmodulin gene regions. Furthermore, South African isolates were morphologically compared to those isolates from the U.S.A., and the type specimen of C. zeae-maydis.
MATERIALS AND METHODS
Isolates
Single-conidial isolates were obtained from symptomatic maize leaves, and cultured as explained in Crous (1998). Cultural characteristics and morphology of Cercospora isolates (Table 1) were determined on plates containing 2% malt extract agar (MEA) (20 g/L), 2% potato-dextrose agar (PDA), oatmeal agar (OA), and carnation leaf agar (CLA) [1% water agar (10 g/L) with autoclaved carnation leaves placed onto the medium] (Gams et al. 1998). Plates were incubated at 25 °C under continuous near-UV light, to promote sporulation.
Table 1.
Cercospora isolates used for sequence analysis.
| Species | Accession number1 | Host | Country | Collector | GenBank numbers2(ITS, EF ACT, CAL, HIS) |
|---|---|---|---|---|---|
| Cercospora apii | CBS 114418; CPC 10924 | Apium graveolens | Italy | Meutri | AY840517, AY840484, AY840443, AY840415, AY840382 |
| CBS 116455; CPC 11556* | A. graveolens | Germany | K. Schrameyer | AY840519, AY840486, AY840450, AY840417, AY840384 | |
| CBS 116504; CPC 11579 | A. graveolens | Germany | K. Schrameyer | AY840520, AY840487, AY840451, AY840418, AY840385 | |
| CBS 119.25; CPC 5086 | A. graveolens | — | L. J. Klotz | AY840512, AY840479, AY840443, AY840410, AY840377 | |
| CBS 121.31; CPC 5073 | Beta vulgaris | Austria | — | AY840513, AY840480, AY840444, AY840411, AY840378 | |
| CBS 127.31; CPC 5119 | B. vulgaris | Hungary | — | AY840514, AY840481, AY840445, AY840412, AY840379 | |
| Cercospora beticola | CBS 116456; CPC 11557* | B. vulgaris | Italy | V. Rossi | AY840527, AY840494, AY840458, AY840425, AY840392 |
| CBS 116501; CPC 11576 | B. vulgaris | Iran | A. A. Ravanlou | AY840528, AY840495, AY840459, AY840426, AY840393 | |
| CBS 116502; CPC 11577 | B. vulgaris | Germany | S. Mittler | AY840529, AY840496, AY840460, AY840427, AY840394 | |
| CBS 116.47; CPC 5074 | B. vulgaris | Netherlands | G. E. Bunschoten | AY752135, AY752168, AY752196, AY752227, AY752258 | |
| CBS 124.31; CPC 5070 | B. vulgaris | Romania | — | AY840523, AY840490, AY840454, AY840421, AY840388 | |
| CBS 126.31; CPC 5064 | B. vulgaris | Germany | — | AY840525, AY840492, AY840456, AY840423, AY840390 | |
| CPC 5125 | B. vulgaris | New Zealand | C. F. Hill | AY752137, AY752170, AY752198, AY752229, AY752260 | |
| CPC 5128 | B. vulgaris | New Zealand | C. F. Hill | AY752138, AY752171, AY752199, AY752230, AY752261 | |
| CPC 10168 | B. vulgaris | New Zealand | C. F. Hill | AY840533, AY840500, AY840464, AY840431, AY840398 | |
| Cercospora canescens | ATCC 32779 | Vigna radiata | Taiwan | — | AY266164, —, —, —, — |
| Cercospora sorghi | — | Sorghum bicolor | U.S.A., Texas | — | AF291707, —, —, —, — |
| Cercospora sorghi var. maydis | — | Zea mays | U.S.A., North Carolina | — | AF297233, —, —, —, — |
| — | Z. mays | Kenya | — | AF297232, —, —, —, — | |
| Cercospora sp. | CPC 12062 | Z. mays | South Africa, KwaZulu-Natal | P. Caldwell | DQ185071, DQ185083, DQ185095, DQ185107, DQ185119 |
| Cercospora zeae-maydis | — | Z. mays | U.S.A., Indiana | — | AF291709, —, —, —, — |
| CBS 117755 = A358 | Z. mays | U.S.A., Indiana | B. Fleener | DQ185072, DQ185084, DQ185096, DQ185108, DQ185120 | |
| CBS 117756 = A359 | Z. mays | U.S.A., Delaware | B. Fleener | DQ185073, DQ185085, DQ185097, DQ185109, DQ185121 | |
| CBS 117757* = A360 | Z. mays | U.S.A., Wisconsin | B. Fleener | DQ185074, DQ185086, DQ185098, DQ185110, DQ185122 | |
| CBS 117758 = A361 | Z. mays | U.S.A., Iowa | B. Fleener | DQ185075, DQ185087, DQ185099, DQ185111, DQ185123 | |
| CBS 117759 = A362 | Z. mays | U.S.A., Tennessee | B. Fleener | DQ185076, DQ185088, DQ185100, DQ185112, DQ185124 | |
| CBS 117760 = A363 | Z. mays | U.S.A., Pennsylvania | B. Fleener | DQ185077, DQ185089, DQ185101, DQ185113, DQ185125 | |
| CBS 117761 = A364 | Z. mays | U.S.A., Indiana | B. Fleener | DQ185078, DQ185090, DQ185102, DQ185114, DQ185126 | |
| CBS 117762 = A365 | Z. mays | U.S.A., Missouri | B. Fleener | DQ185079, DQ185091, DQ185103, DQ185115, DQ185127 | |
| CBS 117763 = A367 | Z. mays | U.S.A., Iowa | B. Fleener | DQ185080, DQ185092, DQ185104, DQ185116, DQ185128 | |
| Cercospora zeina | CBS 118820 = CPC 11995* | Z. mays | South Africa, KwaZulu-Natal | P. Caldwell | DQ185081, DQ185093, DQ185105, DQ185117, DQ185129 |
| CPC 11998 | Z. mays | South Africa, KwaZulu-Natal | P. Caldwell | DQ185082, DQ185094, DQ185106, DQ185118, DQ185130 | |
| — | Z. mays | U.S.A., North Carolina | — | AF291710, —, —, —, — |
CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CPC: Culture collection of Pedro Crous, housed at CBS.
ITS: internal transcribed spacer region, EF: partial elongation factor 1-alpha gene, ACT: partial actin gene, CAL: partial calmodulin gene, HIS: partial histone H3 gene.
Ex-type cultures.
DNA phylogeny
Isolates of C. zeae-maydis, C. beticola, C. apii, and an unidentified Cercospora sp. (Table 1) were used for phylogenetic analysis. The protocol of Lee & Taylor (1990) was used to isolate genomic DNA from fungal mycelium of monoconidial cultures grown on MEA in Petri dishes. The primers ITS1 and ITS4 (White et al. 1990) were used to amplify part (ITS) of the nuclear rRNA operon spanning the 3' end of the 18S rRNA gene, the first internal transcribed spacer (ITS1), the 5.8S rRNA gene, the second ITS region and the 5' end of the 28S rRNA gene. To obtain additional sequence information, four other loci were also sequenced. Part of the elongation factor 1-α gene (EF) was amplified with primers EF1-728F and EF1-986R, part of the actin gene (ACT) with primers ACT-512F and ACT-783R, and part of the calmodulin gene (CAL) with primers CAL-228F and CAL-737R (Carbone & Kohn 1999). Part of the histone H3 gene (HIS) was amplified with primers CylH3F and CylH3R (Crous et al. 2004a). Sequencing was done with the same PCR primers. The PCR conditions, sequence alignment and subsequent phylogenetic analysis followed the methods of Crous et al. (2004b). The new sequences were added to a subset of the alignment (TreeBASE matrix M2038) of Crous et al. (2004b) and additional sequences were obtained from GenBank. Sequence data were deposited in GenBank and alignments in TreeBASE (S1509, M2712).
Development of a species-specific diagnostic test
The histone H3 gene was found to be most effective in separating the three species described in the present study. Therefore, this area was targeted for the development of a species-specific diagnostic test. Primers CylH3F and CylH3R were used as external primers and their amplification product functions as a positive control. Three species-specific primers were designed for C. zeae-maydis, C. zeina sp. nov. and an undescribed Cercospora species, respectively: CzeaeHIST (5'-TCGACTCGTCTTTCACTTG-3'), CzeinaHIST (5'-TCGAGTGGCCCTCACCGT-3') and CmaizeHIST (5'-TCGAGTCACTTCGACTTCC-3'); all of them species-specific. These internal, species-specific primers, together with the external primers, were used in separate PCR reactions in a total volume of 12.5 μl, containing 1 μl of diluted genomic DNA, 1× PCR buffer, 2 mM MgCl2, 48 μM of each of the dNTPs, 0.7 pmol CylH3F, 3 pmol of CylH3R, 4 pmol of the specific internal primer and 0.7 units (Bioline) Taq polymerase. The amplification reactions were done on a GeneAmp PCR System 9600 (Perkin-Elmer, Norwalk, Connecticut). The initial denaturation step was done at 94 °C for 5 min, followed by 15 cycles of denaturation at 94 °C (20 s), annealing at 58 °C (30 s) and elongation at 72 °C (40 s) as well as 25 cycles of denaturation at 94 °C (20 s), annealing at 55 °C (30 s) and elongation at 72 °C (40 s). A final elongation step at 72 °C (5 min) was included to ensure that full length products are obtained. The PCR products were separated on a 1 % agarose gel and visualized under UV-light after ethidium bromide staining.
Taxonomy
Morphological examinations were made from cultures sporulating on CLA, as well as on host material. Structures were mounted in lactic acid, and 30 measurements at × 1000 magnification were made of each structure. The 95 % confidence levels were determined and the extremes of spore measurements given in parentheses. Colony colours were noted after 3 wk growth on MEA, PDA and OA at 25 °C in the dark, using the colour charts of Rayner (1970). All cultures studied are maintained in the culture collection of the Centraalbureau voor Schimmelcultures (CBS), Utrecht, the Netherlands. Type specimens were deposited at the National Collection of Fungi in Pretoria (PREM), South Africa (Table 1).
RESULTS
DNA phylogeny
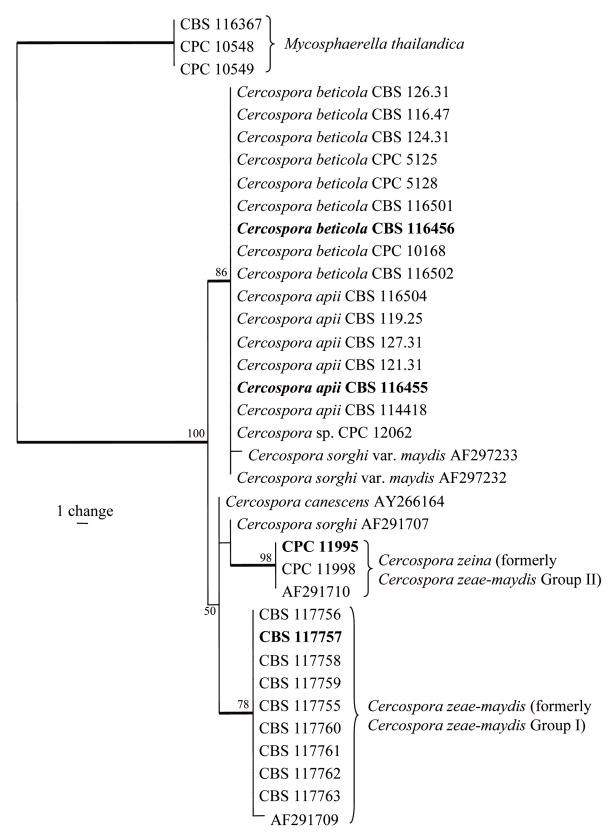
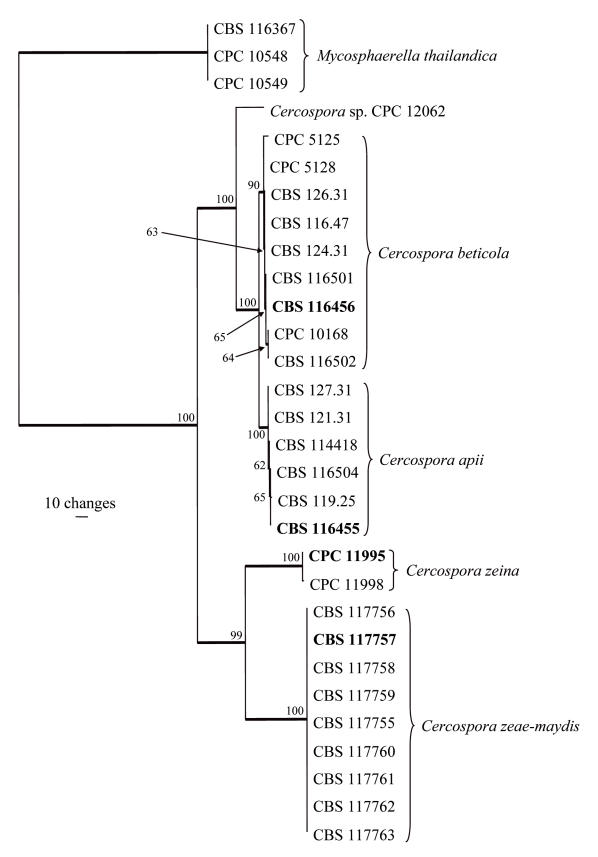
Approximately 500, 310, 230, 320 and 400 bases were determined for ITS, EF, ACT, CAL, and HIS loci, respectively, of the isolates listed in Table 1. Because sequences for the last four loci were not available for other isolates, a separate tree that included more isolates was generated using only ITS sequences (Fig. 1). A partition homogeneity test showed that all loci could be combined (p = 0.747) into a single analysis (Fig. 2).
Fig. 1.
One of six most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the ITS sequence alignment. The scale bar shows a single change, and bootstrap support values from 1000 replicates are shown at the nodes. Thickened lines indicate the strict consensus branches, and ex-type strains are shown in bold print. The tree was rooted to three Mycosphaerella thailandica strains.
Fig. 2.
One of two most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the combined ITS, elongation factor 1-alpha, actin, calmodulin and histone H3 sequence alignment. The scale bar shows 10 changes, and bootstrap support values from 1000 replicates are shown at the nodes. Thickened lines indicate the strict consensus branches, and type strains are shown in bold print. The tree was rooted to three Mycosphaerella thailandica strains.
The ITS data matrix contained 36 taxa (including the three outgroup isolates) and 487 characters including alignment gaps. Of these characters, 40 were parsimony-informative, one was variable and parsimony-uninformative, and 446 are constant. Neighbour-joining analysis using three substitution models (uncorrected “p”, Jukes-Cantor and HKY85) on the sequence data yielded trees with similar topology and bootstrap values. Parsimony analysis of the alignment yielded six most parsimonious trees (TL = 44 steps; CI = 0.955; RI = 0.986; RC = 0.942), one of which is shown in Fig. 1. Three distinct clades were obtained. The first clade (86 % bootstrap support) contained C. apii and C. beticola together with two isolates of C. sorgh var. maydis and an undescribed Cercospora sp. (CPC 12062) from Zea mays in South Africa. The second clade (98 % bootstrap support) contained three isolates of the new species (C. zeina, formerly C. zeae-maydis Group II). The isolates of C. sorghi var. sorghi and C. canescens had ITS sequences similar to those of C. zeae-maydis Group II (= C. zeina), but there was no bootstrap support for this branch. The third clade (78 % bootstrap support) contained isolates of C. zeae-maydis (formerly C. zeae-maydis Group I). The neighbour-joining and parsimony analyses provided trees with similar topologies (data not shown).
The combined data matrix contained 30 taxa (including the three outgroup taxa) and 1643 characters including alignment gaps. Of these characters, 406 were parsimony-informative, 10 were variable and parsimony-uninformative, and 1227 were constant. Parsimony analysis of the alignment yielded two most parsimonious trees (TL = 519 steps; CI = 0.948; RI = 0.986; RC = 0.935), one of which is shown in Fig. 2. Three distinct clades were obtained, the first (100 % bootstrap support) containing clades with C. beticola (90 % bootstrap support) and C. apii (100 % bootstrap support) with Cercospora sp. CPC 12062 as a sister taxon (100 % bootstrap support). Similar to the ITS tree, the C. zeina and C. zeae-maydis isolates formed distinct and well-supported clades (each with a bootstrap support value of 100 %). Neighbour-joining analysis using the three substitution models on the sequence data yielded trees with similar topology and bootstrap values to that obtained using parsimony (data not shown).
Development of a species-specific diagnostic test
Easy and rapid identification of C. zeae-maydis, C. zeina and the new Cercospora sp. is possible using three multiplex PCR amplifications. A 389 bp fragment, which serves as the positive control, is present for all three species, while the second 284 bp fragment is only observed for the Cercospora species recognised by the specific internal primer (Fig. 3). Primers CzeaeHIST, CzeinaHIST, and CmaizeHIST are therefore specific for C. zeae-maydis, C. zeina and the Cercospora sp., respectively, and can be used for their identification and detection.
Fig. 3.
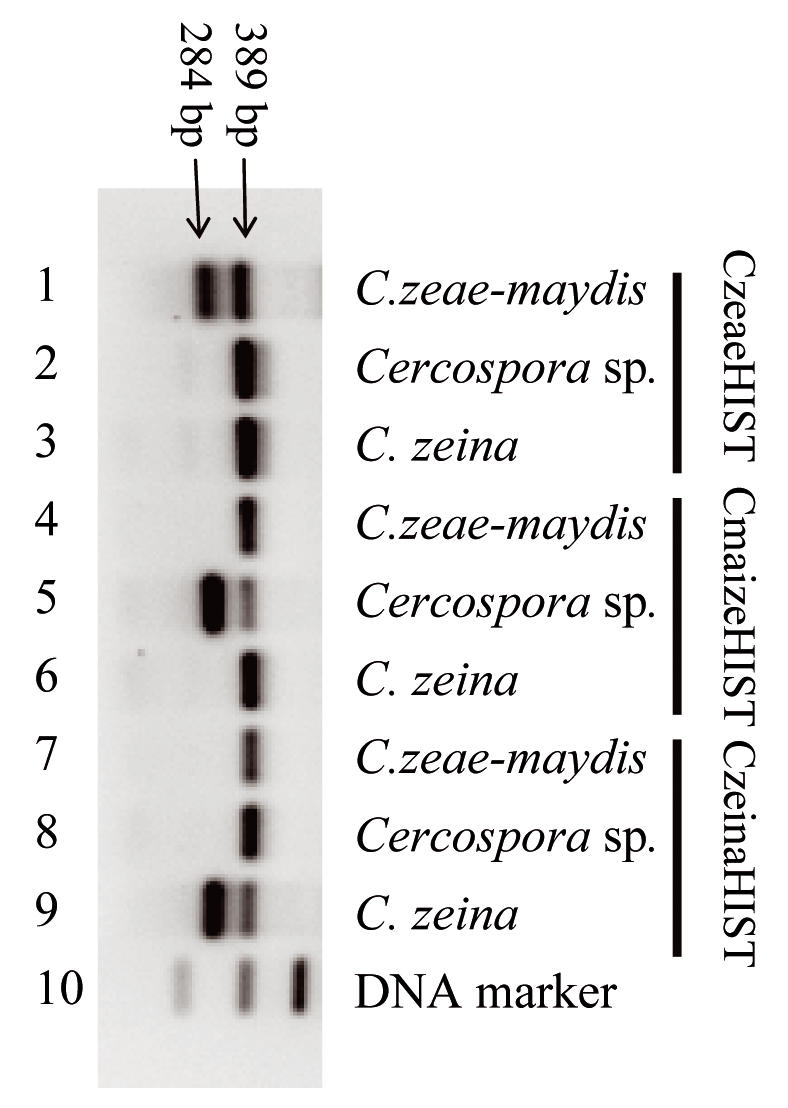
Identification of C. zeae-maydis, an unidentified Cercospora sp. and C. zeina using the species-specific primers. Lane 10 contains the DNA marker. The 389 bp fragment, which acts as the positive control, is present in all PCR amplifications (lanes 1–9). The species-specific fragment (284 bp) is observed when the amplification reaction contains C. zeae-maydis DNA and primer CzeaeHIST (lane 1, strain CBS 117757), Cercospora sp. DNA and primer CmaizeHIST (lane 5, strain CPC 12062) or C. zeina DNA and primer CzeinaHIST (lane 9, strain CPC 11995).
Taxonomy
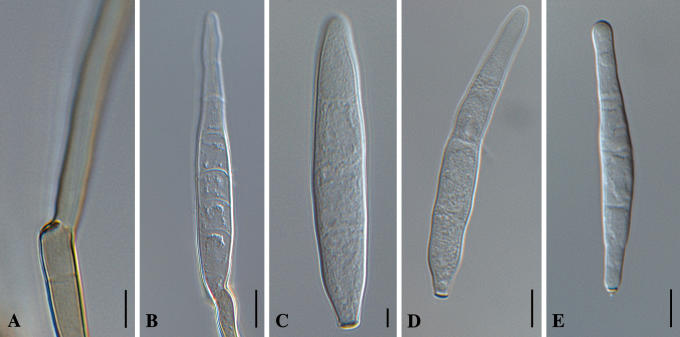
Cercospora zeae-maydis Tehon & E.Y. Daniels, Mycologia 17: 248. 1925. Fig. 4.
Fig. 4.
Cercospora zeae-maydis. A. Conidiophore with darkened, refractive conidiogenous locus. B. Germinating conidium. C–E. Conidia in vitro. Bars = 10 μm.
Leaf spots oblong, forming extended streaks or irregular, greyish to brownish spots, shape and size variable, often with a narrow brown border line or margin. Caespituli amphigenous, mostly hypophyllous, punctiform to subeffuse, brown. Mycelium internal. Stromata lacking or small, with a few swollen substomatal brown cells. Conidiophores in small to moderately large fascicles (3–14), emerging through the stomata, usually divergent, erect, straight, subcylindrical to flexuous, distinctly geniculate–sinuous, unbranched, 40–180 × 4–8 μm, obscurely (0–)1–8-septate, uniformly pale olivaceous to medium brown, thin-walled, smooth; conidiogenous cells integrated, terminal, occasionally intercalary, 10–40 μm long, conidiogenous loci conspicuously thickened and darkened, 2–3 μm wide. Conidia solitary, broadly obclavate–subcylindrical, 30–100 × 4–9 μm, 1–10-septate, hyaline, thin-walled, smooth, apex obtuse, base obconically truncate, hila somewhat thickened and darkened, 2–3 μm wide (based on type specimen).
Specimens examined: U.S.A., Illinois, Alexander Co., McClure, on Zea mays, 29 Aug. 1924, P.A. Young (ILLS 4276) holotype, BPI 442569 isotype; Indiana, Princeton, 2003, B. Fleener, YA-03 = A358 = CBS 117755; Delaware, 1997, B. Fleener, DE-97 = A359 = CBS 117756; Wisconsin, Janesville, 2002, B. Fleener, epitype designated here, CBS H-17774, JV-WI-02 = A360 = CBS 117757, culture ex-type; Iowa, Johnston, 2004, B. Fleener, JH-IA-04 = A361 = CBS 117758; Tennessee, Union City, 1999, B. Fleener, UC-TN-99 = A362 = CBS 117759; Pennsylvania, New Holland, 1999, B. Fleener, NH-PA-99 = A363 = CBS 117760; Indiana, Princeton, 1999, B. Fleener, PR-IN-99 = A364 = CBS 117761; Missouri, Dexter, 2000, B. Fleener, DEXTER-MO-00 = A365 = CBS 117762; Iowa, Reinbeck, 1999, B. Fleener, RENBECK-IA-99 = A367 = CBS 117763.
Cultural characteristics: Colonies on PDA reaching 15–25 mm diam after 3 wk, and forming ample spermatogonia; colonies on MEA erumpent, with sparse aerial mycelium; margins smooth, but irregular; surface olivaceous-grey with irregular patches of white or smoke-grey; reverse iron-grey; colonies fertile. On OA colonies spreading with moderate aerial mycelium; margins smooth but irregular; surface red with patches of white and pale olivaceous-grey; fertile.
Substrate: Zea mays.
Distribution: Azerbaijan, Brazil, Cameroon, Canada, China, Colombia, Congo, Costa Rica, Ecuador, Ethiopia, Georgia, Guatemala, Kenya, Malawi, Mexico, Mozambique, Nigeria, Panama, Peru, South Africa, Swaziland, Tanzania, Trinidad and Tobago, Uganda, USA (CO, DE, IA, IL, KS, KY, MD, MN, NC, OH, PA, SC, TN, VA, WI, WV), Venezuela, Zambia, Zimbabwe (Crous & Braun 2003).
Cercospora zeina Crous & U. Braun, sp. nov. MycoBank MB500863. Fig. 5.
Fig. 5.
Cercospora zeina. A–C. Close-up of grey leaf spot lesions on maize. D. Heavily infected plant. E–G. Conidiophores fascicles on leaf surface. H–J. Conidiophores. K, N. Conidiogenous cells giving rise to conidia. L–M, O–Q. Conidia. R–U. Scanning electron micrographs of conidiophores and conidia. V. Conidiogenous cell showing thickened loci. Scale bars: G–Q = 10 μm, R = 100 μm, S = 50 μm, T–U = 8 μm, V = 5 μm.
Cercospora zeae-maydis affinis, a qua imprimis differt conidiophoris brevioribus (ad 100 μm longis), conidiis late fusiformibus, coloniis in cultura crescentibus tardioribus, sine pigmento rubro.
Leaf spots amphigenous, confined by leaf veins, 2–3 mm wide, variable in length from 5–40 mm; lesions becoming confluent, pale grey to pale brown; borders indistinct, chlorotic in younger leaf spots. Caespituli fasciculate, amphigenous, punctiform to subeffuse, grey to brown on leaves, up to 120 μm high and wide. Mycelium internal, consisting of pale brown, septate, branched, smooth hyphae, 3–4 μm wide. Stromata lacking or small, a few swollen substomatal cells, brown, up to 30 μm diam. Conidiophores aggregated (3–20) in loose to semi-dense fascicles arising from the upper cells of an inconspicuous brown stroma, emerging through stomata, usually divergent, erect, straight, subcylindrical to flexuous, distinctly geniculate–sinuous, unbranched or branched above, 40–100 × 5–7 μm, 1–5-septate, uniformly pale olivaceous to medium brown, thin-walled, smooth; conidiogenous cells integrated, terminal, 40–60 × 5–6 μm, with several conidiogenous loci that are conspicuously thickened, darkened and refractive, 2–3 μm wide. Conidia solitary, broadly fusiform, (40–)60–75(–100) × (6–)7–8(–9) μm, (1–)3–5(–10)-septate, hyaline, thin-walled, smooth, apex subobtuse, base subtruncate, hila somewhat thickened, darkened and refractive, 2–3 μm wide (based on type specimen).
Specimen examined: South Africa, KwaZulu-Natal, Pietermaritzburg, on Zea mays, 2005, P. Caldwell, CBS H-17775 holotype, CBS 118820 = CPC 11995, culture ex-type.
Cultural characteristics: Colonies on PDA reaching 10–15 mm diam after 3 wk, and forming spermatogonia; colonies on MEA erumpent, with sparse aerial mycelium; margins smooth, but irregular; surface olivaceous-grey with irregular patches of white or iron-grey; reverse iron-grey; colonies fertile. On OA colonies are spreading with moderate whitish aerial mycelium; margins smooth but irregular, olivaceous-grey; fertile.
Substrate: Zea mays.
Distribution: South Africa, Uganda, U.S.A. (NC, NY, OH, VA), Zambia, Zimbabwe (Wang et al. 1998, Dunkle & Levy 2000).
Notes: Cercospora zeae-maydis has conidia of similar dimensions to those of C. zeina. However, C. zeina can be distinguished by having shorter conidiophores (up to 100 μm) and more broadly fusiform conidia, versus longer conidiophores (up to 180 μm) and broadly obclavate–subcylindrical conidia of C. zeae-maydis. Colonies of C. zeina grow more slowly in culture and lack the red pigment associated with cercosporin production, typical of C. zeae-maydis (Goodwin et al. 2001).
DISCUSSION
In a recent review of grey leaf spot of maize, Ward et al. (1999) discussed the complexities and importance of this disease in the U.S.A., as well as in Africa. Several papers have commented on the disease being associated with two or more species (Wang et al. 1998, Dunkle & Levy 2000, Goodwin et al. 2001). A review of the literature suggests that there are two possible species complexes associated with grey leaf spot, namely the C. sorghi complex (C. sorghi and C. sorghi var. maydis), and the C. zeae-maydis complex (Groups I and II).
The description of C. zeina has now resolved some of this taxonomic uncertainty, by demonstrating that Group II is, in fact, a distinct species (C. zeina) and that Group I, to which the name C. zeae-maydis applies, apparently does not occur in South Africa. Further collections from other African countries, as well as other locations in South Africa would be required, however, to determine if C. zeae-maydis is truly absent from the continent.
Grey leaf spot disease was first recorded from South Africa in 1988 (Ward et al. 1997). The possible source of inoculum was later postulated by Ward et al. (1999) to have been from infested maize residues imported from the U.S.A. However, as argued by Dunkle & Levy (2000), if this was indeed the case, such inoculum would have more likely contained C. zeae-maydis, which dominates over C. zeina throughout most of the maize-producing areas of the eastern and midwestern U.S.A. Given the distribution of C. zeina throughout Africa and the fact that there is more genetic diversity of the pathogen in Africa than in the U.S.A. (Dunkle & Levy 2000), it was thought to be more likely that C. zeina was introduced to the U.S.A. from Africa, than vice versa. Dunkle & Levy (2000) also considered a third possibility, namely that C. zeina was introduced to Africa and the U.S.A. on another host, as maize is not native to Africa. However, the most likely hypothesis may be that C. zeina is indeed native to Africa, but that it has jumped from another indigenous host (such as sorghum) onto maize. It is interesting to note that the ITS sequence of the C. zeina isolates was more similar to that of an isolate of C. sorghii var. sorghi than to that of the presumably American species C. zeae-maydis. Although they are morphologically distinct, further comparisons between C. zeina and C. sorghi are needed.
Although species of Mycosphaerella and their anamorphs are generally assumed to be host-specific (Corlett 1991, Crous & Braun 2003), some species have been observed to also have the ability to colonise hosts other than those on which they are assumed to be primary pathogens. This was recently observed for the greasy leaf-spot pathogen of Citrus, Mycosphaerella citri Whiteside, which was isolated from other hosts such as Acacia and Musa (Crous et al. 2004b). This finding subsequently led to the formulation of the pogo stick hypothesis (Crous & Groenewald 2005), where species of Mycosphaerella can jump to another host as a secondary colonizer, where they sporulate on lesions of the primary Mycosphaerella pathogen, producing enough inoculum to enable them to continue the search for their real host.
A further interesting finding was the isolation of a single, fast-growing isolate from grey leaf spot lesions caused by C. zeina. Although it was originally suspected that this isolate may represent C. zeae-maydis (fast growing and forming a red pigment in agar), this has proven to not be the case. Morphologically this isolate (CPC 12062) appeared more similar to isolates in the Cercospora apii complex (C. apii and C. beticola). Although only a few of the species in this complex are known from culture, CPC 12062 proved distinct based on DNA sequence data when compared to the more than 100 sequences currently available in our unpublished database. This isolate may represent an unrelated pathogen from another host that has “jumped” onto maize (Crous & Groenewald 2005). By using the PCR-based method described here as a diagnostic tool, it is relatively easy to identify the three Cercospora species on maize that are treated in this study.
Both C. zeae-maydis and C. zeina have the ability to form ample spermatogonia on host tissue as well as in culture. Although there has been an earlier report of a possible Mycosphaerella teleomorph (Latterell & Rossi 1977), this has remained unconfirmed. Wang et al. (1998) were unable to find evidence of the MAT-2 mating type idiomorph in isolates of Cercospora zeae-maydis, and our current mating studies with isolates of C. zeae-maydis and C. zeina have also given negative results. Further population-level studies are thus needed to determine the level of variation present in populations, and whether sexual reproduction occurs within populations of these two fungi. Published results do not support the existence of cryptic sex, however, as Wang et al. (1998) reported the variation to be rather low in populations of both species.
Acknowledgments
The authors are grateful to Dr G. Munkvold for making isolates of Cercospora zeae-maydis available for study. Dr. L. Tiedt (Potchefstroom University) is thanked for providing some of the SEM photographs used.
Taxonomic novelties: Cercospora zeina Crous & U. Braun sp. nov.
References
- Carbone I, Kohn LM (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Chupp, C. (1954). A monograph of the fungus genus Cercospora. Ithaca, New York. Published by the author.
- Corlett M (1991). An annotated list of the published names in Mycosphaerella and Sphaerella. Mycologia Memoir 18, 1–328. [Google Scholar]
- Crous PW (1998). Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1–170. [Google Scholar]
- Crous PW, Braun U (2003). Mycosphaerella and its anamorphs. 1. Names published in Cercospora and Passalora. CBS Biodiversity Series 1: 1–571. [Google Scholar]
- Crous PW, Groenewald JZ (2005). Hosts, species and genotypes: opinions versus data. Australasian Plant Pathology 34: 463–470. [Google Scholar]
- Crous PW, Groenewald JZ, Risede J-M, Hywel-Jones NL (2004a). Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology 50: 415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Pongpanich K, Himaman W, Arzanlou M, Wingfield MJ (2004b). Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Studies in Mycology 50: 457–469. [Google Scholar]
- Dunkle LD, Levy M (2000). Genetic relatedness of African and United States populations of Cercospora zeae-maydis. Phytopathology 90: 486–490. [DOI] [PubMed] [Google Scholar]
- Gams W, Hoekstra ES, Aptroot A (eds) (1998). CBS Course of Mycology. 4th ed. CBS, Baarn, Netherlands.
- Goodwin SB, Dunkle LD, Zisman VL (2001). Phylogenetic analysis of Cercospora and Mycosphaerella based on the internal transcribed spacer region of ribosomal DNA. Phytopathology 91: 648–658. [DOI] [PubMed] [Google Scholar]
- Latterell FM, Rossi AE (1977). Further evidence for the genetic relationship between Cercospora zeae-maydis and a species of Mycosphaerella. Proceedings of the 2nd International Mycological Congress: 374.
- Latterell FM, Rossi AE (1983). Gray leaf spot of corn: a disease on the move. Plant Disease 67: 842–847. [Google Scholar]
- Lee SB, Taylor JW (1990). Isolation of DNA from fungal mycelia and single spores. In: PCR Protocols: a guide to methods and applications. (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, San Diego, California: 282–287.
- Nutter FW Jr., Jenco JH (1992). Development of critical-point yield loss models to estimate yield losses in corn caused by Cercospora zeae-maydis. Phytopathology 82: 994. [Google Scholar]
- Rayner RW (1970). A mycological colour chart. CMI and British Mycological Society. Kew, Surrey, England.
- Wang J, Levy M, Dunkle LD (1998). Sibling species of Cercospora associated with gray leaf spot of maize. Phytopathology 88: 1269–1275. [DOI] [PubMed] [Google Scholar]
- Ward JMJ, Nowell DC (1998). Integrated management for the control of maize gray leaf spot. Integrated Pest Management Reviews 3: 1–12. [Google Scholar]
- Ward JMJ, Laing MD, Rijkenberg FHJ (1997). Frequency and timing of fungicide applications for the control of gray leaf spot of maize. Plant Disease 81: 41–48. [DOI] [PubMed] [Google Scholar]
- Ward JMJ, Stromberg EL, Nowell DC, Nutter FW Jr. (1999). Gray leaf spot: a disease of global importance in maize production. Plant Disease 83: 884–895. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications. (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, San Diego, California: 315–322.