Abstract
The present study compares all known species of Cylindrocladium that have clavate vesicles. Several isolates were obtained from baited soils collected in various parts of the world, while others were associated with leaf litter or symptomatic plant hosts. Isolates were compared based on morphology, as well as DNA sequence data from their β-tubulin and histone gene H3 regions. Cylindrocladium australiense and Cy. ecuadoriae, are described as new species, a decision based on morphology and molecular data. A group of isolates associated with toppling disease of banana in the West Indies is identified as Cy. flexuosum. An epitype is designated for Cy. ilicicola, and a new name, Curvicladiella, proposed to replace the anamorphic genus Curvicladium, which is a homonym.
Keywords: Ascomycetes, Calonectria, Cylindrocladium, Hypocreales, leaf spots, soil fungi, systematics
INTRODUCTION
Members of the genus Calonectria De Not. (Ca.) (Nectriaceae, Hypocreales, Ascomycetes) and their Cylindrocladium Morgan (Cy.) anamorphs are commonly associated with a wide range of plant disease symptoms (Crous 2002). The current paper represents the second in a series assessing the taxonomy of species of Cylindrocladium, by integrating morphology with DNA sequence data and sexual compatibility studies (Crous et al. 2004).
Cylindrocladium species with clavate vesicles are well-known pathogens from a wide range of hosts in most subtropical to tropical countries (Crous & Wingfield 1992, Crous et al. 1995, 1997, 1999, 2000, Kang et al. 2001, Crous 2002). In the current study, we obtained numerous isolates of Cylindrocladium from baited soils collected in tropical areas. Further Cylindrocladium isolates were obtained from a biotic complex including root rot fungi and plant-parasitic nematodes associated with toppling disease of banana (Risède & Simoneau 2001). Previous studies have shown that isolates resembling Cy. gracile (Bugn.) Boesew. were pathogenic to banana, and associated with stem lesions, root breakage and toppling disease (Risède & Simoneau 2001, 2004). The aim of the present study was to analyze all available Cylindrocladium strains with clavate vesicles using morphology and DNA sequence analysis of their β-tubulin and histone H3 gene regions in order to resolve the status of Cylindrocladium species with clavate vesicles. A further aim was to identify the Cylindrocladium sp. associated with toppling disease of banana.
MATERIALS AND METHODS
Isolates
Isolates were obtained from plant hosts, or baited from soil as explained in Crous (2002). Cultural characteristics and morphology were determined on plates containing 2 % malt extract agar (MEA) (20 g/L), and carnation leaf agar (CLA) [1 % water agar (10 g/L) with autoclaved carnation leaves placed onto the medium] in the other (Gams et al. 1998). Plates were incubated for 7 d at 25 °C under continuous near-UV light, to promote sporulation.
DNA phylogeny
Genomic DNA was isolated from fungal mycelia collected from the plates using the isolation protocol of Lee & Taylor (1990). Two loci were amplified and sequenced as explained in Crous et al. (2004), namely, part of the β-tubulin gene, amplified with primers T1 (O'Donnell & Cigelnik 1997) and CYLTUB1R (Crous et al. 2004); and part of the histone H3 gene using primers CYLH3F and CYLH3R (Crous et al. 2004).
The sequences generated in this study were added to other sequences obtained from GenBank (http://www.ncbi.nlm.nih.gov) and TreeBASE (http://www.treebase.org) and the alignment was assembled using Sequence Alignment Editor v. 2.0a11 (Rambaut 2002) with manual adjustments for improvement made visually where necessary. Sequences for Cylindrocladiella peruviana (Bat., J.L. Bezerra & M.M.P. Herrera) Boesew. and Cylindrocladiella lageniformis Crous, M.J. Wingf. & Alfenas were added to the alignments as outgroups.
The phylogenetic analyses of sequence data were done using PAUP (Phylogenetic Analysis Using Parsimony) v. 4.0b10 (Swofford 2002). Phylogenetic analysis of both datasets in PAUP consisted of distance (using the uncorrected “p”, Jukes-Cantor and HKY85 substitution models) and parsimony analysis as described in Crous et al. (2004). Sequences were deposited in GenBank (Table 1) and the alignments in TreeBASE (S1508, M2711).
Table 1.
Isolates of Cylindrocladium (Calonectria) species studied.
| Species | Isolate number1,2 | β-tubulin3 | Histone H33 | Host | Country | Collector |
|---|---|---|---|---|---|---|
| Ca. avesiculata (Cy. avesiculatum) | ATCC 38226; CPC 2373T | AF333392 | DQ190620 | Ilex vomitoria | U.S.A. | S.A. Alfieri |
| Ca. clavata (Cy. flexuosum) | CBS 112675; SLU2 | DQ190547 | DQ190621 | Musa sp. | Saint Lucia | J.M. Risède |
| CBS 112676; Gua9 | DQ190548 | DQ190622 | Musa sp. | Guadeloupe | J.M. Risède | |
| CBS 114557; CPC 2536T | AF333396 | DQ190623 | Callistemon viminalis | U.S.A. | N.E. El-Gholl | |
| CBS 114666; CPC 2537T | DQ190549 | DQ190624 | — | U.S.A. | N.E. El-Gholl | |
| CPC 11347; Mar8 | DQ190550 | DQ190625 | Musa sp. | Martinique | J.M. Risède | |
| CPC 11348; Mar23 | DQ190551 | DQ190626 | Musa sp. | Martinique | J.M. Risède | |
| CPC 11349; Mar11 | DQ190552 | DQ190627 | Musa sp. | Martinique | J.M. Risède | |
| CPC 11350; SLU5 | DQ190553 | DQ190628 | Musa sp. | Saint Lucia | J.M. Risède | |
| CPC 11351; Gua12 | DQ190554 | DQ190629 | Musa sp. | Saint Lucia | J.M. Risède | |
| CPC 11352 | DQ190555 | DQ190630 | Musa sp. | Martinique | J.M. Risède | |
| Ca. colhounii (Cy. colhounii) | CBS 111205; CPC 1330 | DQ190556 | — | — | Indonesia | M.J. Wingfield |
| CBS 111367; CPC 1339 | AF232851 | DQ190631 | Eucalyptus sp. | Indonesia | M.J. Wingfield | |
| CBS 112739; CPC 4082 | DQ190557 | DQ190632 | Eucalyptus grandis | U.S.A. | M.J. Wingfield | |
| CBS 112948; CPC 4669 | DQ190558 | DQ190633 | — | U.S.A. | — | |
| CBS 112951; CPC 4717 | DQ190559 | DQ190634 | Eleaeocarpus angustifolius | Australia | C. Pearce & B. Paulus | |
| CBS 114036; CPC 10718 | DQ190560 | DQ190635 | Rhododendron sp. | U.S.A. | C.S. Hodges | |
| CBS 114660; CPC 2407 | DQ190561 | DQ190636 | Arachis pintoi | Australia | D. Hutton | |
| CBS 114695; CPC 2424 | DQ190562 | DQ190637 | A. pintoi | Australia | D. Hutton | |
| CBS 114704; CPC 2422 | DQ190563 | DQ190638 | A. pintoi | Australia | D. Hutton | |
| CBS 293.79 | DQ190564 | DQ190639 | — | Indonesia | — | |
| CPC 681 | AF232852 | DQ190640 | Soil | Thailand | M.J. Wingfield | |
| CPC 705 | AF232854 | DQ190641 | Soil | South Africa | M.J. Wingfield | |
| CPC 1237 | AF232853 | DQ190642 | Eucalyptus sp. | South Africa | M.J. Wingfield | |
| Ca. gracilipes (Cy. graciloideum) | CBS 111040; CPC 1159 | DQ190565 | DQ190643 | — | Colombia | M.J. Wingfield |
| CBS 111141; CPC 1211T | DQ190566 | DQ190644 | Eucalyptus sp. | Colombia | M.J. Wingfield | |
| CBS 115674; CPC 1153 | AF333406 | DQ190645 | Soil | Colombia | M.J. Wingfield | |
| Ca. gracilis (Cy. pseudogracile) | AR2677T | AF232858 | DQ190646 | Manilkara sp. | Brazil | — |
| CBS 111284; CPC 1483 | DQ190567 | DQ190647 | — | Brazil | P.W. Crous | |
| CBS 114454; CPC 1588 | AF232864 | DQ190648 | Soil | Brazil | P.W. Crous | |
| Cy. hawksworthii | CBS 111870; MUCL 30866; CPC 2405T | AF333407 | DQ190649 | — | Mauritius | A. Peerally |
| Ca. indusiata (Cy. theae) | ATCC 48895; CPC 2383 | AF232861 | — | Rhododendron cv. Kingfisher | U.S.A. | — |
| CBS 111399; CPC 1620 | DQ190568 | DQ190650 | — | Ecuador | M.J. Wingfield | |
| CBS 111705; CPC 1713 | DQ190569 | DQ190651 | Rumohra adiantiformis | U.S.A. | J.Y. Uchida | |
| CBS 114175; CPC 1712 | DQ190570 | DQ190652 | Strelitzia nicolaii | U.S.A. | J.Y. Uchida | |
| CBS 114684; UFV 16A; CPC 2446 | AF232862 | DQ190653 | Rhododendron sp. | U.S.A. | N.E. El-Gholl | |
| Ca. leguminum (Cy. leguminum) | CBS 728.68; IMI 299578T | AF389837 | DQ190654 | — | — | M.B. Figueiredo |
| Ca. macroconidialis (Cy. macroconidiale) | CBS 114880; CPC 307T | AF232855 | DQ190655 | Eucalyptus grandis | South Africa | P.W. Crous |
| CPC 413 | AF232856 | DQ190656 | Eucalyptus sp. | South Africa | P.W. Crous | |
| Ca. madagascariensis (Cy. madagascariense) | CBS 114539; CPC 2321 | AF333416 | — | Soil | Madagascar | J.E. Taylor |
| CBS 114571; CPC 2253 | DQ190571 | DQ190657 | — | Madagascar | P.W. Crous | |
| CBS 114572; CPC 2252T | DQ190572 | DQ190658 | — | Madagascar | P.W. Crous | |
| CPC 2322 | AF333417 | — | Soil | Congo | J. Roux | |
| Ca. multiseptata (Cy. multiseptatum) | CBS 112682; CPC 1589T | DQ190573 | DQ190659 | Eucalyptus sp. | Indonesia | M.J. Wingfield |
| CPC 1602; CMW 4054 | AF210866 | — | Eucalyptus sp. | Indonesia | M.J. Wingfield | |
| Ca. reteaudii (Cy. reteaudii) | CBS 112143; CPC 3200 | DQ190574 | DQ190660 | Eucalyptus sp. | Vietnam | M.J. Dudzinski & P.Q. Thu |
| CBS 112144; CPC 3201T | AF389833 | DQ190661 | E. camaldulensis | Vietnam | M.J. Dudzinski & P.Q. Thu | |
| CBS 112146; CPC 3213 | AF389835 | DQ190662 | E. urophylla | Australia | B. Brown | |
| CBS 112147; CPC 3214 | AF389830 | DQ190663 | E. camaldulensis | Vietnam | M.J. Dudzinski & P.Q. Thu | |
| CBS 112149; CPC 3216 | AF389832 | DQ190664 | E. urophylla | Australia | M. Ramsden | |
| CBS 112151; CPC 3202 | DQ190575 | DQ190665 | E. urophylla | Australia | C. Hanwood | |
| CBS 112153; CPC 3205 | AF389831 | DQ190666 | E. camaldulensis | Vietnam | M.J. Dudzinski & P.Q. Thu | |
| CBS 112155; CPC 3210 | AF389834 | DQ190667 | E. pellita | Australia | P.Q. Thu & K.M. Old | |
| CBS 112634; CPC 4233 | DQ190576 | DQ190668 | Xanthorrhoea australis | Australia | T. Baigent | |
| CBS 112955; CPC 4716 | DQ190577 | DQ190669 | Ficus pleurocarpa | Australia | C. Pearce & B. Paulus | |
| CBS 113582; CPC 516 | AF389846 | DQ190670 | Eucalyptus sp. | Thailand | M.J. Wingfield | |
| CBS 113583; CPC 759 | AF389847 | DQ190671 | Eucalyptus sp. | Madagascar | P.W. Crous | |
| CBS 113925; ATCC 16550; IMI 114953; CPC 2366 | AF389843 | DQ190672 | Scoiopendrium sp. | — | — | |
| CBS 582.50; CPC 3701 | AF389836 | DQ190673 | Seedling of Hibiscus sabdariffa | Indonesia | K.B. Boedijn & J. Reitsma | |
| CPC 3217 | AF389829 | DQ190674 | E. camaldulensis | Vietnam | M.J. Dudzinski & P.Q. Thu | |
| Ca. rumohrae (Cy. rumohrae) | CBS 111431; UFV 218; CPC 1716T | AF232871 | DQ190675 | Rumohra adiantiformis | Panama | A.C. Alfenas |
| CBS 114529; CPC 1603 | AF232873 | DQ190676 | Adiantum sp. | Netherlands | R. Pieters | |
| UFV 215; CPC 1831 | AF232872 | DQ190677 | Rumohra adiantiformis | Panama | A.C. Alfenas | |
| Cy. penicilloides | CBS 174.55; CPC 2388T | AF333414 | — | Prunus sp. | Japan | Tubaki |
| Ca. pteridis (Cy. pteridis) | CBS 111778; UFV 43; CPC 2451 | AF232859 | DQ190678 | — | Brazil | J.C. Dianese |
| CBS 111793; ATCC 34395; CPC 2372T | DQ190578 | DQ190679 | Arachnoides adiantiformis | U.S.A. | — | |
| CBS 111867; CPC 2447 | DQ190579 | DQ190680 | Pinus caribaea | Spain | T.L. Krugner | |
| CBS 111871; UFV 10-A; CPC 2443 | DQ190580 | DQ190681 | Pinus sp. | Spain | T.L. Krugner | |
| CPC 2190 | AF232860 | DQ190682 | Eucalyptus sp. | Brazil | P.W. Crous | |
| CPC 2869 | AF333415 | — | Eucalyptus sp. | Brazil | P.W. Crous | |
| CPC 11284 | DQ190581 | DQ190683 | E. grandis | Brazil | A.C. Alfenas | |
| CPC 11285 | DQ190582 | DQ190684 | E. grandis | Brazil | A.C. Alfenas | |
| CPC 11286 | DQ190583 | DQ190685 | E. grandis | Brazil | A.C. Alfenas | |
| CPC 11435 | DQ190584 | DQ190686 | Eucalyptus sp. | Brazil | A.C. Alfenas | |
| CPC 11569 | DQ190585 | DQ190687 | Eucalyptus sp. | Brazil | A.C. Alfenas | |
| CPC 11618 | DQ190586 | DQ190688 | Tillandsia wagneriana | Netherlands | C.F. Hill | |
| CPC 11619 | DQ190587 | DQ190689 | Guzmania wittmackii | Netherlands | C.F. Hill | |
| CPC 12072 | DQ190588 | DQ190690 | — | New Zealand | C.F. Hill | |
| CPC 12114 | DQ190589 | DQ190691 | Drosera sp. | Netherlands | J. Dijksterhuis | |
| Cy. acicola | CBS 114812 | DQ190590 | DQ190692 | Diffuse leaf lesions of Phoenix canariensis | New Zealand | H. Pearson |
| CBS 114813 | DQ190591 | DQ190693 | Diffuse leaf lesions of P. canariensis | New Zealand | H. Pearson | |
| CPC 10898T | DQ190592 | DQ190694 | Pinus sp. | New Zealand | M. Dick | |
| Cy. angustatum | CBS 112133; P99-1321; CPC 3152 | DQ190593 | DQ190695 | Tillandsia capitata | U.S.A. | R.M. Leahy |
| CBS 114544; P99-0454; CPC 2347T | AF207543 | DQ190696 | T. capitata | U.S.A. | N.E. El-Gholl | |
| CBS 114766; CPC 4522 | DQ190594 | DQ190697 | T. tricolor | Guatemala | C.F. Hill | |
| CBS 114767; CPC 4523 | DQ190595 | DQ190698 | T. tricolor | Guatemala | C.F. Hill | |
| Cy. australiense | CBS 112954; CPC 4714T | DQ190596 | DQ190699 | Ficus pleurocarpa | Australia | C. Pearce & B. Paulus |
| Cy. clavatum (synonym of Cy. gracile) | CBS 111776; ATCC 22833; CPC 2479T | AF232850 | DQ190700 | Pinus caribaea | Brazil | C.S. Hodges |
| Cy. curvisporum | CPC 763T | AF333394 | DQ190701 | Soil | Madagascar | P.W. Crous |
| CPC 765 | AF333395 | AY725664 | Soil | Madagascar | P.W. Crous | |
| Cy. ecuadoriae | CBS 111383; CPC 1587 | DQ190597 | DQ190702 | Soil | Brazil | P.W. Crous |
| CBS 111393; CPC 1627 | DQ190598 | DQ190703 | Soil | Ecuador | M.J. Wingfield | |
| CBS 111394; CPC 1628 | DQ190599 | DQ190704 | Soil | Ecuador | M.J. Wingfield | |
| CBS 111406; CPC 1635T | DQ190600 | DQ190705 | Soil | Ecuador | M.J. Wingfield | |
| CBS 111412; CPC 1648 | DQ190601 | DQ190706 | Soil | Ecuador | M.J. Wingfield | |
| CBS 111425; CPC 1657 | DQ190602 | DQ190707 | Soil | Ecuador | M.J. Wingfield | |
| Cy. gordoniae | CBS 112142; ATCC 201837; CPC 3136T | AF449449 | DQ190708 | Gordonia lasianthus | U.S.A. | D. Chiappini |
| Cy. gracile | CBS 110676; CPC 623 | AF333405 | DQ190709 | Soil | Brazil | M.J. Wingfield |
| CBS 111129; CPC 921 | DQ190603 | DQ190710 | Soil | Brazil | M.J. Wingfield | |
| CBS 111382; CPC 1586 | AF232863 | — | Soil | Brazil | P.W. Crous | |
| CBS 111390; CPC 1616 | DQ190604 | DQ190711 | Soil | Ecuador | M.J. Wingfield | |
| CBS 111456; CPC 1918 | DQ190605 | DQ190712 | Soil | Brazil | A.C. Alfenas | |
| CBS 111458; CPC 1910 | DQ190606 | DQ190713 | Soil | Brazil | A.C. Alfenas | |
| CBS 111465;CPC 1902 | DQ190607 | DQ190714 | Soil | Brazil | A.C. Alfenas | |
| CBS 111468; CPC 1905 | DQ190608 | DQ190715 | Soil | Brazil | A.C. Alfenas | |
| CBS 111474; CPC 1908 | DQ190609 | DQ190716 | Soil | Brazil | A.C. Alfenas | |
| CBS 111475; IMI 167580; CPC 1967 | AF333404 | DQ190717 | Camellia sinensis | Mauritius | A. Peerally | |
| CBS 111476; CPC 1909 | DQ190610 | DQ190718 | Soil | Brazil | A.C. Alfenas | |
| CBS 111478; CPC 1921 | DQ190611 | DQ190719 | Soil | Brazil | A.C. Alfenas | |
| CBS 111869; PC 551197; CPC 2409T | AF232857 | DQ190720 | Argyreia sp. | South East Asia | — | |
| CBS 112673; Cam 14 | DQ190612 | DQ190721 | Musa sp. | Cameroon | J.M. Abadie | |
| CBS 112694; CPC 617 | DQ190613 | DQ190722 | Soil | Brazil | M.J. Wingfield | |
| CBS 114167; CPC 1912 | DQ190614 | DQ190723 | Soil | Brazil | A.C. Alfenas | |
| CBS 114171; CPC 1891 | DQ190615 | DQ190724 | Soil | Brazil | A.C. Alfenas | |
| CBS 115680; CPC 922 | DQ190616 | DQ190725 | Soil | Brazil | M.J. Wingfield | |
| CBS 115769; CPC 920 | DQ190617 | DQ190726 | Soil | Brazil | M.J. Wingfield | |
| Cy. hurae | CBS 114182; UFV 216; CPC 1714 | DQ190618 | DQ190727 | Rumohra adiantiformis | Brazil | A.C. Alfenas |
| CBS 114551; CPC 2344 | AF333408 | DQ190728 | R. adiantiformis | U.S.A. | N.E. El-Gholl | |
| FTCC 1002; CPC 2395 | AF232866 | DQ190729 | R. adiantiformis | U.S.A. | N.E. El-Gholl | |
| FTCC 1003; CPC 2419 | AF232867 | DQ190730 | R. adiantiformis | U.S.A. | N.E. El-Gholl | |
| Cylindrocladium sp. | CBS 112957; CPC 4710 | DQ190619 | DQ190731 | Eleaeocarpus angustifolius | Australia | I. Steer & B. Paulus |
CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CPC: Pedro Crous working collection housed at CBS; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, U.K.; ATCC: American Type Culture Collection, Virginia, U.S.A.; UFV: Univeridade Federal de Viçosa, Brazil; CMW: Mike Wingfield collection housed at FABI, Pretoria, South Africa; FTCC: Food Technology Culture Collection, Malaysian Agricultural Research and Development Institute, Food Technology Centre, MARDI, GPO Box 12301, Kuala Lumpur, 50774, Malaysia; MUCL: Mycotheque de l'Université Catholique de Louvain, Louvain-la-Neuve, Belgium; PC: Laboratoire de Cryptogamie, Paris, France.
All ex-type cultures are indicated with a superscript “T”.
GenBank accession numbers.
Taxonomy
Morphological examinations were made from cultures sporulating on CLA. Structures were mounted in lactic acid, and 30 measurements at × 1000 magnification were made of each structure. The 95 % confidence levels were determined, and the extremes of spore measurements given in parentheses. Colony reverse colours were noted after 6 d on MEA at 25 °C in the dark, using the colour charts of Rayner (1970) for comparison. All cultures studied are maintained in the culture collection of the Centraalbureau voor Schimmelcultures (CBS), Utrecht, the Netherlands (Table 1).
RESULTS
DNA phylogeny
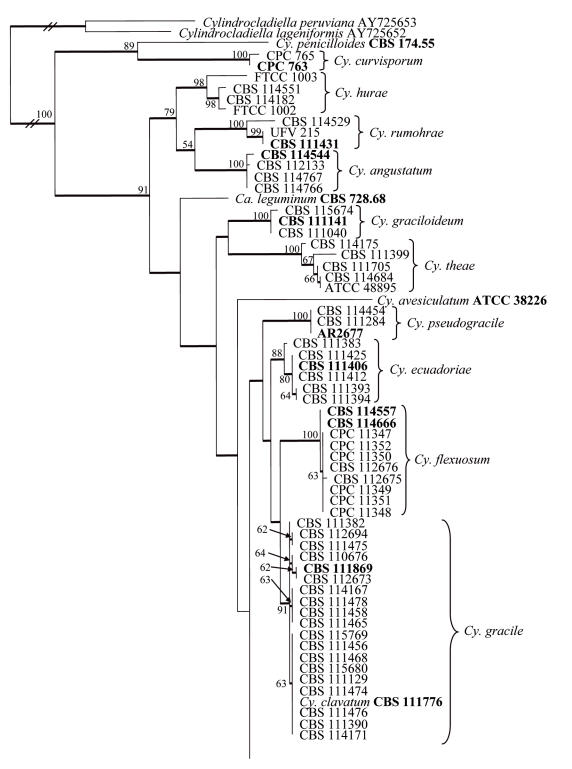
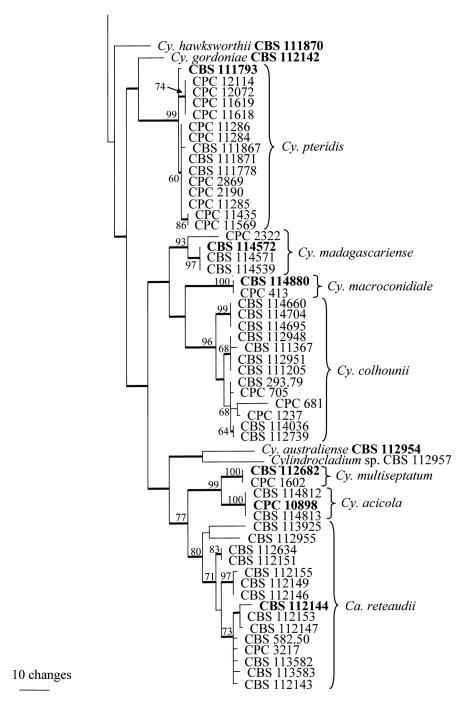
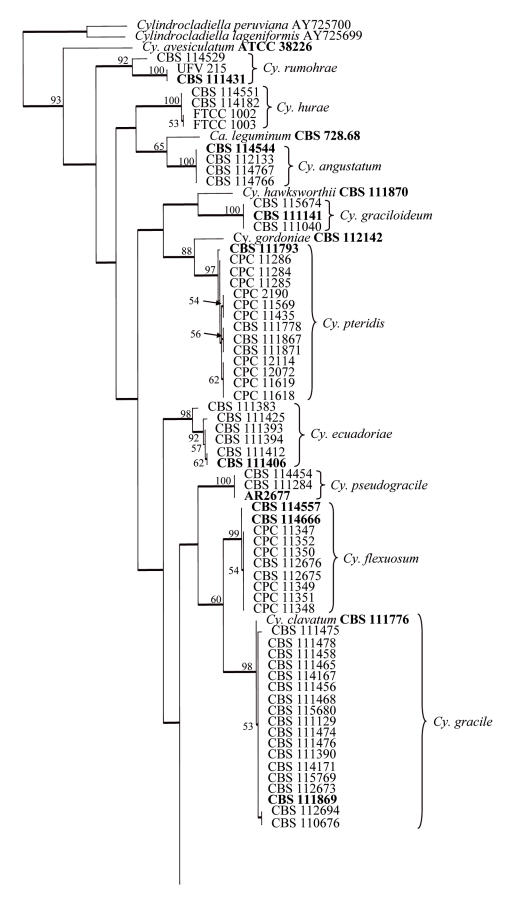
Approximately 550 bases of the β-tubulin gene were determined for the isolates indicated in Table 1. The manually adjusted alignment contained 123 isolates (including the two outgroups) and 533 characters including alignment gaps. Of these characters, 220 were parsimony-informative, 60 were variable and parsimony-uninformative, and 253 were constant. Neighbour-joining analysis using the three substitution models, as well as parsimony analysis, yielded trees in which the same clades were supported. In some analyses, for example between the uncorrected “p” and HKY85 substitution models, the basal order of the clades were different (data not shown). Parsimony analysis of the alignment yielded 657 most parsimonious trees (TL = 881 steps; CI = 0.529; RI = 0.853; RC = 0.451), one of which is shown in Fig. 1. Most of these trees resulted from the reordering of taxa within the Cy. colhounii Peerally and Ca. reteaudii (Bugn.) C. Booth clades. All taxa from the same species clustered in well-supported clades, namely Cy. curvisporum Crous & D. Victor (100 % bootstrap support), Cy. hurae (Linder & Whetzel) Crous (98 %), Cy. rumohrae El-Gholl & Alfenas (100 %), Cy. angustatum Crous & El-Gholl (100 %), Cy. graciloideum Crous & M.R.A. Mchau (100 %), Cy. theae (Petch.) Subram. (100 %), Cy. pseudogracile Crous (100 %), Cy. ecuadoriae Crous & M.J. Wingf. (88 %), Cy. flexuosum Crous (100 %), Cy. gracile (Bugn.) Boesew. (91 %), Cy. pteridis F.A. Wolf (99 %), Cy. madagascariense Crous (93 %), Cy. macroconidiale (Crous, M.J. Wingf. & Alfenas) Crous (100 %), Cy. colhounii (96 %), Cy. multiseptatum Crous & M.J. Wingf. (100 %), Cy. acicola Gadgil & M. Dick (100 %) and Ca. reteaudii (80 %). All species represented by a single taxon were placed as unsupported sister taxa to the other clades in the tree. The only exception was Cy. penicilloides (Tubaki) Tubaki, which grouped with the Cy. curvisporum clade with a bootstrap support value of 89 %. Association with support values were also observed between some clades, for example the clades containing Cy. hurae, Cy. rumohrae and Cy. angustatum grouped with a bootstrap support value of 79 %.
Fig. 1.
One of 657 most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the β-tubulin sequence alignment. The scale bar shows 10 changes and bootstrap support values from 1000 replicates are shown at the nodes. Thickened lines indicate branches present in the strict consensus tree. The tree was rooted to two Cylindrocladiella species.
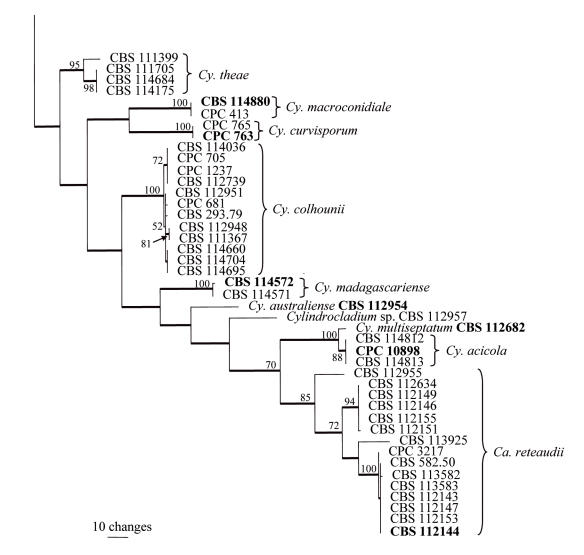
Approximately 480 bases of the histone gene were determined for the isolates in Table 1. The manually adjusted alignment contained 115 isolates (including the two outgroups), and for each taxon 425 characters including alignment gaps were analysed. Of these characters, 168 were parsimony-informative, 9 were variable and parsimony-uninformative, and 248 were constant. Neighbour-joining analysis using the three substitution models, as well as parsimony analysis, yielded trees in which the same clades were supported. For distance analysis, the Jukes-Cantor and HKY85 substitution models yielded trees with identical topologies, but the tree obtained from the uncorrected “p” model had rearrangements at the deep nodes when compared with the other two trees (data not shown). Parsimony analysis of the alignment yielded four most parsimonious trees (TL = 917 steps; CI = 0.382; RI = 0.868; RC = 0.331), one of which is shown in Fig. 2. All of these trees resulted from reordering of taxa within the Cy. colhounii clade. As with the β-tubulin tree, taxa from the same species clustered together in well-supported clades (Fig. 2). Clade order was not supported at the deeper nodes.
Fig. 2.
One of four most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the histone H3 sequence alignment. The scale bar shows 10 changes and bootstrap support values from 1000 replicates are shown at the nodes. Thickened lines indicate branches present in the strict consensus tree. The tree was rooted to two Cylindrocladiella species.
Taxonomy
Calonectria clavata Alfieri, El-Gholl & E.L. Barnard, Mycotaxon 48: 206. 1993.
Anamorph: Cylindrocladium flexuosum Crous, Syst. Appl. Microbiol.18: 248. 1995.
Macroconidiophores consisting of a stipe, a penicillate arrangement of fertile branches, a stipe extension, and a terminal vesicle; stipe septate, pale brown at base, hyaline, smooth, septate, 60–260 × 5–7 μm; stipe extensions septate, straight to flexuous, 120–450 μm long, 3–4 μm wide at apical septum, terminating in a narrowly clavate vesicle, 4–5 μm diam. Conidiogenous apparatus 70–120 μm long, 25–60 μm wide; primary branches aseptate or 1-septate, 30–65 × 4–6 μm; secondary branches aseptate or 1-septate, 30–50 × 3–6 μm, tertiary and quaternary branches aseptate, 15–30 × 3–5 μm, each terminal branch producing 1–4 phialides; phialides elongate doliiform to reniform, hyaline, aseptate, 10–20 × 4–5 μm, apex with minute periclinal thickening and inconspicuous collarette. Conidia cylindrical, rounded at both ends, straight, (55–)68–75(–95) × (5–)6(–7) μm (av. = 70 × 6 μm), 1-septate (but up to 5-septate at germination), lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime (description based on isolates obtained from Musa).
Specimens examined: U.S.A. Florida, Lake Placid, roots and stems of Callistemon viminalis, 5 Apr. 1978, C.P. Seymour & E.L. Barnard, PREM 51721 holotype of Cy. flexuosum, P078-1543 = ATCC 66389 = STE-U 2536 = CBS 114557culture ex-type, heterothallic mating with P078-1261 = STE-U 2537 = CBS 114666, Florida, Lee County, root debris in non-sterilized peat, 4 Mar. 1978, D. Ferrin, Aug. 1989, N.E. El-Gholl, FLAS F55430, holotype of Ca. clavata. Guadeloupe, Musa sp., J.M. Risède & Ph. Simoneau, Gua12 = CPC 11351, CPC 11352 = CBS 119338, Gua9 = CBS 112676. Martinique, Musa sp., J.M. Risède & Ph. Simoneau, Mar11 = CPC 11349 = CBS 119336, Mar23 = CPC 11348 = CBS 119335, Mar8 = CPC 11347 = CBS 119334. Saint Lucia, Musa sp., SLU2 = CBS 112675, SLU5 = CPC 11350 = CBS 119337.
Cultural characteristics: See Crous (2002).
Substrates and distribution: Musa spp., Guadeloupe, Martinique, Saint Lucia; Callistemon viminalis, and root debris in peat U.S.A. (Florida) (Crous 2002).
Notes: Cylindrocladium flexuosum is known to have conidia that are straight to curved, (44–)50–70(–80) × (4–)5–6 μm (av. = 65 × 5 μm) and 1(–3)-septate. The isolates obtained from Musa differ from the ex-type strains by having conidia that are up to 7 μm wide. Although we originally suspected the Musa isolates to represent an undescribed taxon, they clustered in the same clade as those of Cy. flexuosum. None of the isolates were able to mate, and since its original description, it has not proven possible to reproduce perithecia of Calonectria clavata in culture.
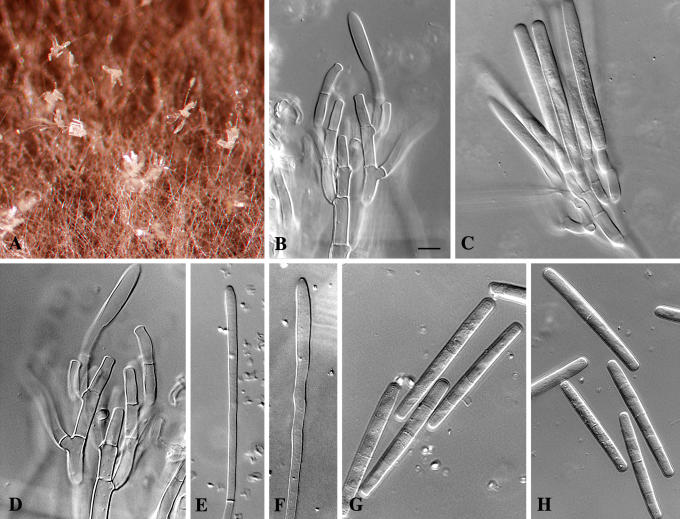
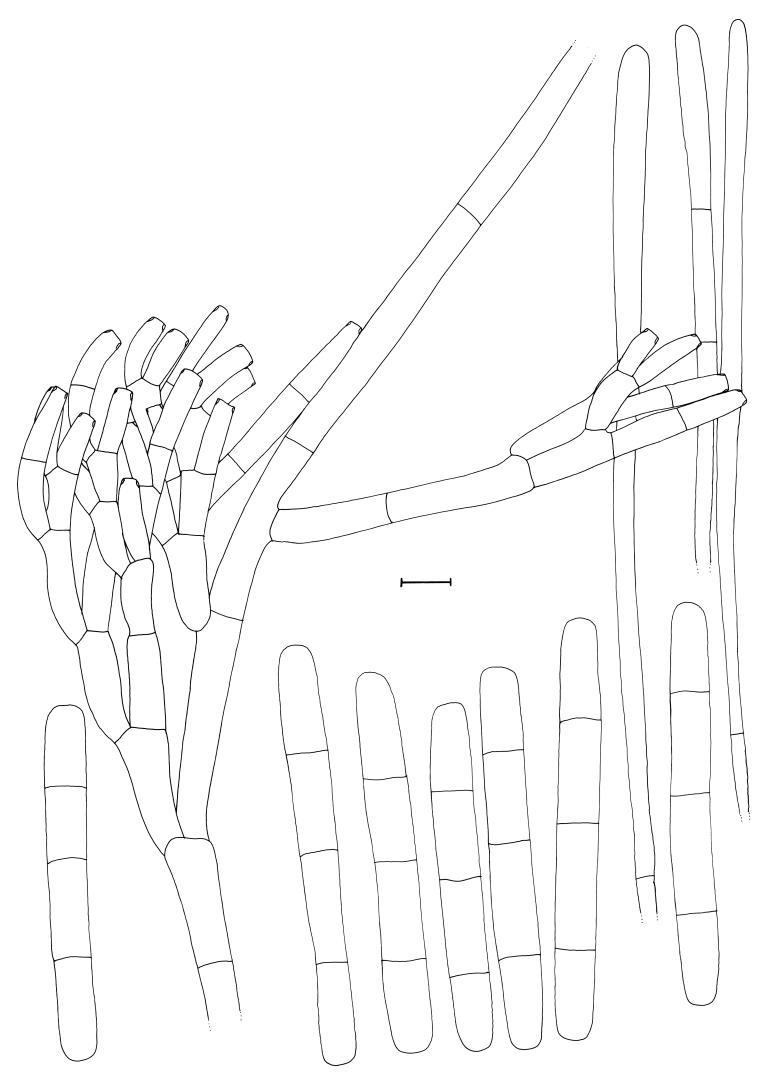
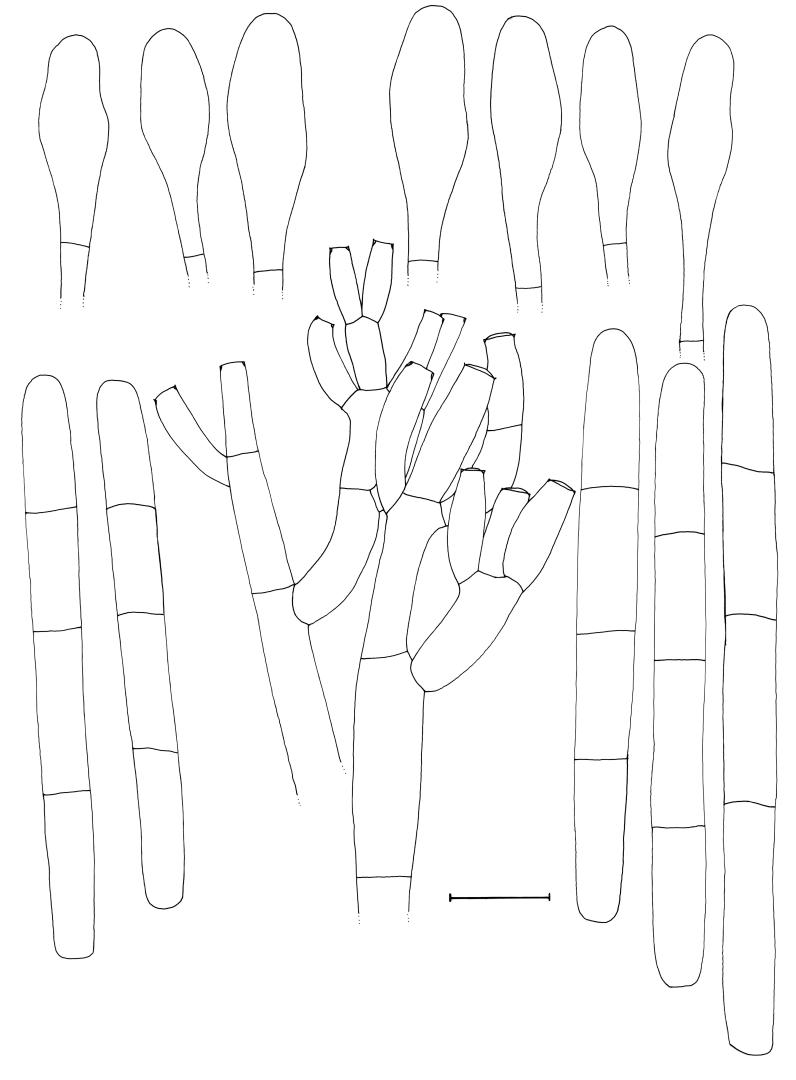
Cylindrocladium australiense Crous & K.D. Hyde, sp. nov. MycoBank MB500864. Figs 3, 4.
Fig. 3.
Cylindrocladium australiense. A. Sporulation on MEA. B–D. Conidiophores on CLA. E–F. Clavate vesicles. G–H. Three-septate conidia. Scale bar = 10 μm.
Fig. 4.
Cylindrocladium australiense. Penicillate conidiophore, clavate vesicles and conidia. Scale bar = 10 μm.
Etymology: Named after the country from which it was collected.
Cylindrocladio colhounii simile sed conidiis latioribus, (48–)57–68(–75) × (6–)6.5(–7) μm, distinguendum.
Teleomorph unknown. Conidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, a stipe extension, and a terminal vesicle; stipe septate, hyaline, smooth, 60–150 × 6–7 μm; stipe extensions septate, straight to flexuous, 300–450 μm long, 2.5–3 μm wide at the apical septum, terminating in a clavate vesicle, (3.5–)5(–6) μm diam. Conidiogenous apparatus 40–80 μm long, and 40–60 μm wide; primary branches aseptate or 1-septate, 15–30 × 5–7 μm; secondary branches aseptate, 12–20 × 5–6 μm, tertiary and additional branches (–6), aseptate, 10–15 × 5–6 μm, each terminal branch producing 1–4 phialides; phialides cylindrical to allantoid, hyaline, aseptate, 10–15 × 3.5–4.5 μm; apex with minute periclinal thickening and inconspicuous collarette. Conidia cylindrical, rounded at both ends, straight, (48–)57–68(–75) × (6–)6.5(–7) μm (av. = 63 × 6.5 μm), (1–)3-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Megaconidia and microconidia unknown.
Specimen examined: Australia, Queensland, Topaz, Atherton Tablelands, Ficus pleurocarpa, 2 Apr. 2001, C. Pearce & B. Paulus, holotype CBS H-17872, culture ex-type CBS 112954 = CPC 4714.
Cultural characteristics: Colonies fast growing with abundant white aerial mycelium; surface and reverse sienna (13i), with moderate numbers of chlamydospores.
Substrate: Ficus pleurocarpa.
Distribution: Australia.
Notes: This species can be confused with taxa in the Cylindrocladium colhounii Peerally species complex that form 3-septate conidia of similar dimensions, and yellow Calonectria perithecia. It can be distinguished by having wider conidia (48–)57–68(–75) × (6–)6.5(–7) μm than Cy. colhounii [(45–)60–70(–80) × (4–)5(–6) μm], and Cy. madagascariense Crous [(42–)52–58(–65) × (3.5–)4–5 μm]. Another species that needs to be compared to Cy. australiense is Cy. theae (Petch) Subram., which again has larger conidia (65–)70–88(–96) × 5–6(–7) μm, and also forms megaconidia and a Calonectria teleomorph with red perithecia in culture (Crous 2002).
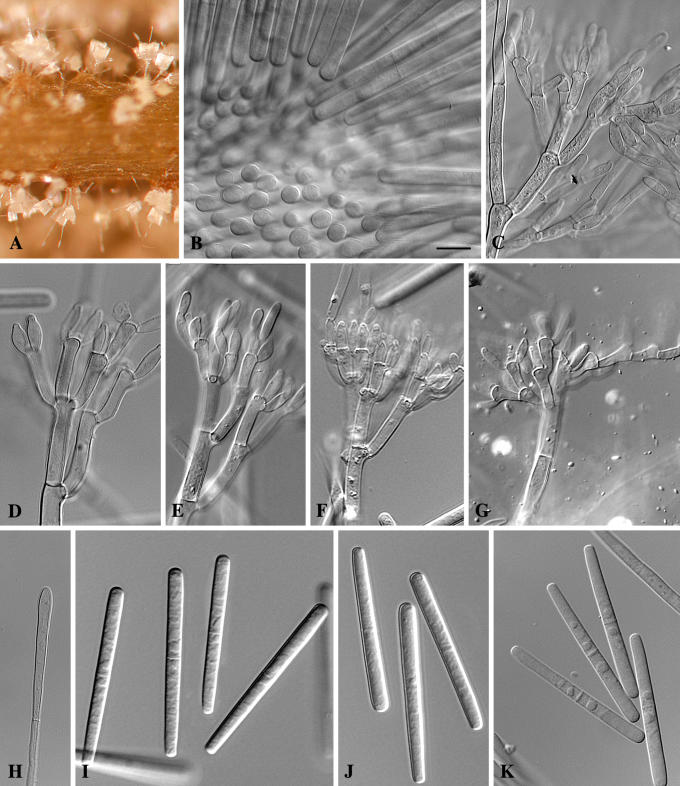
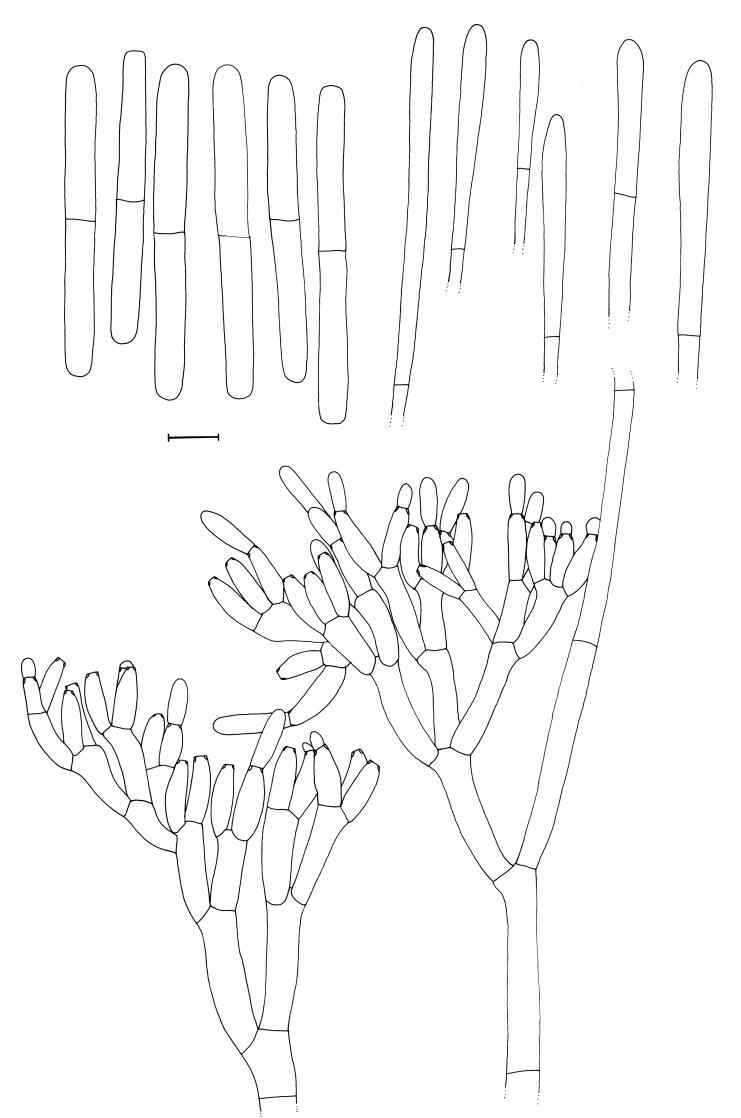
Cylindrocladium ecuadoriae Crous & M.J. Wingf., sp. nov. MycoBank MB500865. Figs 5, 6.
Fig. 5.
Cylindrocladium ecuadoriae. A. Sporulation on CLA. B. Conidial packet. C–G. Conidiophores. H. Clavate vesicle. I–K. One-septate conidia. Scale bar = 10 μm.
Fig. 6.
Cylindrocladium ecuadoriae. Penicillate conidiophores, clavate vesicles and conidia. Scale bar = 10 μm.
Etymology: Named after Ecuador, where it appears quite commonly in soil.
Cylindrocladio gracili simile, sed conidiis angustioribus, (45–)48–55(–65) × (4–)4.5(–5) μm, distinguendum.
Teleomorph unknown. Conidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, a stipe extension, and a terminal vesicle; stipe septate, hyaline, smooth, 60–100 × 5–7 μm; stipe extensions septate, straight to flexuous, 200–300 μm long, 2–3 μm wide at the apical septum, terminating in a clavate vesicle, (3–)4(–5) μm diam. Conidiogenous apparatus 30–100 μm long and wide; primary branches aseptate or 1-septate, 20–30 × 3–5 μm; secondary branches aseptate, 15–25 × 3–5 μm, tertiary branches aseptate, 12–17 × 3–5 μm, additional branches (–7), aseptate, 10–15 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 7–15 × 3–4 μm; apex with minute periclinal thickening and inconspicuous collarette. Conidia cylindrical, rounded at both ends, straight, (45–)48–55(–65) × (4–)4.5(–5) μm (av. = 51 × 4.5 μm), 1(–3)-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Megaconidia and microconidia unknown.
Specimens examined: Ecuador, soil, 20 Jun. 1997, M.J. Wingfield, holotype CBS H-17871, culture ex-type CBS 111406 = CPC 1635; CBS 111394 = CPC 1628; CBS 111412 = CPC 1648; CBS 111393 = CPC 1627; CBS 111425 = CPC 1657. Brazil, Belém, Cpatu, soil, 1996, P.W. Crous, CBS 111383 = CPC 1587.
Cultural characteristics: Colonies sienna on the surface, and umber in reverse; chlamydospores extensive, dense, occurring throughout the medium, forming microsclerotia, with moderate to extensive sporulation on the aerial mycelium.
Substrate: Soil.
Distribution: ?Brazil, Ecuador.
Notes: When first isolated, isolates of Cy. ecuadoriae were observed to also form a few conidia that were 3-septate when studied on CLA. Presently, however, strains seem to have lost this ability and only form 1-septate conidia. The same phenomenon was also observed in the strain obtained from Brazil (CBS 111383). Although the Brazilian strain clusters close to those obtained from Ecuador, its conidia are somewhat shorter (av. 44 μm) than those from Ecuador (av. 51 μm), and it might very well end up representing a cryptic species closely related to Cy. ecuadoriae.
Cylindrocladium ecuadoriae is morphologically similar to others in the Cy. gracile (Bugn.) Boesew. species complex. Its conidia are (45–)48–55(–65) × (4–)4.5(–5) μm (av. = 51 × 4.5 μm), thus longer and wider than those of Cy. graciloideum Crous & G.R.A. Mchau [(35–)40–48(–60) × 4–5(–6) μm (av. = 45 × 4.5 μm)], narrower than those of Cy. gracile [(38–)40–55(–65) × (3.5–)4–5(–6) μm (av. = 53 × 4.5 μm)], and shorter than those of Cy. flexuosum Crous [(44–)50–70(–80) × (4–)5–6 μm (av. = 65 × 5 μm)]. In the past, isolates of Cy. ecuadoriae were treated as representative of Cy. pseudogracile Crous, which has conidia of similar dimensions of [(40–)53–58(–65) × (3.5–)4–5 μm (av. = 56 × 4.5 μm)]and are 1(–3)-septate. Cylindrocladium ecuadoriae can be distinguished from Cy. pseudogacile based on its lower average conidial length, and the absence of a Calonectria state in culture (Crous 2002).
DISCUSSION
Several studies in recent years have focused on resolving the taxonomy of Cylindrocladium spp. with clavate vesicles (Crous et al. 1995, 1997, 1999, 2000, Kang et al. 2001, Crous 2002). In a study focusing on taxa with sphaeropedunculate vesicles, Crous et al. (2004) described nine new species from the Cy. floridanum species complex. Contrary to what we expected, only two new species could be resolved from all Cylindrocladium strains with clavate vesicles available to us. Part of the reason for this could be that the this complex has been studied in more detail than that with sphaeropedunculate vesicles, but also that Calonectria teleomorphs are more common among species with sphaeropedunculate vesicles than those with clavate vesicles, causing more variation. Some taxa, for instance Cy. colhounii and Cy. reteaudii, proved to be quite variable for the loci sequenced. However, we believe that it is currently premature to split these species into more taxa, and that additional isolates and more loci will have to be investigated to fully resolve their status.
A further aim of the current study was to resolve the Cy. pteridis F.A. Wolf or Cy. gracile-like isolates associated with toppling disease of banana (Risède & Simoneau 2001, 2004). Although we originally expected these strains to represent an undescribed species, we were surprised to find that both β-tubulin and histone H3 datasets placed them in Cy. flexuosum (teleomorph Ca. clavata). This was rather unexpected, as conidia of Cy. flexuosum are [(44–)50–70(–80) × (4–)5–6 μm (av. 65 × 5 μm)] (Crous 2002), while those of the banana isolates are [(55–)68–75(–95) × (5–)6–7 μm (av. 70 × 6 μm)]. Notwithstanding this discrepancy, these isolates clustered together in a clade (100 % support) in both data sets, suggesting that the original ex-type strains, which have narrower, slightly curved conidia, might be atypical for what is commonly seen in this species. Although several attempts have been made over the years to redo the crosses between the two mating testers of Cy. flexuosum, or to mate them with the newly collected isolates from banana, none of the matings proved successful. These findings suggest, however, that the Cy. gracile-like isolates associated with toppling disease of banana should be attributed to Cy. flexuosum, and that the latter species is morphologically more variable than originally expected (Crous et al. 1995).
The description of Cy. australiense from Australia adds yet another species to the Cy. colhounii/madagascariense/theae complex. It appears, however, that there are yet more Australian species awaiting description, as CBS 112957, isolated from Eleaeocarpus angustifolius in Queensland (Table 1), also clustered apart from any known taxon. Vesicles were clavate, and conidia 3-septate, 60–90 × 5–6 μm. We chose not to name this species, as the strain sporulated rather poorly, making it difficult to determine its range of morphological variation on CLA.
Isolates of Cylindrocladium ecuadoriae have until recently been treated under the name Cy. pseudogracile. Given the significant overlap in general conidial dimensions, this is not surprising, as these two species are rather similar, and can only be distinguished once the mean conidial dimensions have been determined. The single Brazilian isolate, CBS 11383, which again has smaller conidia than both Cy. ecuadoriae and Cy. pseudogracile, suggests that there may be yet more cryptic taxa within this complex that need to be resolved.
Acknowledgments
The authors acknowledge collections made by various researchers, without which this study would not have been possible. We are especially grateful to C. Pearce, B. Paulus, H.A. van der Aa, A.C. Alfenas and M.J. Wingfield, who placed numerous collections from diverse locations at our disposal for study. Bart van Asten (CBS) is thanked for the sequencing of isolates.
APPENDIX
Calonectria pyrochroa(Desm.) Sacc., Michelia 1: 308. 1878.
≡ Nectria pyrochroa Desm., Pl. Crypt. France Ed. 2: 372. 1856.
= Calonectria daldiniana De Not., Comment. Soc. Crittogam. Ital. 2: 477. 1867.
= Ophionectria puiggarii Speg., Bol. Acad. Nac. Ci. 11: 532. 1889.
= Nectria abnormis Henn., Hedwigia 36: 219. 1897.
Anamorph: Cylindrocladiumilicicola (Hawley) Boedijn & Reitsma, Reinwardtia 1: 57. 1950. Fig. 7.
Fig. 7.
Cylindrocladium ilicicola (epitype). Conidiophore, vesicles and conidia. Scale bar = 10 μm.
≡ Candelospora ilicicola Hawley, Proc. Roy. Irish Acad. 31: 11. 1912.
= Tetracytum lauri Vanderw., Parasitica 1: 145. 1945. (as “laurii”).
Macroconidiophores consisting of a stipe, a penicillate arrangement of fertile branches, a stipe extension, and a terminal vesicle; stipe septate, hyaline, smooth, up to 70 μm long, 5–6 μm wide; stipe extensions septate, straight to flexuous, 160–210 μm long, 3–4 μm wide at apical septum, terminating in an obpyriform to broadly ellipsoidal vesicle, 5–8 μm diam. Conidiogenous apparatus with primary branches that are aseptate or 1-septate, 15–20 × 3–5 μm; secondary branches aseptate, 10–20 × 3–5 μm, tertiary branches aseptate, rarely observed, 8–15 × 3–5 μm, each terminal branch producing 2–4 phialides; phialides doliiform to reniform, hyaline, aseptate, 9–15 × 3–4 μm, apex with minute periclinal thickening and inconspicuous collarette. Conidia cylindrical, rounded at both ends, straight, (50–)63–68(–70) × 5(–6) μm, (1–)3-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Megaconidia and microconidia unknown.
Specimens examined: Ireland, Clare Island, Ilex aquifolium, Hawley, K 61269!, holotype of Cy. ilicicola, IMI 76542 isotype. Netherlands, South-East Limburg, Vijlenerbos, Vijlen, Ilex aquifolium, Aug. 1970, H.A van der Aa, epitype designated here CBS H-15110, ex-epitype culture CBS 749.70.
Cultural characteristics: Cultures sterile, white.
Substrate and distribution: See Crous (2002).
Notes: The genus Calonectria is based upon Calonectria pyrochroa (= Ca. daldiniana), which is linked to a Cylindrocladium ilicicola anamorph (Rossman 1979, Brayford & Chapman 1987, Crous 2002). All cultures thus far collected by us, and thought to be representative of Cy. ilicicola, have turned out to represent other species, and hence no authentic cultures of Cy. ilicicola have as yet been obtained. A strain not previously studied by us was recently retrieved from the CBS collection (CBS 749.70). Although the isolate sporulated poorly, it was accompanied by a very good specimen, which proved to be identical to the original holotype collection. We therefore designate this specimen as epitype, thereby obtaining an authentic strain of Cy. ilicicola for further study.
Curvicladiella Decock & Crous, nom. nov. MycoBank MB500866.
≡ Curvicladium Decock & Crous, Mycologia 90: 276. 1998 [non Curvicladium Enroth, 1993].
Type species: Curvicladiella cignea (Decock & Crous) Decock & Crous
Curvicladiella cignea (Decock & Crous) Decock & Crous, comb. nov. MycoBank MB500867.
≡ Curvicladium cigneum Decock & Crous, Mycologia 90: 277. 1998.
Specimens examined: French Guiana, Matoury, first part of the Lamirande trail, on decaying leaf of unknown angiosperm, 23 Jan. 1997, C. Decock FG2240, MUCL 40269 = CPC 1595 = CBS 109167 (ex-type culture); on decaying seed of unknown angiosperm, 20 Jan. 1997, C. Decock FG2158, MUCL 40268 = CPC 1594 = CBS 109168.
Notes: It was recently brought to our attention (J. Bischoff, NCBI), that the generic name “Curvicladium”, which was proposed by Decock & Crous (1998) for a anamorphic fungus collected from leaf litter in French Guiana, was already occupied for a species of moss (Enroth 1993). A new name is thus called for, and herewith we propose Curvicladiella Decock & Crous, to replace Curvicladium Decock & Crous (1998).
Taxonomic novelties: Cylindrocladium australiense Crous & K.D. Hyde sp. nov., Cylindrocladium ecuadoriae Crous & M.J. Wingf. sp. nov., Curvicladiella Decock & Crous nom. nov., Curvicladiella cignea (Decock & Crous) Decock & Crous comb. nov.
References
- Brayford D, Chapman AU (1987). Cylindrocladium ilicicola on cuttings of evergreen ornamental shrubs in the U.K. Plant Pathology 36: 413–414. [Google Scholar]
- Crous PW (2002). Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera. APS Press, St. Paul, MN, U.S.A.
- Crous PW, Groenewald JZ, Risède J-M, Simoneau P, Hywel-Jones NL (2004). Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology 50: 415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Kang JC, Schoch CL, Mchau GRA (1999). Phylogenetic relationships of Cylindrocladium pseudogracile and Cylindrocladium rumohrae with morphologically similar taxa, based on morphology and DNA sequences of internal transcribed spacers and β-tubulin. Canadian Journal of Botany 77: 1813–1820. [Google Scholar]
- Crous PW, Korf A, Van Zyl WH (1995). Nuclear DNA polymorphisms of Cylindrocladium species with 1-septate conidia and clavate vesicles. Systematic and Applied Microbiology 18: 224–250. [Google Scholar]
- Crous PW, Theron L, Van Zyl WH (1997). Delineation of Cylindrocladium species with 1–3-septate conidia and clavate vesicles based on morphology and rDNA RFLPs. Mycological Research 101: 210–214. [Google Scholar]
- Crous PW, Schoch CL, El-Gholl N, Schubert TS, Leahy RM (2000). Cylindrocladium angustatum sp. nov., a new leaf spot pathogen of Tillandsia capitata from Florida, U.S.A. Mycoscience 41: 521–526. [Google Scholar]
- Crous PW, Wingfield MJ (1992). Cylindrocladium leucothoes and C. hederae, synonyms of C. reteaudii. South African Journal of Botany 58: 397–400. [Google Scholar]
- Decock C, Crous PW (1998). Curvicladium gen. nov., a new hyphomycete genus from French Guiana. Mycologia 90: 276–281. [Google Scholar]
- Enroth J (1993). Notes on the Neckeraceae (Musci). 18. Description of Curvicladium, a new genus from Southern and Southeastern Asia. Annales Botanici Fennici 30: 109–117. [Google Scholar]
- Gams W, Hoekstra ES, Aptroot A (eds) (1998). CBS Course of Mycology. 4th ed. CBS, Baarn, Netherlands.
- Kang JC, Crous PW, Old KM, Dudzinski MJ (2001). Non-conspecificity of Cylindrocladium quinqueseptatum and Calonectria quinqueseptata based on beta-tubulin gene phylogeny and morphology. Canadian Journal of Botany 79: 1241–1247. [Google Scholar]
- Lee SB, Taylor JW (1990). Isolation of DNA from fungal mycelia and single spores. In: PCR Protocols: A guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, San Diego, California: 282–287.
- O'Donnell K, Cigelnik E (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. [DOI] [PubMed] [Google Scholar]
- Rambaut A (2002). Sequence Alignment Editor. Version 2.0. Department of Zoology, University of Oxford, Oxford.
- Rayner RW (1970). A mycological colour chart. CMI and British Mycological Society. Kew, Surrey, England.
- Risède J-M, Simoneau P (2001). Typing Cylindrocladium species by analysis of ribosomal DNA spacers polymorphism: application to field isolates from the banana rhizosphere. Mycologia 93: 494–504. [Google Scholar]
- Risède J-M, Simoneau P (2004). Pathogenic and genetic diversity of soilborne isolates of Cylindrocladium from banana cropping systems. European Journal of Plant Pathology 110: 139–154. [Google Scholar]
- Rossman AY (1979). Calonectria and its type species, C. daldiniana, a later synonym of C. pyrochroa. Mycotaxon 8: 321–328. [Google Scholar]
- Swofford DL (2002). PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland, MA, U.S.A.