Abstract
Black foot disease is a serious disease of grapevine crops in most areas where vines are grown. Mainly two species of Cylindrocarpon, C. destructans and C. macrodidymum, are associated with this disease. Recent studies have revealed a tremendous molecular variation within the former but only slight molecular variation within the latter, indicating that C. destructans presents a complex of several species The present study elucidates the taxonomic status of C. destructans-like isolates associated with black foot disease of grapevines. Grapevine isolates were studied morphologically, subjected to DNA analyses of their ITS and partial β-tubulin genes, and were mated in all combinations in vitro. Cylindrocarpon destructans strains isolated from grapevines in Europe and South Africa appeared morphologically and genetically identical, and had identical ITS and partial β-tubulin gene sequences. Phylogenetic analyses placed these strains in a clade closely related but clearly distinct from other clades with C. destructans-like anamorphs obtained from various herbaceous or woody hosts. Only the ex-type strain of Cylindrocarpon liriodendri had identical sequences to strains isolated from grapevines, and could also not be distinguished by morphological characters. The grapevine isolates are therefore reidentified here as Cylindrocarpon liriodendri. Cylindrocarpn liriodendri formed perithecia in heterothallic conditions and the holomorph of this species is described as Neonectria liriodendri sp. nov. Neonectria liriodendri is genetically distinct from the ex-type strain of Neonectria radicicola, which originated from Cyclamen in Sweden. Both ex-type strains also differ from at least two other clades comprising additional C. destructans-like strains. Many of these strains originated from Panax sp., which is the host of the type of C. destructans. Our phylogenetic analyses indicate that C. destructans is not the anamorph of N. radicicola and that N. liriodendri, N. radicicola and several C. destructans-like taxa may have evolved independently within the same phylogenetic species complex.
Keywords: β-tubulin gene, black foot disease, Cylindrocarpon, internal transcribed spacer regions, Nectriaceae, phylogeny, systematics, Vitis
INTRODUCTION
In recent years, two species of Cylindrocarpon Wollenw. have been associated with black foot disease of grapevines (Vitis spp.). Cylindrocarpon destructans (Zinnsm.) Scholten [anamorph of Neonectria radicicola (Gerlach & L. Nilsson) Mantiri & Samuels] was first recorded on grapevine in France in 1961 (Maluta & Larignon 1991). Since then it has been isolated from diseased vines in Tasmania (Sweetingham 1983), Sicily (Grasso 1984), Portugal (Rego 1994, Rego et al., 2000, 2001), Pennsylvania, U.S.A. (Gugino & Travis 2003), New Zealand and South Africa (Halleen et al. 2004). Various unidentified species of Cylindrocarpon have also been isolated from young vines and from declining vines with basal rot or root necrosis in Australia (Edwards & Pascoe 2004), Chile (Auger et al. 1999), Greece (Rumbos & Rumbou 2001), Spain (Armengol et al. 2001) and South Africa (Fourie et al. 2000, Fourie & Halleen 2001). In a recent taxonomic study, a second species, newly described as C. macrodidymum Schroers, Halleen & Crous (anamorph of Neonectria macrodidyma Halleen, Schroers & Crous) was associated with the disease. These isolates were obtained from grapevines in South Africa, Tasmania, New Zealand and Canada (Halleen et al. 2004). It is possible that C. macrodidymum was earlier incorrectly identified on grapevines as Cylindrocarpon obtusisporum (Cooke & Harkn.) Wollenw. (Grasso & Magnano di San Lio 1975, Scheck et al. 1998). Furthermore, Campylocarpon Halleen, Schroers & Crous, a newly described genus, which is Cylindrocarpon-like in morphology, has also been associated with the disease in South Africa (Halleen et al. 2004).
Booth (1966) artificially segregated Cylindrocarpon species into four groups based on the presence or absence of microconidia and chlamydospores. Cylindrocarpon magnusianum (Sacc.) Wollenw. (+ chlamydospores; – microconidia), which is the anamorph of the type species of Neonectria Wollenw., C. cylindroides Wollenw. (– chlamydospores; – microconidia), which is the type species of the genus Cylindrocarpon, and members of Cylindrocarpon species predominantly connected with teleomorphs of the Nectria mammoidea W. Phillips & Plowr. group (– chlamydospores; + microconidia) were core members of three of these anamorphic groups delineated by Booth (1966). A fourth group was centred on C. destructans (+ chlamydospores; + microconidia), which generally is accepted as the anamorph of Neon. radicicola. Rossman et al. (1999), Mantiri et al. (2001) and Brayford et al. (2004) recently transferred representatives of all “Nectria” groups with Cylindrocarpon anamorphs into Neonectria. Mantiri et al. (2001) and Brayford et al. (2004) analysed mitochondrial small subunit (SSU) ribosomal DNA (rDNA) sequence data of some of the species and concluded that the Neonectria/Cylindrocarpon species grouped together by this reclassification were monophyletic. However, these authors also found that this overall Neonectria/Cylindrocarpon clade included distinct subclades corresponding to at least three of the four groups delineated by Booth (1966). Significant molecular variation among taxa with Cylindrocarpon-like anamorphs was found by Seifert et al. (2003), in a study on fungi causing root rot of ginseng (Panax quinquefolius) and other hosts, encountered significant molecular variation particularly among Cylindrocarpon destructans-like strains and suggested that Neon. radicicola/C. destructans may present a complex of various species. Halleen et al. (2004) added an additional phylogenetic clade mainly comprising of root and rootstock pathogens of grapevines, that conformed well to the morphological concept of C. destructans. Although Halleen et al. (2004) referred to the primary causal organism of black foot disease of grapevine as C. destructans, the ex-type strain of Neon. radicicola CBS 264.65 did not form part of the clade comprising of grapevine isolates, nor did isolates from Panax, which is the host from which Booth (1966) selected the neotype of C. destructans. The aim of the present study was to determine the correct identity of C. destructans-like isolates occurring on grapevines. In order to do this, strains isolated from grapevines in several countries were subjected to DNA analyses of their ITS and β-tubulin genes and to mating studies in vitro.
MATERIALS AND METHODS
Isolates
Cylindrocarpon destructans strains, previously isolated from diseased grapevines in Portugal (Rego 1994, Rego et al. 2001), France, South Africa and New Zealand (Halleen et al. 2004), were obtained from the collection of the Centraalbureau voor Schimmelcultures in Utrecht, the Netherlands (CBS) (Table 1). Disease symptoms associated with these isolates include various forms of decline as well as typical black foot symptoms.
Table 1.
Cylindrocarpon and Neonectria isolates included in this study.
| Species | Isolate number1 | Host | Country | Collector | GenBank numbers3(ITS, TUB) |
|---|---|---|---|---|---|
| N. liriodendri / C. liriodendri | CBS 110.812; IMI 303645 | Liriodendron tulipifera | U.S.A. | — | DQ178163, DQ178170 |
| CBS 112591; CPC 3673 | Vitis vinifera | France | P. Larignon | AY677262, AY677245 | |
| CBS 112596; CPC 3994 | Vitis vinifera | South Africa | F. Halleen | AY677264, AY677239 | |
| CBS 112602; CPC 3998 | Vitis vinifera | South Africa | F. Halleen | AY677267, AY677242 | |
| CBS 112610; CPC 3674 | Vitis vinifera | France | P. Larignon | AY677270, AY677244 | |
| CBS 117526; Cy 68 | Vitis vinifera | Portugal | C. Rego | DQ179164, DQ178171 | |
| CBS 117527; Cy 76 | Vitis vinifera | Portugal | C. Rego | DQ178165, DQ178172 | |
| CBS 117640; IMI 357400; Cy 1 | Vitis vinifera | Portugal | C. Rego | DQ178166, DQ178173 | |
| N. ditissima / C. heteronema | CBS 117751; KIS 10463 | Malus sp. | Slovenia | H.J. Schroers & R. Mavec | DQ178167, — |
| CBS 117752; KIS 10462 | Malus sp. | Slovenia | H.J. Schroers & R. Mavec | DQ178168, — | |
| CPC 12078 | Malus sp. | The Netherlands | P.W. Crous | DQ178169, — |
CBS: Centraaibureau voor Schimmelcultures, Utrecht, The Netherlands; CPC: Culture collection of Pedro Crous, housed at CBS; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, U.K.; KIS: Agricultural Institute of Slovenia, Ljubljana, Slovenia.
Ex-type cultures.
ITS: internal transcribed spacer region, TUB: partial β-tubulin gene.
DNA phylogeny
Mycelium was grown in tubes with 2 mL of complete medium (Raper & Raper 1972) and DNA was extracted using the FastDNA® Kit (Bio 101, Carlsbad, CA, U.S.A.). PCR amplification and sequencing of the partial β-tubulin gene introns and exons and the ITS rDNA, was performed as described by Halleen et al. (2004). Newly generated sequences have been deposited in GenBank (Table 1).
Additional sequences were obtained from GenBank and added to the alignment. Sequences were manually aligned using Sequence Alignment Editor v. 2.0a11 (Rambaut 2002). In phylogenetic trees, downloaded sequences are indicated by their GenBank accession numbers; newly generated sequences are indicated by CBS strain numbers. A member of Campylocarpon (Halleen et al. 2004) was used as outgroup. Two datasets were created; analysis of the datasets in PAUP* 4.0b10 (Swofford 2002) consisted of distance (using the uncorrected “p”, Jukes-Cantor and HKY85 substitution models) and parsimony analyses as described by Halleen et al. (2004). For the parsimony analyses, heuristic searches were performed with 100 random taxon additions. Two gaps of more than 10 characters each (caused by the outgroup sequence) were coded as a single character in the ITS alignment in TreeBASE (S1511, M2716).
Taxonomy
Strains were grown in darkness or under continuous near-ultraviolet (nuv) light (400–315 nm) (Sylvania Blacklight-Blue, Osram Nederland B.V., Alphen aan den Rijn, the Netherlands) at 20 °C. Media used were synthetic nutrient-poor agar (SNA) with and without the addition of a 1 × 3 cm piece of filter-paper to the colony surface, potato-dextrose agar (Difco PDA, Becton Dickinson, Sparks, MD, U.S.A.), oatmeal agar (OA), and carnation leaf agar (CLA) (Gams et al. 1998), and malt extract agar (MEA) (Sigma-Aldrich Chemie BV, Zwijndrecht, the Netherlands) using 9 cm diam Petri dishes. Growth rates and colony diameters of cultures incubated in darkness were measured on PDA. Characters such as size and shape of conidia, phialides, and chlamydospores were determined from strains grown on SNA, PDA, or CLA after 14–21 d. Structures were mounted in lactic acid, and 30 measurements at × 1000 magnification were made of each structure. The 95 % confidence levels were calculated, and the extremes of spore measurements given in parentheses. Images were taken from slides mounted in lactic acid. Macroscopic characters of colonies were described after 14 d; colour names are from Rayner (1970). Cardinal temperatures for growth were assessed on PDA incubated for 7 d in the dark at 4, 10, 15, 20, 25, 30 and 35 °C. Mating experiments were performed on minimal salt medium at 25 °C, using autoclaved birch toothpicks as explained by Guerber & Correll (2001). Three replicates were done for each cross. Petri dishes were sealed with Parafilm (Pechiney Plastic Packaging, Menasha, WI, USA), incubated in a single layer under a mixture of cool-white fluorescent and nuv light, and observed at weekly intervals for a total period of 8 wk. Two strains were considered sexually compatible if they produced perithecia with viable, exuding masses of ascospores within this time.
RESULTS
DNA phylogeny
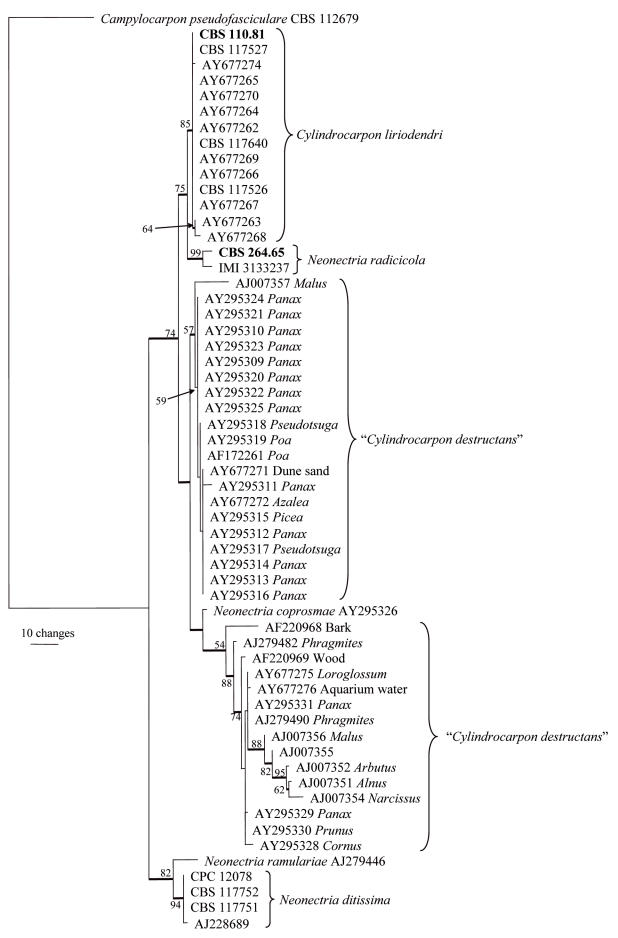
The manually adjusted ITS alignment contained 59 taxa and 472 characters including alignment gaps. Of the 472 characters, 47 were parsimony-informative, 60 were variable and parsimony-uninformative, and 365 were constant. Parsimony analysis of the ITS data yielded 61 most parsimonious trees [tree length (TL) = 224 steps; consistency index (CI) = 0.795; retention index (RI) = 0.924; rescaled consistency index (RC) = 0.734], one of which is shown in Fig. 1. The topologies of the trees generated with neighbour-joining analyses using the three substitution models were identical to each other and were also similar to the trees obtained using parsimony (data not shown). In the tree (Fig. 1), isolates that cluster with the ex-type strain of C. liriodendri grouped with a bootstrap support value of 85 %. Two isolates of Neon. radicicola (99 % bootstrap support) formed the closest sister clade (75 % bootstrap support). Isolates of “C. destructans” form two poorly supported clades (57 and 54 %, respectively) separated by Neon. coprosmae. The final clade is in a basal position and contains isolates of N. ditissima (94 % bootstrap support) with N. ramulariae as closest sister (82 % bootstrap support).
Fig. 1.
One of 61 most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the ITS sequence alignment. The scale bar shows 10 changes and bootstrap support values from 1000 replicates are shown at the nodes. Thickened lines indicate the strict consensus branches and ex-type strains are shown in bold print. The host genus or source is indicated next to the GenBank accession numbers for the taxa in the “C. destructans” complex. The tree was rooted to Campylocarpon pseudofasciculare AY677306.
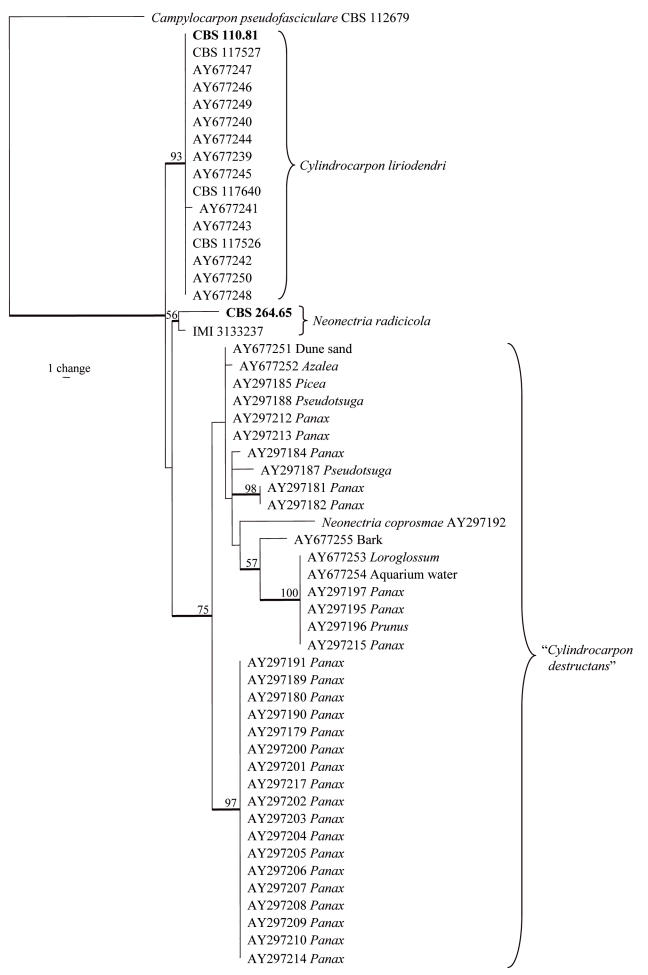
The manually adjusted β-tubulin alignment contained 55 taxa and 327 characters including alignment gaps. Of the 327 characters, 33 were parsimony-informative, 39 were variable and parsimony-uninformative, and 255 were constant. Parsimony analysis of the β-tubulin data yielded 180 most parsimonious trees (TL = 103 steps; CI = 0.864; RI = 0.959; RC = 0.829), one of which is shown in Fig. 2. The topology of the trees generated with neighbor-joining analysis using the three substitution models and the trees obtained using parsimony only differed in the order of the isolates in the “C. destructans” clade (data not shown). As with the ITS tree, isolates of C. liriodendri form a well-defined clade (bootstrap support value of 93 %), but the two isolates of Neon. radicicola group together in a poorly supported clade (56 % bootstrap support). Isolates in the “C. destructans” complex clade (75 % bootstrap support) are mainly in basal positions with a small number of defined clades with high bootstap support values. Neonectria coprosmae is also included in this clade.
Fig. 2.
One of 180 most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the β-tubulin sequence alignment. The scale bar shows a single change and bootstrap support values from 1000 replicates are shown at the nodes. Thickened lines indicate the strict consensus branches and type strains are shown in bold print. The host genus or source is indicated next to the GenBank accession numbers for the taxa in the “C. destructans” complex. The tree was rooted to Campylocarpon pseudofasciculare AY677214.
Taxonomy
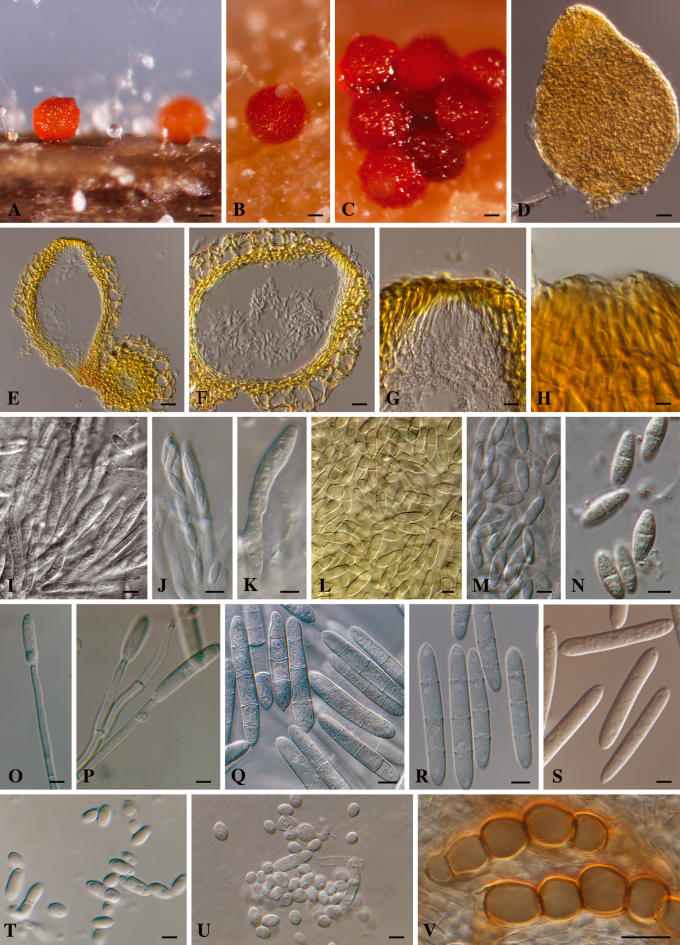
Neonectria liriodendriHalleen, Rego & Crous, sp. nov. MycoBank MB500226. Figs 3A–V, 4.
Fig. 3.
Neonectria liriodendri and its Cylindrocarpon liriodendri anamorph. A–C. Perithecia on beach toothpicks. D. Perithecium mounted in lactic acid. E–H. Sections though perithecia, showing wall anatomy and ostiolar area. I–K. Asci. L–N. Ascospores. O–P. Conidiophores. Q–S. Macroconidia. T–U. Subcylindrical, ellipsoid and ovoid microconidia. V. Chlamydospores in chains. Scare bars: A = 90 μm, B–C = 70 μm, D = 30 μm, E = 80 μm, F = 26 μm, G–H = 20 μm, I–K = 5 μm, L–N = 3 μm, O–S = 5 μm, T–U = 4 μm, V = 15 μm.
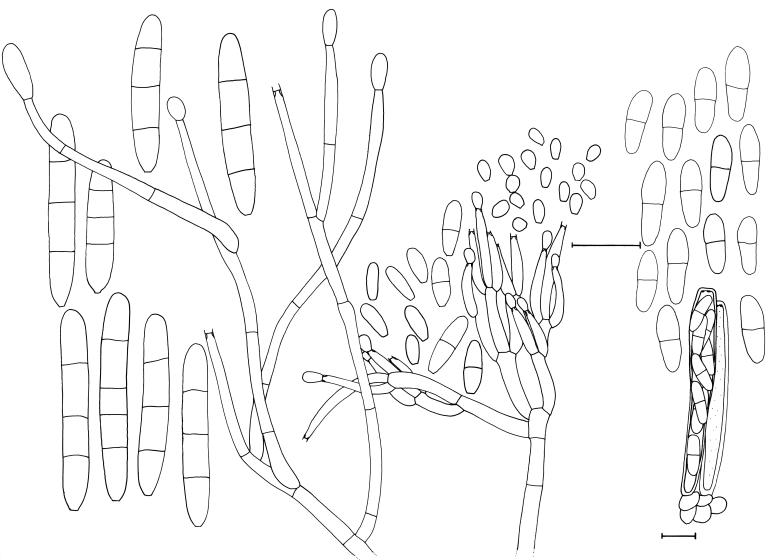
Fig. 4.
Neonectria liriodendri and its Cylindrocarpon liriodendri anamorph (CBS H-17776). Scale bar = 10 μm.
Anamorph:Cylindrocarpon liriodendriJ.D. MacDon. & E.E. Butler, Plant Disease 65: 156. 1981.
Neonectriae radicicolae similis sed ascosporis levibus vel verruculosis, et peritheciis levibus vel verruculosis distincta. Ascosporae (7–)9–11(–14) × (2.5–)3–3.5(–4) μm.
Perithecia (not known from nature) formed heterothallically in vitro, disposed solitarily or in groups of up to six, developing directly on the agar surface or on sterile pieces of beach wood or pine needles, ovoid to obpyriform, with a flattened apex, up to 70 μm wide, orange to red, becoming purple-red in 3 % KOH (positive colour reaction), smooth to warted, up to 300 μm diam and high; with minute stroma of dark red pseudoparenchymatous cells; perithecial wall consisting of two regions; outer region 15–30 μm thick, composed of 1–3 layers of angular to subglobose cells, 10–25 × 8–17 μm; cell walls up to 1 μm thick; inner region 10–15 μm thick, composed of cells that are flat in transverse optical section and angular to oval in subsurface optical face view, 7–15 × 3–5 μm; perithecial warts consisting of globose to subglobose cells, 15–30 × 15–20 μm in surface view. Asci narrowly clavate to cylindrical, 45–60 × 5–6 μm, 8-spored; apex subtruncate, with a minutely visible ring. Ascospores medianly 1-septate, ellipsoidal to oblong ellipsoidal, somewhat tapering towards both ends, smooth to finely warted, hyaline, become pale brown with age, (7–)9–11(–14) × (2.5–)3–3.5(–4) μm.
Conidiophores simple or complex, sporodochial. Simple conidiophores arising laterally or terminally from the aerial mycelium or erect, arising from the agar surface, solitary to loosely aggregated, unbranched or sparsely branched, 1–6-septate, rarely consisting only of the phialide, 40–160 μm long; phialides monophialidic, cylindrical, 20–40 × 3–4 μm, 2–2.5 μm near the aperture. Sporodochial conidiophores aggregated in pionnote sporodochia, irregularly branched; phialides cylindrical, mostly widest near the base, 15–30 × 2.5–3.5 μm, 2–2.5 μm wide near the aperture. Micro- and macroconidia present on both types of conidiophores. Macroconidia predominating, formed by both types of conidiophores, predominantly (1–)3-septate, straight or sometimes slightly curved, cylindrical, mostly with a visible, basal or slightly laterally displaced hilum; 3-septate macroconidia, (24–)35–40(–55) × (4.5–)5.5–6(–6.5) μm (n = 116). Microconidia sparsely produced on all media, 0–1-septate, ellipsoidal to subcylindrical to ovoid, more or less straight, with a minutely or clearly visible lateral hilum; aseptate subcylindrical to ellipsoidal microconidia, 5–15 × 2.5–4 μm; aseptate ovoid microconidia, 3–5 × 3–4 μm, formed predominently on dense, penicillately branched conidiophores on CLA and twigs, and then also without subcylindrical to ellipsoidal microconidia; occurring on other media as a mixture with ovoid microconidia. Conidia formed in heads on simple conidiophores, as hyaline masses on simple as well as complex conidiophores. Chlamydospores common, medium brown, ovoid to ellipsoid, mostly in short, intercalary chains, 10–20 × 10–17 μm.
Specimens of Neonectria liriodendri examined obtained from crossings: CBS H-17776, heterothallic mating of CBS 117527 × CBS 112596, holotype of Neonectria liriodendri; CBS H-17781, mating of CBS 117526 × CBS 117527; CBS H-17780, mating of CBS 117527 × CBS 110.81; CBS H-17779, mating of CBS 117527 × CBS 112596; CBS H-17778, mating of CBS 117527 × CBS 112602; CBS H-17777, mating of CBS 117527 × CBS 112610.
Strains examined: Portugal, Vitis vinifera, coll./isol. C. Rego, CBS 117526, 117527. France, from Vitis vinifera, coll./isol. P. Larignon, CBS 112591, 112610. South Africa, from Vitis vinifera, coll./isol. F. Halleen (CBS 112596, 112602). U.S.A., California, from Liriodendron tulipifera, CBS 110.81, ex-type strain of Cylindrocarpon liriodendri.
Cultural characteristics: Colonies on PDA (surface and reverse) cinnamon to sepia, with sparse aerial mycelium. On OA dark brick to fawn (surface and reverse). Minimum temperature for growth < 4 °C; optimum temperature 20–25 °C, at which PDA colonies reach 30–42 mm diam after 7 d in the dark; maximum temperature between 30–35 °C. Yellow pigmentation not observed.
Host range and distribution: Vitis vinifera (France, Portugal, New Zealand, South Africa), Cyclamen sp. (The Netherlands), Liriodendron tulipifera (U.S.A., California).
Habitat: Typically isolated from roots and rootstocks of grapevines, causing black foot disease. The ex-type culture was obtained from Liriodendron tulipifera in California, where it caused root rot, while another was associated with bulb rot of a Cyclamen sp. in the Netherlands.
Phylogenetic affinity: Nectriaceae, Hypocreales.
DISCUSSION
Species of Cylindrocarpon Wollenw. are commonly isolated from soil and regarded to be saprobes or weak pathogens of a wide range of herbaceous and woody plants (Brayford 1993). Since C. destructans was first reported from grapevine in France in 1961, it has been recognised as a pathogen of grapevines cultivated in various countries of different continents (Sweetingham 1983, Grasso 1984, Rego 1994, Fourie et al. 2000, Gugino and Travis, 2003). In a recent taxonomic study revising Cylindrocarpon spp. associated with grapevines, the primary organism causing the black foot disease was identified as C. destructans (Halleen et al. 2004). Halleen et al. (2004) also described a species now known as C. macrodidymum. Although difficult to prove, it is likely that strains pathogenic to grapevine roots but in older literature identified as C. obtusisporum (Grasso & Magnano di San Lio 1975, Scheck et al. 1998) belong to this species. Furthermore, two Cylindrocarpon-like species were removed from Cylindrocarpon and classified as Campylocarpon (Halleen et al. 2004). Although all four species have been found to cause disease on grapevines, C. destructans proved to be the species most commonly isolated from diseased vines, and could possibly be more important than the other pathogens in this disease complex. Further research is currently underway, however, to investigate this aspect.
Several pathogenicity studies have previously been conducted with isolates from the “C. destructans” clade. Oliveira et al. (1998) inoculated rooted cuttings of the grapevine cultivar Seara Nova by dipping the roots in a spore suspension of “C. destructans” (Cy1 = CBS 117640). Typical black foot disease symptoms were observed within 60 d. Similar results were obtained when rooted cuttings of `99Richter' rootstock were inoculated with 12 “C. destructans” isolates, two of which were Cy 68 (CBS 117526) and Cy 76 (CBS 117527). Inoculation significantly reduced plant height and the number of roots, whilst isolate CBS 117526 was considered to be one of the most virulent isolates evaluated (Rego et al. 2001). Inoculation of 6-mo-old potted grapevine rootstocks (cv. Ramsey) with isolate CBS 112597 resulted in death of 27.5 % of the plants 60 d after inoculation, whilst the remaining plants suffered a dramatic reduction in root and shoot mass (Halleen et al. 2004).
Cylindrocarpon liriodendri was first associated with root rot of tulip poplar (Liriodendron tulipifera) in California. Affected plants appeared severely stunted and the root systems were covered with black, dry, scabby lesions that completely girdled or rotted off distal portions of some roots (MacDonald & Butler 1981). MacDonald & Butler (1981) reported that C. liriodendri does not form microconidia. Therefore, it was accepted to not be part of the C. destructans-complex. Our observations are contrasting those of MacDonald & Butler (1981) because the ex-type strain (CBS 110.81) did form microconidia; also, sequences of CBS 110.81 were identical to other isolates from vines (formerly identified as C. destructans) that also formed microconidia in culture. If the C. destructans isolates occurring on grapevines were in fact C. liriodendri, this raised the question as to the identity of Neon. radicicola and its purported anamorph, C. destructans, and the originally described C. radicicola Wollenw. Neonectria radicicola was originally described from rotting bulbs of Cyclamen persicum collected in Sweden, of which an ex-type culture was available for study (CBS 264.65) (Gerlach & Nilsson 1963). The anamorph linked to this species is C. destructans, which Booth (1966) based on a North American neotype from Kentucky, collected on Panax ginseng (CUP 11985), for which there is no culture available. In a recent study, Seifert et al. (2003) showed that there was more than one C. destructans-like species on Panax. Here it is shown that none of these clades are identical to the ex-type strains of Neon. radicicola or C. liriodendri. Cylindrocarpon liriodendri is a name available for the grapevine pathogen, which clusters in its own well supported clade, for which the name Neon. liriodendri is introduced to accommodate its teleomorph.
To fully resolve the taxonomic status of the species present in the C. destructans species complex, however, detailed mating studies with all clades in this complex, and additional sequence data of other loci need to be generated. This work is currently in progress and will be reported on in future studies.
Taxonomic novelty: Neonectria liriodendri Halleen, Rego & Crous sp. nov.
References
- Armengol J, Vicent A, Torné L, García-Figueres F, García-Jiménez J (2001). Fungi associated with esca and grapevine declines in Spain: a three-year survey. Phytopathologia Mediterranea 40: S325–S329. [Google Scholar]
- Auger J, Droguett A, Esterio M (1999). The red globe decline. In: Proceedings of the 1st International Workshop on Grapevine Trunk Diseases: Esca and grapevine declines. Siena, Italy, 1–3 October 1999: 23.
- Booth C (1966). The genus Cylindrocarpon. Mycological Papers 104: 1–56. [Google Scholar]
- Brayford D (1993). Cylindrocarpon. In: Methods for research on soilborne phytopathogenic fungi. (Singleton LL, Mihail JD, Rush M, eds). APS Press, St. Paul, U.S.A.: 103–106.
- Brayford D, Honda BM, Mantiri FR, Samuels GJ (2004). Neonectria and Cylindrocarpon: the Nectria mammoidea group and species lacking macroconidia. Mycologia 96: 572–597. [PubMed] [Google Scholar]
- Edwards J, Pascoe IG (2004). Occurrence of Phaeomoniella chlamydospora and Phaeoacremonium aleophilum associated with Petri disease and esca in Australian grapevines. Australasian Plant Pathology 33: 273–279. [Google Scholar]
- Fourie PH, Halleen F (2001). Diagnose van swamsiektes en hul betrokkenheid by terugsterwing van jong wingerd. Wynboer 149: 19–23. [Google Scholar]
- Fourie PH, Halleen F, Volkmann AS (2000). Fungi associated with grape wood, root and trunk diseases: A summary of the 1999–2000 results from the diagnostic service at Nietvoorbij. Proceedings of the 2nd International Viticulture & Enology Congress, November 8–10, 2000, Cape Town, South Africa.
- Gams W, Hoekstra ES, Aptroot A, eds. (1998). CBS Course of Mycology. 4th edn. Centraalbureau voor Schimmelcultures, Baarn.
- Gerlach W, Nilsson L (1963). Beiträge zur kenntnis der gattung Cylindrocarpon Wr. V. Nectria radicicola n. sp., die bisher unbekannte hauptfruchtform von Cylindrocarpon radicicola Wr. Phytopathologische Zeitschrift 48: 251–257. [Google Scholar]
- Grasso S (1984). Infezioni di Fusarium oxysporum e di Cylindrocarpon destructans associate a una moria di giovani piante di vite in Sicilia. Informatore Fitopatologico 1: 59–63. [Google Scholar]
- Grasso S, Magnano Di San Lio G (1975). Infections of Cylindrocarpon obtusisporum on grapevines in Sicily. Vitis 14: 36–39. [Google Scholar]
- Guerber JC, Correll JC (2001). Characterization of Glomerella acutata, the teleomorph of Colletotrichum acutatum. Mycologia 93: 216–229. [Google Scholar]
- Gugino BK, Travis J.W (2003). Suppression of Cylindrocarpon destructans utilizing composted soil amendments. Phytopathology 93: S31. [Google Scholar]
- Halleen F, Schroers H-J, Groenewald JZ, Crous PW (2004). Novel species of Cylindrocarpon (Neonectria) and Campylocarpon gen. nov. associated with black foot disease of grapevines (Vitis spp.). Studies in Mycology 50: 431–455. [Google Scholar]
- MacDonald JD, Butler EE (1981). Cylindrocarpon root rot of tulip poplar. Plant Disease 65: 154–157. [Google Scholar]
- Maluta D-R, Larignon P (1991). Pied-noir: mieux vaut prévenir. Viticulture 11: 71–72. [Google Scholar]
- Mantiri FR, Samuels GJ, Rahe JE, Honda BM (2001). Phylogenetic relationship in Neonectria species having Cylindrocarpon anamorphs inferred from mitochondrial ribosomal DNA sequences. Canadian Journal of Botany 79: 334–340. [Google Scholar]
- Oliveira H, Nascimento T, Rego C (1998). Crown gall and Cylindrocarpon black-foot diseases of grapevine in Portugal. In: Proceedings of the 19th International Geisenheim workshop on grapevine grafting. Geisenheim, Germany 2–4 July 1998: 23–34.
- Rambaut A (2002). Sequence Alignment Editor. Version 2.0. Department of Zoology, University of Oxford, Oxford.
- Raper JR, Raper CA (1972). Genetic analysis of the life cycle of Agaricus bisporus. Mycologia 64: 1088–1117. [Google Scholar]
- Rayner RW (1970). A mycological colour chart. CMI and British Mycological Society. Kew, Surrey, England.
- Rego MC (1994). Nova e grave micose da videira em Portugal. Agente responsável: Cylindrocarpon destructans (Zins.) Scholten. Publicação do Laboratório de Patologia Vegetal Veríssimo de Almeida 67: 1–4. [Google Scholar]
- Rego C, Oliveira H, Carvalho A, Phillips A (2000). Involvement of Phaeoacremonium spp. and Cylindrocarpon destructans with grapevine decline in Portugal. Phytopathologia Mediterranea 39: 76–79. [Google Scholar]
- Rego C, Nascimento T, Oliveira H (2001). Characterisation of Cylindrocarpon destructans isolates from grapevines in Portugal. Phytopathologia Mediterranea 40: S343–S350. [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, Lowen R (1999). Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42: 1–260. [Google Scholar]
- Rumbos I, Rumbou A (2001). Fungi asociated with esca and young grapevine decline in Greece. Phytopathologia Mediterranea 40: S330–S335. [Google Scholar]
- Scheck HJ, Vasquez SJ, Fogle D, Gubler WD (1998). Grape growers report losses to black-foot and grapevine decline. California Agriculture 52: 19–23. [Google Scholar]
- Seifert KA, McMullen CR, Yee D, Reeleder RD, Dobinson KF (2003). Molecular differentiation and detection of ginseng-adapted isolates of the root rot fungus Cylindrocarpon destructans. Phytopathology 93: 1533–1542. [DOI] [PubMed] [Google Scholar]
- Sweetingham M (1983). Studies on the nature and pathogenicity of soilborne Cylindrocarpon spp. Ph.D. Thesis, University of Tasmania.
- Swofford DL (2002). PAUP 4.0b10: Phylogenetic analysis using parsimony. Sinauer Associates, Sunderland, MA, U.S.A.