Abstract
Cryphonectria havanensis is a fungus associated with Eucalyptus species in Cuba and Florida (U.S.A.). Until recently, there have been no living cultures of C. havanensis and it has thus not been possible to assess its taxonomic status. Isolates thought to represent this fungus have, however, emerged from surveys of Eucalyptus in Mexico and Hawaii (U.S.A.). Results of this study showed that these isolates represent C. havanensis but reside in a genus distinct from Cryphonectria sensu stricto, which is described here as Microthia. Isolates of an unidentified fungus occurring on Myrica faya in the Azores and Madeira also grouped in Microthia and were identical to other M. havanensis isolates. Cryphonectria coccolobae, a fungus occurring on sea grape (Coccoloba uvifera) in Bermuda and Florida, was found to be morphologically identical to Microthia and is transferred to this genus, but as a distinct species. Surveys for M. coccolobae on sea grape in Florida, yielded a second diaporthalean fungus from this host. This fungus is morphologically and phylogenetically distinct from M. coccolobae and other closely related taxa and is described as Ursicollum fallax gen. et sp. nov. Phylogenetic analyses in this study have also shown that isolates of C. eucalypti, a pathogen of Eucalyptus in South Africa and Australia, group in a clade separate from all other groups including that representing Cryphonectria sensu stricto. This difference is supported by the fact that Cryphonectria eucalypti has ascospore septation different to that of all other Cryphonectria species. A new genus, Holocryphia, is thus erected for C. eucalypti.
Keywords: Cryphonectria coccolobae, Cryphonectria eucalypti, Cryphonectria havanensis, Diaporthales, phylogeny
INTRODUCTION
Cryphonectria havanensis (Bruner) M.E. Barr was first described from Eucalyptus spp. (Myrtaceae, Myrtales) in Cuba (Bruner 1916). Bruner (1916) found this fungus on bark of dead, injured or healthy Eucalyptus trees, but it did not appear to cause disease. Cryphonectria havanensis was also found on dead branches of mango (Mangifera indica, Anacardiaceae, Sapindales) and avocado (Persea gratissima, Lauraceae, Laurales) lying on the ground in the vicinity of the Eucalyptus trees (Bruner 1916). Besides these exotic hosts, fruiting structures of C. havanensis were also found on the bark of jobo (Spondias mombin, Anacardiaceae, Sapindales), a plant native to Cuba (Bruner 1916).
Barnard et al. (1987) found C. havanensis on Eucalyptus plantations in Florida. The fungus was, however, reported as Cryphonectria gyrosa (Berk. & Broome) Sacc., a name previously used for the species (Kobayashi 1970, Hodges 1980). The identification of the fungus as C. havanensis was based on the presence of orange stromata, as well as conidial and ascospore dimensions that resembled those of the type specimen from Cuba. Chrysoporthe cubensis (Bruner) Gryzenh. & M.J. Wingf., a fungus previously known as Cryphonectria cubensis (Bruner) Hodges (Gryzenhout et al. 2004) and a serious pathogen of Eucalyptus spp. (Wingfield 2003), was also found in the same plantations (Barnard et al. 1987). Cryphonectria havanensis was mainly associated with dead coppice shoots in stands of Eucalyptus grandis while Chr. cubensis was the causal agent of basal cankers and death of coppice shoots (Barnard et al. 1987).
Other than reports from tropical or sub-tropical areas of the world such as Cuba and Florida, the name C. havanensis has also been used for collections of a fungus from Eucalyptus globulus in Japan (Kobayashi & Itô 1956, Kobayashi 1970). The fungus referred to as C. havanensis in Japan is also known from other host genera besides Eucalyptus (Kobayashi 1970), namely species of Quercus (Fagaceae, Fagales), Betula (Betulaceae, Fagales) and Pyrus (Rosaceae, Rosales). A recent study employing DNA sequence comparisons (Myburg et al. 2004a) showed that the fungus referred to as C. havanensis in Japan is the same as Cryphonectria nitschkei (G. H. Otth) M.E. Barr. The study by Myburg et al. (2004a) did not, however, consider whether C. nitschkei is the same as the fungus referred to as C. havanensis from Cuba, where C. havanensis was originally described (Bruner 1916).
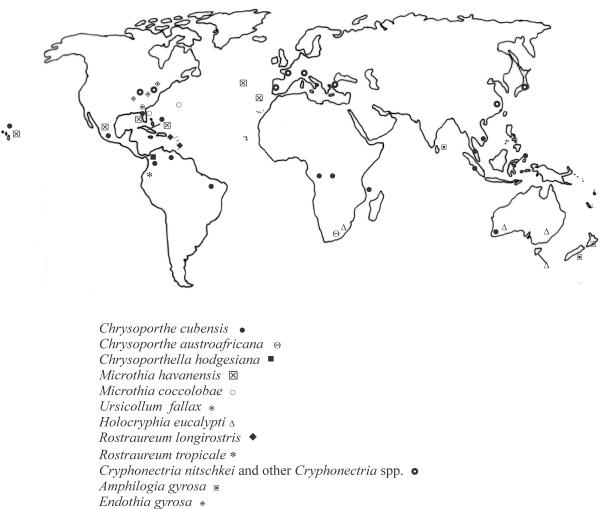
Cryphonectria havanensis and four other fungi in the Diaporthales with orange stromatic tissue are known from islands in the Caribbean Sea and Atlantic Ocean (Fig. 1). Chrysoporthe cubensis is well-known from several countries in Central and South America (Gryzenhout et al. 2004), including Cuba (Bruner 1917) where C. havanensis was first discovered. Cryphonectria coccolobae (Vizioli) Micales & Stipes occurs as a saprobe on twigs, branches and seeds of Coccoloba uvifera (sea grape, Polygonaceae, Polygonales) from Bermuda (Vizioli 1923) and Florida (Micales & Stipes 1987, Barnard et al. 1993). In the Azores and Madeira, an unidentified species of Cryphonectria has been associated with cankers on Myrica faya (Myricaceae, Myricales) (Gardner & Hodges 1990, Hodges & Gardner 1992). Another closely related species, Cryphonectria longirostris (Earle) Micales & Stipes, occurs in Puerto Rico and Trinidad (Earle 1901, Roane 1986). This fungus is saprobic and has recently been transferred to the new genus Rostraureum (Gryzenhout et al. 2005a). Rostraureum also includes a second new species, Rostraureum tropicale Gryzenh. & M.J. Wingf., which is a pathogen of Terminalia ivorensis trees in Ecuador (Gryzenhout et al. 2005a).
Fig. 1.
Map showing the distribution of the various taxa in the Diaporthales with orange stromata. Only locations verified with sequence data are shown.
The correct identity of C. havanensis and its phylogenetic relationship with species of Cryphonectria and closely related genera remained unresolved (Myburg et al. 2004b). This is largely due to the absence of isolates that could, with reasonable certainty, be attributed to this species. The same problem was true for C. coccolobae (Myburg et al. 2004b), which has been suspected to be a synonym of C. havanensis (Hodges & Gardner 1990). The relationship between C. havanensis and the fungus attributed to this species from Japan (Myburg et al. 2004a) also remains to be resolved.
Recently, fungi closely resembling C. havanensis were found on Eucalyptus spp. in Mexico and Hawaii, where this fungus had not been known previously. These collections included cultures and specimens on bark and enabled us to reconsider questions relating to the identity and the phylogenetic position of C. havanensis.
MATERIALS & METHODS
Symptoms and collection of samples
Fruiting structures thought to represent C. havanensis were collected from cankers and dead trees on the stems of E. grandis and an unidentified Eucalyptus sp. on the island of Kauai (Hawaii, U.S.A.). Fruiting structures of Chr. cubensis were also found on the stems of the same Eucalyptus spp. in the plantation, but were associated with cankers on living trees. Chrysoporthe cubensis was also common on cankered E. grandis trees on the island of Hawaii. Specimens of this fungus previously examined from the Hawaiian Islands were all from Kauai (Hodges et al. 1979, Myburg et al. 2003), and collections made in this study represent the first record of Chr. cubensis from the island of Hawaii.
Bark tissue bearing orange fruiting structures resembling C. havanensis was also collected from cankers on E. grandis in Las Chiapas, Mexico. An additional isolate from Mexico was received from Dr. E.L. Barnard (Florida Division of Forestry, FDACS, Gainesville, Florida). An isolate (ATCC 60862 = CMW 14332) representing C. havanensis (collected as C. gyrosa) from Eucalyptus plantations in Florida, linked to the study of Barnard et al. (1987), was acquired from the American Type Culture Collection (ATCC). Isolates and specimens (Tables 1, 2) linked to the report of a Cryphonectria species from M. faya in the Azores (Gardner & Hodges 1990) were also included in this study. This collection also included authentic isolates (Table 1) of C. parasitica from Castanea sativa in the Azores (Gardner & Hodges 1990). Unfortunately, no isolates of C. havanensis could be obtained from Cuba despite surveys aimed at re-collecting the fungus in that country.
Table 1.
Isolates sequenced in this study (in bold) and previously published sequences.
| Species identity | Isolate numbera | Alternative isolate numbera | Host | Origin | Collector | GenBank accession numbersb |
|---|---|---|---|---|---|---|
| Microthia havanensis | CMW 14332 | ATCC 60862 | Eucalyptus grandis | Florida (U.S.A.) | E.L. Barnard & K. Old | DQ368734, DQ368739, DQ368740 |
| CMW 14550 | CBS 115855 | Eucalyptus saligna | Mexico | C.S. Hodges | DQ368735, DQ368741, DQ368742 | |
| CMW 11297 | CBS 115765 | Eucalyptus sp. | Mexico | E.L. Barnard | AY 214319, AY 214247, AY 214283 | |
| CMW 11298 | - | Eucalyptus sp. | Mexico | C.S. Hodges | AY 214320, AY 214248, AY 214284 | |
| CMW 11301 | - | Myrica faya | Azores | C.S. Hodges & D.E. Gardner | AY 214323, AY 214251, AY 214287 | |
| CMW 11300 | - | M. faya | Madeira | C.S. Hodges | AY 214322, AY 214250, AY 214286 | |
| CMW 14551 | CBS 115841 | M. faya | Madeira | C.S. Hodges | DQ368736, DQ368743, DQ368744 | |
| CMW 10879 | CBS 115758 | Eucalyptus sp. | Kauai, Hawaii (U.S.A.) | M.J. Wingfield | DQ368737, DQ368745, DQ368746 | |
| CMW 10885 | CBS 115760 | Eucalyptus sp. | Kauai, Hawaii (U.S.A.) | M.J. Wingfield | DQ368738, DQ368747, DQ368748 | |
| Amphilogia gyrosa | CMW 10469 | E67, CBS 112922 | Elaeocarpus dentatus | New Zealand | G. Samuels | AF 452111, AF 525707, AF 525714 |
| CMW 10470 | E68, CBS 112923 | El. dentatus | New Zealand | G. Samuels | AF 452112, AF 525708, AF 525715 | |
| Chrysoporthe cubensis | CMW 10639 | CBS 115747 | E. grandis | Colombia | C.A. Rodas | AY 263419, AY 263420, AY 263421 |
| CMW 10669 | CBS 115751 | Eucalyptus sp. | Republic of Congo | J. Roux | AF 535122, AF 535124, AF 535126 | |
| CMW 11006 | CBS 115732 | Eucalyptus sp. | Kauai, Hawaii (U.S.A.) | M.J. Wingfield | DQ368719, DQ368723, DQ368724 | |
| CMW 11008 | CBS 115733 | Eucalyptus sp. | Kauai, Hawaii (U.S.A.) | M.J. Wingfield | DQ368718, DQ368721, DQ368722 | |
| CMW 10889 | CBS 118666 | Hawaii, Hawaii (U.S.A.) | M.J. Wingfield | DQ368720, DQ368725, DQ368726 | ||
| CMW 1856 | - | Eucalyptus sp. | Kauai, Hawaii (U.S.A.) | - | AY 083999, AY 084010, AY 084022 | |
| CMW 8651 | CBS 115718 | Syzygium aromaticum | Sulawesi, Indonesia | M.J. Wingfield | AY 084002, AY 084014, AY 084026 | |
| Chrysoporthella hodgesiana | CMW 9994 | CBS 115729 | Tibouchina semidecandra | Colombia | R. Arbelaez | AY 956968, AY 956975, AY 956976 |
| CMW 10641 | CBS 115854 | T. semidecandra | Colombia | R. Arbaleaz | AY 692322, AY 692326, AY 692325 | |
| Chrysoporthe austroafricana | CMW 2113 | CBS 112916 | E. grandis | South Africa | M.J. Wingfield | AF 046892, AF 273067, AF 273462 |
| CMW 9327 | CBS 115843 | Tibouchina granulosa | South Africa | M.J. Wingfield | AF 273473, AF 273060, AF 273455 | |
| Rostraureum tropicale | CMW 9971 | CBS 115725 | Terminalia ivorensis | Ecuador | M.J. Wingfield | AY 167426, AY 167431, AY 167436 |
| CMW 10796 | CBS 115757 | Te. ivorensis | Ecuador | M.J. Wingfield | AY 167428, AY 167433, AY 167438 | |
| Holocryphia eucalypti | CMW 7036 | CRY62, CBS 119478 | Eucalyptus sp. | South Africa | I. van der Westhuizen | AF 232878, AF 368341, AF 368340 |
| CMW 7037 | CRY45, CBS 119477 | Eucalyptus delegatensis | Australia | K.M. Old | AF 232880, AF 368343, AF 368342 | |
| CMW 7038 | CRY909, CBS 119475 | Eucalyptus globulus | Australia | M.J. Wingfield | AF 232881, AF 368345, AF 368344 | |
| CMW 14545 | CRY103, CBS 115852 | Eucalyptus sp. | South Africa | I. van der Westhuizen | AF 232877c, DQ368730, DQ368731 | |
| CMW 14546 | CRY287, CBS 115838 | Eucalyptus sp. | South Africa | H. Smith | AF 232879c, DQ368732, DQ368733 | |
| CMW 7033 | CBS 115842 | E. grandis | South Africa | M. Venter | DQ368727, DQ368728, DQ368729 | |
| Ursicollum fallax | CMW 18110 | Coccoloba uvifera | Florida (U.S.A.) | C.S. Hodges | - | |
| CMW 18114 | CBS 118661 | Co. uvifera | Florida (U.S.A.) | C.S. Hodges | - | |
| CMW 18115 | CBS 118660 | Co. uvifera | Florida (U.S.A.) | C.S. Hodges | DQ368756, DQ368760, DQ368761 | |
| CMW 18119 | CBS 118663 | Co. uvifera | Florida (U.S.A.) | C.S. Hodges | DQ368755, DQ368758, DQ368759 | |
| CMW 18124 | CBS 118662 | Co. uvifera | Florida (U.S.A.) | C.S. Hodges | DQ368757, DQ368762, DQ368763 | |
| Cryphonectria parasitica | CMW 13749 | MAFF 410158 TFM:FPH Ep1 | Castanea mollisima | Japan | Unknown | AY 697927, AY 697943, AY 697944 |
| CMW 7048 | ATCC 48198, E9 | Quercus virginiana | U.S.A. | F.F. Lombard | AF 368330, AF 273076, AF 273470 | |
| CMW 14547 | CBS 115845 | Castanea sativa | Azores | D.E. Gardner | DQ368749, DQ368751, DQ368752 | |
| CMW 14548 | CBS 115846 | Ca. sativa | Azores | D.E. Gardner | DQ368750, DQ368753, DQ368754 | |
| Cryphonectria radicalis | CMW 10455 | CBS 238.54, E42 | Castanea dentata | Italy | A. Biraghi | AF 452113, AF 525705, AF 525712 |
| CMW 10477 | CBS 240.54, E76 | Quercus suber | Italy | M. Orsenigo | AF 368328, AF 368347, AF 368346 | |
| CMW 10436 | CBS 165.30, E14 | Quercus suber | Portugal | B. d'Oliviera | AF 452117, AF 525703, AF 525710 | |
| CMW 10484 | E83, CBS 112918 | Castanea sativa | Italy | A. Biraghi | AF 368327, AF 368349, AF 368349 | |
| Cryphonectria macrospora | CMW 10463 | E54, CBS 112920 | Castanopsis cuspidata | Japan | T. Kobayashi | AF 368331, AF 368351, AF 368350 |
| CMW 10914 | TFM: FPH E55 | Castanopsis cuspidata | Japan | T. Kobayashi | AY 697942, AY 697973, AY 697974 | |
| Cryphonectria nitschkei | CMW 10785 | 9494 | Quercus sp. | China | M. Milgroom & K. Wang | AF 140246, AF 140252, AF 140258 |
| CMW 13747 | MAFF 410569 TFM:FPH E25 | Quercus serrata | Japan | T. Kobayashi | AY 697937, AY 697963, AY 697964 | |
| CMW 10910d | TFM:FPH E11 | Eucalyptus globulus | Japan | T. Kobayashi | AY 697941, AY 697971, AY 697972 | |
| CMW 11294d | TFM:FPH E57 | Quercus grosseserrata | Japan | T. Kobayashi | AY 214211, AY 214213, AY 214215 | |
| Endothia gyrosa | CMW 2091 | ATCC 48192, E13 | Quercus palustris | U.S.A. | R.J. Stipes | AF 046905, AF 368337, AF 368336 |
| CMW 10442 | E27 | Q. palustris | U.S.A. | R.J. Stipes | AF 368326, AF 368339, AF 368338 | |
| Diaporthe ambigua | CMW 5288 | CBS 112900 | Malus domestica | South Africa | W.A. Smit | AF 543817, AF 543819, AF 543821 |
| CMW 5587 | CBS 112901 | M. domestica | South Africa | W.A. Smit | AF 543818, AF 543820, AF 543822 |
CMW, CRY = Forestry & Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa; ATCC = American Type Culture Collection, Manassas, USA; CBS = Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; TFM:FPH = Forestry and Forest Products Research Institute, Danchi-Nai, Ibaraki, Japan, E or Ep refers to an isolate; (09494) = isolates used in Liu et al. (2003); MAFF = Microorganisms Section, MAFF GeneBank, National Institute of Agrobiological Sciences (NIAS), MAFF GeneBank, Ibaraki, Japan; E = from the culture collection of Prof. R.J. Stipes (Department of Plant Pathology, Virginia Polytechnic Institute & State University, Blacksburg, Virginia, U.S.A.) now housed in the culture collection (CMW) of FABI.
Accession numbers refer to sequence data of the ITS, β-tubulin 1 (primers Bt1a/1b) and β-tubulin 2 (primers Bt2a/2b) regions, respectively.
Only the β-tubulin sequences were obtained in this study, while the ITS sequences were obtained from Venter et al. (2001).
Previously labelled Cryphonectria havanensis.
Table 2.
Herbarium specimens examined in this study.
| Species identity | Herbarium numbera | Linked isolateb | Host | Origin | Collector | Date |
|---|---|---|---|---|---|---|
| Microthia havanensis | BPI 614275 (holotype) | - | Eucalyptus sp. | Santiago de las Vegas, Cuba | S.C. Bruner | 15 Feb. 1916 |
| BPI 614273 | Eucalyptus sp. | Santiago de las Vegas, Cuba | S.C. Bruner | 15 Feb. 1916 | ||
| BPI 614278 | Eucalyptus botryoides | Santiago de las Vegas, Cuba | C.L. Shear | 25 Mar. 1916 | ||
| BPI 614282 | - | Spondias sp. | Santiago de las Vegas, Cuba | C.L. Shear | 28 Mar. 1916 | |
| BPI 614283 | - | Spondias myrobalanus | Earle's Herradura, Cuba | C.L. Shear | 5 Apr. 1916 | |
| BPI 614284 | - | S. myrobalanus | Earle's Herradura, Cuba | C.L. Shear | 5 Apr. 1916 | |
| BPI 614279 | - | Mangifera indica | Santiago de las Vegas, Cuba | C.L. Shear | 6 Apr. 1916 | |
| BPI 614280 | - | Ma. indica | Santiago de las Vegas, Cuba | C.L. Shear | Apr. 1916 | |
| BPI 614281 | - | Ma. indica | Santiago de las Vegas, Cuba | C.L. Shear | 26 Mar. 1916 | |
| PREM 57518 | CMW 11298 | Eucalyptus saligna | Las Chiapas, Mexico | C.S. Hodges | 26 Feb. 1998 | |
| NY 511 | - | Unknown | Puerto Rico | F.J. Seaver & C.E. Chardon | 1923 | |
| PREM 57521 | CMW 10897 | Eucalyptus sp. | Kauai, Hawaii (U.S.A.) | M.J. Wingfield | Sep. 2002 | |
| PREM 57522 | CMW 10885 | Eucalyptus sp. | Kauai, Hawaii (U.S.A.) | M.J. Wingfield | Sep. 2002 | |
| FLAS 54261 | ATCC 60862 | Eucalyptus robusta | Near Palmdale, Glades Co., Florida (U.S.A.) | E.L. Barnard & K. Old | 1984 | |
| FLAS 54263 | - | Eucalyptus grandis | Glades Co., Florida (U.S.A.) | E.L. Barnad & K. Old | 1984 | |
| PREM 57523 | CMW 14551 | Myrica faya | Machico, Madeira | C.S. Hodges | 8 May 2000 | |
| PREM 57524 | CMW 11301c | M. faya | Mosteiro, Island of São Miguel, Azores | C.S. Hodges & D.E. Gardner | ||
| PREM 57525 | CMW 11301c | M. faya | Island of Pico, Azores | C.S. Hodges & D.E. Gardner | 30 Jul. 1992 | |
| PREM 58810 | CMW 11301c | M. faya | Island of Pico, Azores | C.S. Hodges & D.E. Gardner | 31 May 1985 | |
| PREM 58811 | CMW 11301c | M. faya | Island of São Miguel, Azores | C.S. Hodges & D.E. Gardner | 2 Aug. 1992 | |
| PREM 58812 | CMW 11301c | M. faya | Island of Terceiro, Azores | C.S. Hodges & D.E. Gardner | 31 May 1987 | |
| PREM 58813 | CMW 11301c | M. faya | Island of Faial, Azores | C.S. Hodges | 27 May 1985 | |
| Microthia coccolobae | CUP 128 (holotype) | - | Fruit of Coccoloba uvifera | Grape Bay, Bermuda | H.H. Whetzel | 11 Dec. 1921 |
| BPI 613756 (isotype) | - | Fruit of Co. uvifera | Grape Bay, Bermuda | H.H. Whetzel | 11 Dec. 1921 | |
| NY 147 (isotype) | - | Fruit of Co. uvifera | Grape Bay, Bermuda | H.H. Whetzel | 11 Dec. 1921 | |
| CUP 30512 | - | Fruit of Co. uvifera | Grape Bay, Bermuda | H.H. Whetzel | 11 Dec. 1921 | |
| CUP 35078 | - | Calophyllum calaba | Devonshire, Bermuda | Seaver, Whetzel & Ogilvie | 2 Feb. 1926 | |
| CUP 57366 (nr. 326) | - | Bark of Co. uvifera | South Shore, Bermuda | F.J. Seaver & J.M. Waterston | 25 Nov. 1940 | |
| CUP 35081 | - | Conocarpus erecta | Devonshire Bay, Bermuda | Seaver, Whetzel & Ogilvie | 5 Feb. 1926 | |
| CUP 34658 | - | Fruit of Co. uvifera | Elbow Beach, Bermuda | Whetzel, Seaver & Ogilvie | 28 Jan. 1926 | |
| Unknown | CUP 34657 | - | Petioles of Co. uvifera | Hungry Bay, Bermuda | Seaver & Whetzel | 14 Jan. 1926 |
| Ursicollum fallax | PREM 58840 | CMW 18119 | Co. uvifera | Fort Lauderdale, Florida (U.S.A.) | C.S. Hodges | Mar. 2005 |
| PREM 58841 | CMW 18124, CMW 18115 | Co. uvifera | Crandon Park, Key Biscayne, Florida (U.S.A.) | C.S. Hodges | Mar. 2005 | |
| PREM 58842 | CMW 18124, CMW 18115 | Co. uvifera | Key Biscayne, Florida (U.S.A.) | C.S. Hodges | Mar. 2005 | |
| PREM 58843 | CMW 18114 | Co. uvifera | Oakland Park, Florida (U.S.A.) | C.S. Hodges | Mar. 2005 | |
| PREM 58844 | CMW 18110 | Co. uvifera | Oakland Park, Florida (U.S.A.) | C.S. Hodges | Mar. 2005 | |
| Holocryphia eucalypti | PREM 56211 (holotype) | CMW 7034 | GC747 clone of Eucalyptus | Mtubatuba, South Africa | M. Venter | 25 Feb. 1998 |
| PREM 56214 | - | Eucalyptus grandis | Mtubatuba, South Africa | M. Venter | Oct. 1998 | |
| PREM 56216 | - | Eucalyptus grandis | Mtubatuba, South Africa | M. Venter | Oct. 1998 | |
| PREM 56215 (epitype designated here) | CMW 7033 | E. grandis | KwaMbonambi, South Africa | M. Venter | Oct. 1998 | |
| PREM 56212 | - | E. grandis | Sabie, South Africa | J. Roux | Aug. 1998 | |
| PREM 56305 | CMW 7035 | E. saligna | Tzaneen, South Africa | M. Venter | 6 Feb. 1999 | |
| PREM 56217 | CMW 7038 | Eucalyptus globulus | Perth, Australia | M.J. Wingfield | 1997 | |
| Chrysoporthe cubensis | PREM 58814 | CMW 11006, CMW 11008 | Eucalyptus sp. | Kauai, Hawaii (U.S.A.) | M.J. Wingfield | Sep. 2002 |
| PREM 58815 | CMW 10889 | Eucalyptus sp. | Hawaii, Hawaii (U.S.A.) | M.J. Wingfield | Sep. 2002 | |
| Cryphonectria parasitica | CUP 2926 | CMW 10790 | Castanea dentata | New York, U.S.A. | W.A. Murrill | 1907 |
| Cryphonectria nitschkei | TFM: FPH 1045 (holotype) | CMW 10518 | Quercus grosseserrata | Japan | T. Kobayashi | 1954 |
| Cryphonectria havanensisd | TFM:FPH 633 | CMW 10910 | Eucalyptus globulus | Meguro, Japan | T. Kobayashi | 1954 |
| TFM:FPH 2300 | - | Betula sp. | Yoshiwara, Japan | Zinno | 1963 | |
| TFM:FPH 1270 | CMW 13736 | Pyrus sinensis | Inagi, Japan | T. Kobayashi | 1960 | |
| TFM:FPH 1203 | - | Quercus variabilis | Seto, Japan | T. Kobayashi | 1953 | |
| TFM:FPH 1047 | - | Quercus glandulifera | Japan | T. Kobayashi | 1954 |
BPI, U.S. National Fungus Collections, Systematic Botany and Mycology, Beltsville, U.S.A.; PREM, National Collection of Fungi, Pretoria, South Africa; CUP, Plant Pathology Herbarium, Plant Pathology Department, Cornell University, Ithaca, New York, U.S.A.; FLAS, Mycological Herbarium, Department of Plant Pathology, University of Florida, Gainesville, U.S.A.; NY, William and Lynda Steere Herbarium, New York Botanical Garden, Bronx, New York, USA; TFM: FPH, Forestry and Forest Products Research Institute, Norin Kenkyu, Danchi-Nai, Ibaraki, Japan.
CMW = Forestry & Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa.
Isolates originating from same locality and host, but are not necessarily linked to specific specimen.
Specimens labeled as C. havanensis but actually representing C. nitschkei.
During surveys for C. coccolobae on Co. uvifera in Florida, a fungus with distinctive orange fruiting structures was found in the vicinity of Fort Lauderdale, Key Biscayne, Dania and Oakland Park (Tables 1, 2). This fungus was fruiting profusely on branches and twigs, but was not associated with disease symptoms. It was included in this study to determine whether it represents C. coccolobae.
Isolations from fungal structures on bark specimens were made from single conidia and ascospores collected from the apices of pycnidia and perithecia, respectively. The isolates used in this study are maintained in the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa and representative isolates not originally obtained from internationally recognised collections have been deposited with the Centraalbureau voor Schimmelcultures, Utrecht, Netherlands (Table 1). The original bark specimens from which cultures were made have been deposited in the National Collection of Fungi (PREM), Pretoria, South Africa (Table 2).
DNA sequence comparisons
DNA was extracted from isolates grown in malt extract broth (20 g/L malt extract, Biolab, Midrand, South Africa) as described by Myburg et al. (1999). DNA sequences were derived for the internal transcribed spacer (ITS) regions ITS1 and ITS2, including the conserved 5.8S gene of the ribosomal RNA (rRNA) operon, using primer pair ITS1/ITS4 (White et al. 1990), and β-tubulin genes using the primer pairs Bt1a/Bt1b and Bt2a/Bt2b respectively (Glass & Donaldson 1995). For these, the protocols of Myburg et al. (1999) and Myburg et al. (2002), respectively, were followed. Purification of PCR products for subsequent sequence reactions was done using a QIAquick PCR Purification Kit (Qiagen GmbH, Hilden, Germany). Sequence reactions were performed with the same primers used in the PCR reactions, using the ABI PRISM™ Dye Terminator Cycle Sequencing Ready Reaction Kit with AmpliTaq® DNA Polymerase (Perkin-Elmer, Warrington, UK). The sequencing reactions were run on an ABI PRISM 3100™ automated DNA sequencer. Nucleotide sequences were analysed using Sequence Navigator v. 1.0.1 (Perkin-Elmer Applied BioSystems, Inc., Foster City, California, U.S.A.) software.
New sequences were submitted to GenBank (Table 1). These also included sequences obtained in this study of additional Cryphonectria eucalypti M. Venter & M.J. Wingf. isolates to strengthen the C. eucalypti clade presented by Myburg et al. (2004b). This fungus is a pathogen of Eucalyptus trees in South Africa (Van der Westhuizen et al. 1993, Gryzenhout et al. 2003) and Australia (Walker et al. 1985, Yuan & Mohammed 2000). The sequences were compiled into a matrix using a modified data set (TreeBASE accession numbers S1128, M1935) of Myburg et al. (2004b) as a template. Additional sequences from other studies were also added to the data matrix. These included sequences of Chrysoporthella hodgesiana Gryzenh. & M.J. Wingf. (Gryzenhout et al. 2004, Rodas et al. 2005), and those of Cryphonectria parasitica (Murrill) M.E. Barr, Cryphonectria macrospora (Tak. Kobay. & Kaz. Itô) M.E. Barr and C. nitschkei from Japan, including those of isolates referred to as C. havanensis (Myburg et al. 2004b). Sequences representing R. tropicale (Gryzenhout et al. 2005a) and Amphilogia gyrosa (Berk. & Broome) Gryzenh. & M.J. Wingf., the new genus that now contains Cryphonectria gyrosa (Gryzenhout et al. 2005b), were also added. The resultant dataset was deposited with TreeBASE (S1490, M2675).
The alignment was obtained using the web interface (http://timpani.genome.ad.jp/%7Emafft/server/) of the alignment program MAFFT v. 5.667 (Katoh et al. 2002). Phylogenetic analyses were made using PAUP (Phylogenetic Analysis Using Parsimony) v. 4.0b10 (Swofford 2002). A 500 replicate partition homogeneity test (PHT) was done on the rRNA and β-tubulin gene sequence data sets (after the exclusion of uninformative sites) to determine whether they could be analysed collectively (Farris et al. 1994). Phylogenetic analyses included parsimony and distance methods. Maximum parsimony (MP) was inferred using the heuristic search option with the tree-bisection-reconnection (TBR) branch swapping and MULTREES options (saving all optimal trees) effective and a 100 random additions. Gaps inserted during manual sequence alignment were treated as fifth character (NEWSTATE) in the heuristic searches, and missing in distance analyses. Uninformative characters were excluded and remaining characters were reweighted according to the individual Consistency Indices (CI) to reduce the number of trees. For the distance analyses, the correct model for the datasets was found with MODELTEST v. 3.5 (Posada & Crandall 1998). This model was the Tamura-Nei model (TrN+G+I) (Tamura & Nei 1993) with the Gamma distribution shape parameter (G) set to 0.9717 and frequency of invariable sites (I) 0.4643; base frequencies of 0.1903, 0.3411, 0.2301 and 0.2385; and rate matrix of 1, 3.1147, 1, 1, 4.1643, 1. Support for the branch nodes in the various phylogenetic trees was tested with a 1000 replicate bootstrap analysis and is presented as a 70 % majority rule tree.
Morphology
A large number of specimens from different species, hosts and geographical areas were included in the morphological comparisons (Table 2). These included the type specimen of C. havanensis (BPI 614275). Conidiomata and ascostromata were cut from bark specimens, rehydrated (1 min) in boiling water and sectioned with a Leica CM1100 cryostat at –20 °C, 12–14 μm thick. For embedding, Leica mountant (Setpoint Premier, Johannesburg, South Africa) was used, which was dissolved in water after sectioning. Lactic acid (85 %) was used to prepare semi-permanent slides. Hand sections were made with a razor blade to more closely study conidiophore morphology. Fruiting structures were also mounted in 3 % KOH when conidiophores and asci could not easily be observed. Twenty measurements of ascospores, asci, conidia and conidiophores suspended in lactic acid or KOH, were taken for the specimens and these are presented as (min–)(average – std. dev.) – (average + std. dev.)(–max) μm. For the eustromata and perithecia, a size range from the largest and smallest structures was obtained. Colours were assigned to structures using the charts of Rayner (1970).
For growth studies, colony growth was assessed on 90 mm diam plates of MEA (20 g/L malt extract agar, Biolab, Merck, Midrand, South Africa). Four plates were inoculated per isolate. The cultures were grown in the dark at temperatures ranging from 15–35 °C. Two measurements were taken daily for each plate until the plates were fully covered.
RESULTS
DNA sequence comparisons
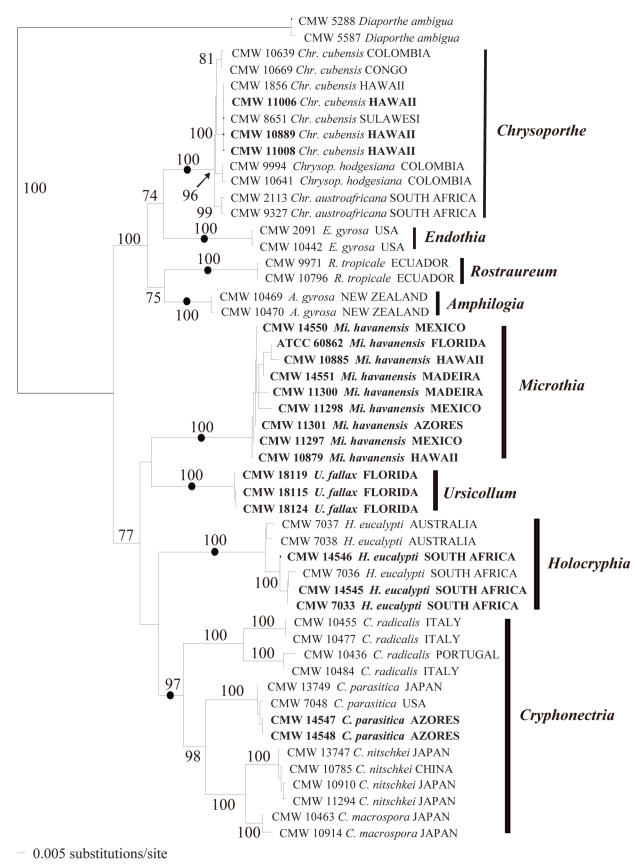
The sequence data set consisted of 51 taxa with sequences from two isolates of Diaporthe ambigua Nitschke (Diaporthales), which reside in a different family in the Diaporthales (Castlebury et al. 2002), as outgroup. The ribosomal DNA dataset (571 bp) consisted of 335 constant, 10 parsimony-uninformative and 226 parsimony-informative characters (g1 = – 0.4143), and the β-tubulin DNA sequence set (966 bp) consisted of 516 constant, 32 parsimony-uninformative and 418 parsimony-informative characters (g1 = – 0.3582). Results generated with the PHT analyses (P = 0.004) indicated that trees obtained with the different gene regions were incongruent. This was because the relationship of the Cryphonectria sensu stricto clade with the other clades was different in each gene tree. Each tree, however, showed the same clades, which were always highly supported with bootstrap values between 90 and 100 %. For this reason we combined the data. The resultant dataset contained 1537 characters.
The heuristic search resulted in six most parsimonious trees (tree length = 1101.9, CI = 0.736, Retention index/RI = 0.943), which differed only in the lengths of the branches. The trees obtained with the distance and parsimony analyses showed identical clades grouping isolates. The same groups of isolates, with high bootstrap values, were obtained when the more variable regions, and thus potentially ambiguously aligned sequences of the introns and ITS1 region, were excluded. The tree obtained with distance analysis based on the complete dataset is presented in Fig. 2.
Fig. 2.
A phylogenetic tree obtained with distance analyses (TrN+G+I model, G = 0.9717, I = 0.4643, base frequencies 0.1903, 0.3411, 0.2301, 0.2385; rate matrix 1, 3.1147, 1, 1, 4.1643, 1) from a combined DNA sequences dataset of the ITS1, 5.8S rRNA gene and ITS2 regions of the ribosomal operon, and β-tubulin genes. Bootstrap confidence levels (> 70 %) are indicated on the branches, and those branches representing genera are marked with a dot. The outgroup taxon is Diaporthe ambigua.
The isolates thought to represent C. havanensis from E. grandis in Mexico (CMW 14550, CMW 11297, CMW 11298), Florida (CMW 14332) and Kauai (CMW 10879, CMW 10885), grouped together (Fig. 2) and formed a discrete clade (bootstrap support 100 %) separate from the clades representing species of Cryphonectria (Sacc.) Sacc., Endothia Fr., Chrysoporthe Gryzenh. & M.J. Wingf., Rostraureum Gryzenh. & M.J. Wingf. and Amphilogia Gryzenh., Glen & M.J. Wingf. The C. havanensis clade (Fig. 2) also included the isolates from M. faya in the Azores (CMW 11301) and Madeira (CMW 14551, CMW 11300). Cryphonectria havanensis isolates from Kauai grouped separately from Chr. cubensis isolates from Kauai (CMW 1856, CMW 11006, CMW 11008) and Hawaii (CMW 10889). The latter isolates all grouped (bootstrap support 100 %) in the South East Asian sub-clade (Myburg et al. 2002) of Chr. cubensis (Fig. 2).
Isolates from Japan and previously assigned to C. havanensis (CMW 10910, CMW 11294), grouped with C. nitschkei isolates (CMW 10785, CMW 13747) in the Cryphonectria clade (bootstrap support 100 %; Fig. 2), as previously reported (Myburg et al. 2004a). They thus resided in a clade separate from isolates believed to represent C. havanensis. Isolates derived from cankers on Castanea sativa (Gardner & Hodges 1990) from the Azores (CMW 14547, CMW 14548) grouped with other C. parasitica isolates (CMW 7048, CMW 13749) in the Cryphonectria clade (bootstrap support 100 %; Fig. 2).
The C. eucalypti isolates formed a discrete clade (bootstrap support 100 %) separate from the clade defining Cryphonectria s. str. (Fig. 2). This clade was also separated from the clades representing other genera. The isolates obtained from Co. uvifera in Florida also formed a clade distinct from those representing the other genera (bootstrap support 100 %), and did not group with the isolates representing C. havanensis.
Morphology
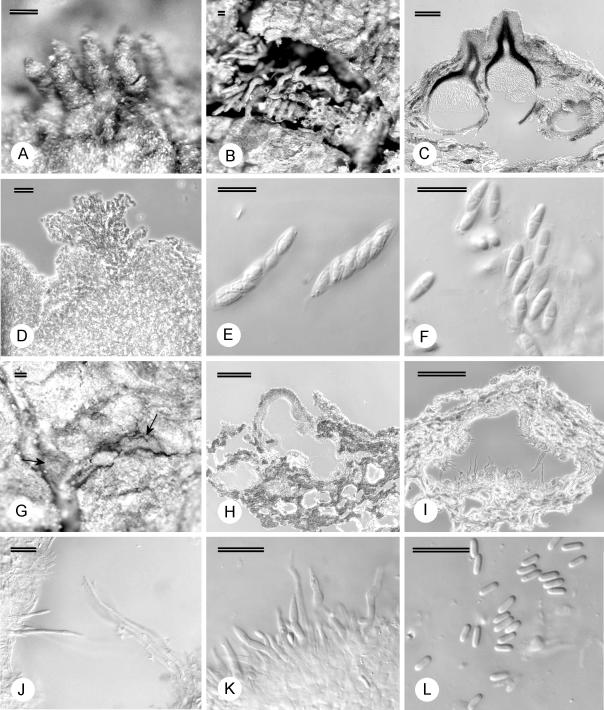
Fruiting structures on the specimens connected to the isolates from Mexico, Florida and Kauai (Fig. 3) were indistinguishable from those on the type specimen of C. havanensis from Cuba. Ascospores [(5.5–)7–9(–10) × (2–)2.5–3(–4) μm], asci [(26.5–)29.5–34.5(–37) × (5–)5.5–7(–8) μm] and conidia [(2.5–)3–4(–5) × 1–1.5 μm] also fell within the range of those reported for the type specimen (Bruner 1916). We are thus confident that the collections from Mexico and Hawaii represent C. havanensis, although the phylogenetic relationship between the fungus in Cuba and the isolates from Mexico and Kauai could not be determined due to the lack of isolates from Cuba. Fruiting structures on herbarium specimens of M. faya from the Azores and Madeira, linked to isolates (CMW 11300, CMW 11301, CMW 14551) that also grouped with those from Mexico and Kauai, were similar to those from Cuba, Hawaii and Mexico (Fig. 3). A specimen from Puerto Rico (NY 511), annotated as C. longirostris but shown by Gryzenhout et al. (2005a) not to represent this species, was also morphologically similar to C. havanensis.
Fig. 3.
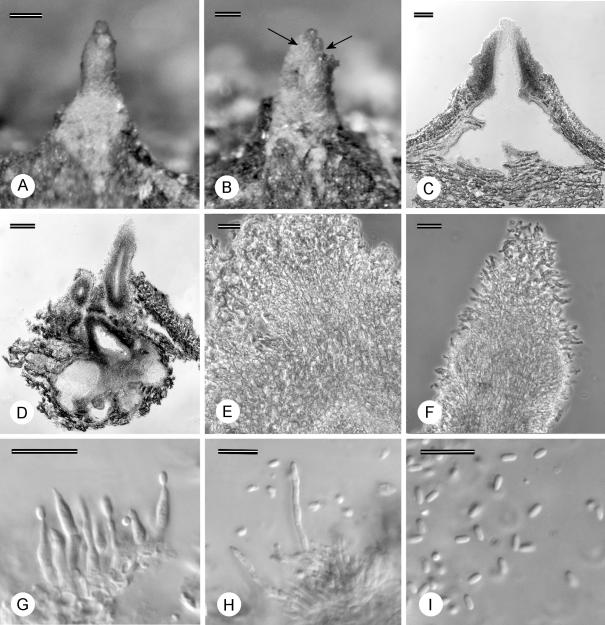
Fruiting structures of Microthia havanensis. A–B. Stereomicrographs of ascostromata C. Longitudinal section through ascostroma. D. Stromatic tissue. E. Asci. F. Ascospores. G. Conidiomata on bark (arrow). H. Longitudinal section of conidioma. I–J. Long conidiophores and sterile paraphyses. K. Conidiophores. L. Conidia. Scale bars A–C, G–I = 100 μm; D=20 μm; E–F, J–L = 10 μm.
Clear differences could be seen between the specimens that represent C. havanensis (originating from Cuba, Florida, Mexico, Puerto Rico, Kauai, Madeira and the Azores), and those previously labeled as C. havanensis from Japan. Non-confluent stromata in the C. havanensis specimens were much smaller (200–650 μm diam above level of bark) than those on specimens from Japan (250–1630 μm diam above the level of the bark). Longitudinally sectioned stromata of the C. havanensis specimens also tended to be more superficial with reduced tissue development (Fig. 3C, H), while structures on specimens from Japan were distinctly semi-immersed with strongly developed, erumpent tissue. Ascostromata on the C. havanensis specimens (Fig. 3A–B) occasionally had long extending perithecial necks (up to 370 μm long) while those from Japan were consistently short (up to 130 μm long). The conidiogenous cells on the C. havanensis specimens also had characteristically long, cylindrical conidiophores up to 57 μm long, with the longest of these being sterile, resembling paraphyses (Fig. 3F–K). These structures differed from conidiophores of the Japanese specimens that were up to 29 μm long. Although the structural differences could also be attributed to different hosts, there are also differences, e.g. the presence of paraphyses, that cannot be attributed to hosts. Thus these differences more likely represent robust characteristics to support the distinct phylogenetic grouping (Fig. 2) of specimens representing C. havanensis s. str. from those of Cryphonectria s. str. and other closely related genera.
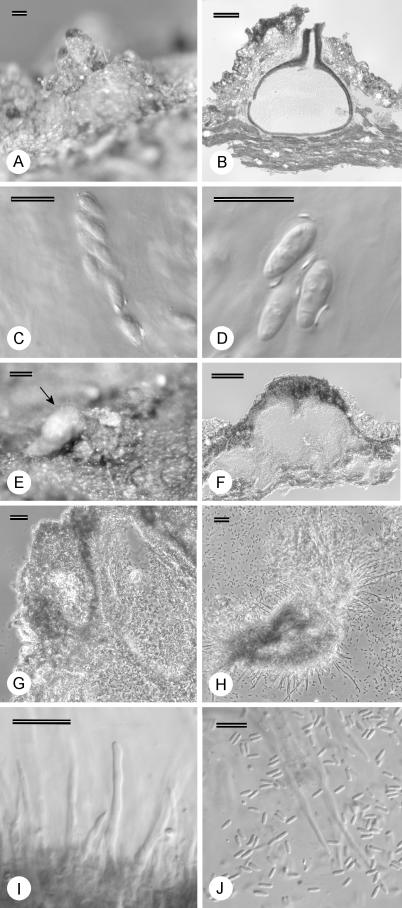
Structures of C. coccolobae on Co. uvifera (Fig. 4) on various specimens were similar to those thought to represent C. havanensis. Conidia [(2.5–)3–4.5(–5.5) × 1–1.5 μm] and ascospores [(6.5–)7.5–9(–10.5) × (2.5–)3–3.5(–4) μm] were similar to those of C. havanensis, and similar long (up to 62 μm) and cylindrical conidiophores, with the longest sterile, were observed (Fig. 4H–J). A specimen with conidia of (3–)3.5–4.5(–5) × 1–1.5 μm and labeled C. coccolobae from bark of Conocarpus erecta (CUP 35081) in Bermuda, also contained structures similar to those of the other C. coccolobae specimens. Fruiting structures on seed, however, differed from those on bark (Table 2) in being superficial and not semi-immersed.
Fig. 4.
Fruiting structures of Microthia coccolobae. A. Ascostroma on bark. B. Longitudinal section through ascostroma. C. Ascus. D. Ascospores. E. Conidioma on bark with spore mass (arrow). F. Longitudinal section of conidioma. G. Stromatic tissue. H. Conidiophores and long paraphyses. I. Conidiophores. J. Conidia and paraphyses. Scale bars A–B, E–F = 100 μm; G–H = 20 μm; C–D, I–J = 10 μm.
Asci measured for the different C. coccolobae specimens [(32.5–)34.5–39(–41) × (5–)7–9.5(–10.5) μm] were longer and wider than those measured for the majority of C. havanensis specimens [(26.5–)29.5–34.5(–37) × (5–)5.5–7(–8) μm]. Ascus size was, however, a variable character since specimen PREM 57518, linked to isolate CMW 11298 grouping with the other C. havanensis isolates, had asci of similar size [(31.5–)32–39(–44.5) × (5.5–)6–7.5(–8.5) μm] to those of the C. coccolobae specimens and thus longer than the other C. havanensis specimens.
The newly collected specimens from Co. uvifera in Florida were morphologically different from those representing C. coccolobae. Conidiomata were pyriform to rostrate, often having a globose base with a long to tapered cylindrical neck or more than one neck (Figs 5A–D, 6A–B). This was different from conidiomata of C. coccolobae which are pulvinate without long necks (Fig. 4E–F). Furthermore, necks of the conidiomata were often covered with short hairs (Fig. 5F). Conidial locules of the Florida specimens (Figs 5D, 6B) also did not contain the long, sterile paraphyses commonly found in locules of C. coccolobae (Fig. 4H–J). No teleomorph was observed for the Florida specimens on the bark.
Fig. 5.
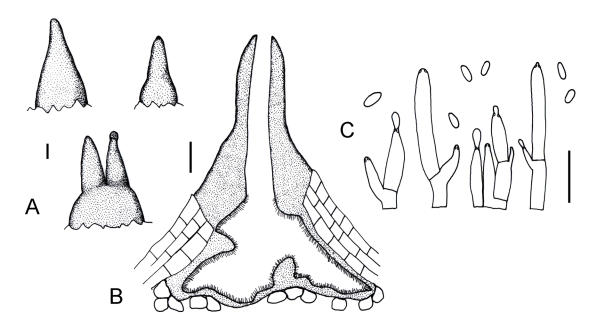
Fruiting structures of Ursicollum fallax. A–B. Conidiomata on bark (necks indicated with arrows). C–D. Longitudinal section through conidioma. E. Tissue at base of conidioma. F. Tissue of neck. G–H. Conidiophores. I. Conidia. Scale bars A–D = 100 μm; E–F = 20 μm; G–I = 10 μm.
Fig. 6.
Line drawings of Ursicollum fallax. A. Conidiomata on bark. B. Longitudinal section through conidioma. C. Conidiophores and conidia. Scale bars A–B = 100 μm; C = 10 μm.
The conidiomata of the Florida specimens did not resemble the anamorphs of Cryphonectria, Endothia, Rostraureum, Amphilogia or Chrysoporthe although that fungus was closely related to these genera in the DNA sequence comparisons. The conidiomata of the fungus from Florida resembled the rostrate conidiomata of Rostraureum (Gryzenhout et al. 2005a) most closely, but could be distinguished from Rostraureum based on conidiomata that are more pyriform in shape, and with necks more cylindrical. Conidiomata of the newly collected fungus from Co. uvifera in Florida also lacked the distinct textura intricata tissue at the junction between neck and base in the conidiomata of Rostraureum (Gryzenhout et al. 2005a). Furthermore, the conidiomatal neck tissue was prosenchymatous (Fig. 5F), and not of textura porrecta as is found in Rostraureum (Gryzenhout et al. 2005a).
One of the specimens labeled as C. coccolobae (CUP 34657) contained a fungus morphologically different from C. coccolobae, but with orange stromatic tissue. This fungus was erroneously illustrated by Waterston (1947) to represent C. coccolobae, an illustration previously used by Seaver & Waterston (1940) in their description of a fungus named Gnomonia pulcherrima Seaver & Waterston. These structures occurred on petioles and twigs of Co. uvifera from Bermuda (Table 2). The fungus differs from C. coccolobae because the perithecial necks extending from the orange stromata are black and not orange as is the case for C. coccolobae. Ascospores are also cylindrical, 1–2-septate, guttulate and 11.5–14.5(–16) × (2.5–)3–4(–5) μm. Fruiting structures of this fungus did not colour purple in KOH and yellow in lactic acid, similar to structures of C. coccolobae. Previously G. pulcherrima was cited as a synonym (Roane 1986) of C. coccolobae, but these are clearly distinct fungi.
Taxonomy
Results of the phylogenetic analyses and morphological comparisons have shown clearly that cultures and specimens believed to represent C. havanensis do not reside in Cryphonectria s. str. but represent a distinct taxonomic group. Based on morphological characteristics, C. havanensis most closely resembles Cryphonectria s. str., but it can be distinguished from species in Cryphonectria s. str. by its smaller and more superficial stromata, and long paraphyses between the conidiophores. Based on our observations of the material representing C. havanensis in this study, we transfer the fungus to a new genus that is closely related to Cryphonectria. The following description is provided.
Microthia Gryzenh. & M.J. Wingf., gen. nov. MycoBank MB500792.
Etymology: Greek, micros, small, and this, a heap, thus referring to the small and pulvinate stromata.
Ascostromata subimmersa vel superficialia, pulvinata, aurantiaca. Ascosporae fusoideae vel ellipsoideae, hyalinae, semel septatae. Stromata anamorpha subimmersa vel superficialia, pulvinata, aurantiaca. Conidiophora cylindrica, subcontracta, saepe longa, cellulis longissimis paraphyses fingentibus. Conidia hyalina, cylindrica, non septata.
Ascostromata semi-immersed to superficial, pulvinate, orange, tissue predominantly prosenchymatous but pseudoparenchymatous at edges. Perithecia dark-walled, with globose to sub-globose bases and slender periphysate necks that emerge at the stromatal surface as black ostioles in papillae covered with orange stromatal tissue. Asci fusiform, floating freely in the perithecial cavity, unitunicate with non-amyloid, refractive apical rings. Ascospores fusoid to ellipsoid, hyaline, 1-septate, often with a slight constriction at the septum.
Anamorphic stromata semi-immersed to superficial, pulvinate, orange, uni- to multilocular and convoluted, locules often occurring in the same stroma that contains perithecia. Conidiophores cylindrical, slightly tapering, often septate with or without lateral branches beneath the septum, hyaline, often long with longest cells sterile and representing paraphyses, conidiogenous cells phialidic. Conidia hyaline, cylindrical, aseptate, expelled through opening at stromatal surface as orange droplets or tendrils.
Typus: Microthia havanensis (Bruner) Gryzenh. & M.J. Wingf., comb. nov.
Microthia havanensis (Bruner) Gryzenh. & M.J. Wingf., comb. nov. MycoBank MB500793. Fig. 3.
Basionym: Endothia havanensis Bruner, Mycologia 8: 241–242. 1916.
≡ Cryphonectria havanensis (Bruner) M.E. Barr, Mycologia Mem. 7: 143. 1978.
Specimens examined: Cuba, Santiago de las Vegas, Eucalyptus sp., 15 Feb. 1916, S.C. Bruner, holotype BPI 614275, BPI 614273; Eucalyptus botryoides, 25 Mar. 1916, C.L. Shear, BPI 614278; Spondias sp., 28 Mar. 1916, C.L. Shear, BPI 614282; Earle' s Herradura, Spondias myrobalanus, 5 Apr. 1916, C.L. Shear, BPI 614283, BPI 614284; Santiago de las Vegas, Mangifera indica, 6 Apr. 1916, C.L. Shear, BPI 614279, BPI 614280, 26 Mar. 1916, C.L. Shear, BPI 614281. Mexico, Las Chiapas, Eucalyptus saligna, 26 Feb. 1998, C.S. Hodges, PREM 57518, living culture CMW 11298. Puerto Rico, 1923, F.J. Seaver & C.E. Chardon, NY 511. U.S.A., Hawaii, Kauai, Eucalyptus sp., Sept. 2002, M.J. Wingfield, PREM 57521, living culture CMW 10879 = CBS 115758, PREM 57522, living culture CMW 10885 = CBS 115760. Florida, Near Palmdale, Glades Co., Eucalyptus robusta, 1984, E.L. Barnard & K.M. Old, FLAS 54261, ATCC 60862; Eucalyptus grandis, 1984, E.L. Barnard & K.M. Old, FLAS 54263. Madeira, Machico, Myrica faya, 8 May 2000, C.S. Hodges, PREM 57523, living culture CMW 14551 = CBS 115841. Azores, Island of São Miguel, Mosteiro, M. faya, C.S. Hodges & D.E. Gardner, PREM 57524, living culture from same locality CMW 11301; Island of Pico, M. faya, 30 Jul. 1992, C.S. Hodges & D.E. Gardner, PREM 57525, living culture from same locality CMW 11301; Island of Pico, M. faya, 31 May 1985, C.S. Hodges & D.E. Gardner, PREM 58810, living culture from same locality CMW 11301; Island of São Miguel, M. faya, 2 Aug. 1992, C.S. Hodges & D.E. Gardner, PREM 58811, living culture from same locality CMW 11301; Island of Terceiro, M. faya, 31 May 1987, C.S. Hodges & D.E. Gardner, PREM 58812, living culture from same locality CMW 11301; Island of Faial, M. faya, 27 May 1985, C.S. Hodges, PREM 58813, living culture from same locality CMW 11301.
Notes: Microthia havanensis and A. gyrosa have been considered as synonyms when the latter fungus was still known as C. gyrosa (Kobayashi 1970, Hodges 1980). Cryphonectria gyrosa has also been known as Endothia tropicalis Shear & N.E. Stevens during the time that Cryphonectria was considered synonymous to Endothia (Shear et al. 1917, Kobayashi & Itô 1956, Kobayashi 1970, Roane 1986). Amphilogia gyrosa is, however, a distinct fungus from M. havanensis, as shown clearly in this study.
Specimens of C. coccolobae resemble those of Mi. havanensis closely and clearly reside in the same genus. Based on the similar spore dimensions, it is also probable that C. coccolobae is conspecific with Mi. havanensis. However, in the absence of isolates that can be used to confirm the phylogenetic relationship of C. coccolobae, we propose that C. coccolobae retain its independent taxonomic status for the present. Specimens representing C. coccolobae are, however, transferred to Microthia since this species clearly does not reside in Cryphonectria s. str.
Microthia coccolobae (Vizioli) Gryzenh. & M.J. Wingf., comb. nov. MycoBank MB500794. Fig. 4.
Basionym: Endothia coccolobae Vizioli, Mycologia 15: 115. 1923 (as E. coccolobii).
≡ Cryphonectria coccolobae (Vizioli) Micales & Stipes, Phytopathology 77: 651. 1987 (as C. coccolobii).
Specimens examined: Bermuda, Grape Bay, fruit of Coccoloba uvifera, 11 Dec. 1921, H.H. Whetzel, holotype CUP 128; Grape Bay, fruit of Co. uvifera, 11 Dec. 1921, H.H. Whetzel, isotypes BPI 613756, NY 147, other specimen CUP 30512; Elbow Beach, Fruit of Co. uvifera, 28 Jan. 1926, Whetzel, Seaver & Ogilvie, CUP 34658; South Shore, bark of Co. uvifera, 25 Nov. 1940, F.J. Seaver & J.M. Waterston, CUP 57366; Devonshire, Calophyllum calaba, 2 Feb. 1926, Seaver, Whetzel & Ogilvie, CUP 35078; Devonshire Bay, Conocarpus erecta, 5 Feb. 1926, Seaver, Whetzel & Ogilvie, CUP 35081.
The fungus collected from Co. uvifera in Florida as part of this study clearly does not represent Mi. coccolobae. DNA sequence and morphological comparisons showed that a new genus should be provided for it and the appropriate description is presented below. No teleomorph could be found on the material, but based on DNA sequence comparisons the fungus clearly belongs to the Diaporthales and is closely related to Cryphonectria and allied genera. It is, however, described as an anamorphic fungus following Art. 59.2 of the International Code of Botanical Nomenclature (Greuter et al. 2000).
Ursicollum Gryzenh. & M.J. Wingf., gen. nov. MycoBank MB500795.
Etymology: Latin, ursus, a bear, and latin, collus, neck. Referring to the hairy neck of the conidioma that reminds of that of a bear.
Conidiomata eustromatica, pyriformia vel rostrata, superficialia, aurantiaca, cum collis uno vel tribus, textura pseudoparenchymatosa sed in collo prosenchymatosa. Conidiophora cylindrica. Conidia cylindrica, hyalina, non septata.
Conidiomata eustromatic, pyriform or rostrate, superficial to slightly immersed in bark, unilocular, internally strongly convoluted, orange, with one to three attenuated or cylindrical necks, tissue pseudoparenchymatous but prosenchymatous in the neck. Conidiophores hyaline, delimited by septa or not, cylindrical, conidiogenous cells phialidic, apical or lateral on branches beneath the septum. Conidia cylindrical, hyaline, aseptate.
Typus: Ursicollum fallax Gryzenh. & M.J. Wingf., sp. nov.
Ursicollum fallax Gryzenh. & M.J. Wingf., sp. nov. MycoBank MB500796. Figs 5, 6.
Etymology: Latin, fallax, false. Refers to the conidiomata that appear to be false ascostromata.
Conidiomata eustromatica, pyriformia vel rostrata, aurantiaca, cum collis attenuatis uno vel tribus, superficialia vel subimmersa. Textura basalis pseudoparenchymatosa, textura collorum prosenchymatosa. Conidiophora cylindrica, apice attenuata an non. Conidia (2.5–)3–4(–5.5) × (1–)1.5(–2) μm, cylindrica, non septata, hyalina.
Conidiomata orange, eustromatic, pyriform to rostrate, with one to three attenuated or cylindrical necks (Figs 5A–B, 6A–B), base 120–400 μm high, 190–550 μm diam, neck up to 400 μm long, 90–180 μm wide, superficial to slightly immersed, unilocular, internally convoluted (Figs 5B–C, 6B). Basal tissue predominantly pseudoparenchymatous (Fig. 5E), neck tissue prosenchymatous (Fig. 5F). Conidiophores hyaline, cylindrical with or without attenuated apex, cells delimited by septa or not, total length of conidiophore (4.5–)5.5–19(–39) μm (Figs 5G–H, 6C). Conidiogenous cells phialidic, apical or lateral on branches beneath the septum, cylindrical to flask-shaped with attenuated apices, 1.5–2(–2.5) μm wide, collarette and periclinal thickening inconspicuous (Figs 5G–H, 6C). Conidia (2.5–)3–4(–5.5) × (1–)1.5(–2) μm, cylindrical, aseptate, hyaline, exuded as orange droplets (Figs 5I, 6C).
Cultural characteristics: on MEA white, fluffy, margins even, optimum for growth 25–30 °C, isolates covering 90 mm diam plates after 5–6 d at optimum growth temperatures.
Substratum: Bark of Coccoloba uvifera.
Distribution: Florida (U.S.A.).
Specimens examined: U.S.A., Florida, Fort Lauderdale, Coccoloba uvifera, 8 Mar. 2005, C.S. Hodges, holotype PREM 58840, culture ex-type CMW 18119 = CBS 118663; Key Biscayne, Coccoloba uvifera, 10 Mar. 2005, C.S. Hodges, PREM 58841, PREM 58842, living cultures CMW 18115 = CBS 118660, CMW 18124 = CBS 118662; Oakland Park, Coccoloba uvifera, 11 Mar. 2005, C.S. Hodges, PREM 58843, living culture CMW 18114 = CBS 118661; Dania, Coccoloba uvifera, 11 Mar. 2005, C.S. Hodges, PREM 58844, living culture CMW 18110.
Phylogenetic analyses based on the collection of isolates treated in this study and that of Gryzenhout et al. (2006), showed that isolates representing C. eucalypti from Australia and South Africa form a clade distinct from other species in Cryphonectria s. str. This phylogenetic grouping is supported by discrete morphological characteristics such as aseptate ascospores and small stromata, which are different to those found in Cryphonectria. Results of this study provide us with strong justification to erect a new genus for C. eucalypti, and a description is provided as follows:
Holocryphia Gryzenh. & M.J. Wingf., gen. nov. MycoBank MB500797.
Etymology: Greek, holo, undivided, crypho-, secret, referring to undivided ascospores and the semi-immersed nature of the stromata.
Ascostromata subimmersa, pulvinata, aurantiaca. Ascosporae cylindricae, interdum allantoideae, hyalinae, non septatae. Stromata anamorpha subimmersa, pulvinata, aurantiaca. Conidiophora cylindrica, basibus inflatis an non, attenuatae; paraphyses inter conidiophora adsunt. Conidia hyalina, cylindrica, non septata.
Ascostromata semi-immersed, pulvinate, orange, pseudoparenchymatous tissue at the edge of stromata, prosenchymatous tissue in the centre. Perithecia dark-walled, with globose to sub-globose bases and slender periphysate necks that emerge at the stromatal surface as black ostioles in papillae covered with orange stromatal tissue. Asci fusiform, floating freely in the perithecial cavity, unitunicate with non-amyloid, refractive apical rings. Ascospores cylindrical, occasionally allantoid, hyaline, aseptate.
Anamorphic stromata erumpent, semi-immersed, pulvinate, orange, uni- to multilocular and convoluted, locules often occurring in same stroma that contains perithecia. Conidiophores cylindrical with or without inflated bases, tapering, often septate with or without lateral branches beneath a septum, hyaline, paraphyses occurring between conidiophores, conidiogenous cells phialidic. Conidia hyaline, cylindrical, aseptate, expelled through an opening at the stromatal surface as orange droplets or tendrils.
Typus: Holocryphia eucalypti (M. Venter & M.J. Wingf.) Gryzenh. & M.J. Wingf., comb. nov.
Holocryphia eucalypti (M. Venter & M.J. Wingf.) Gryzenh. & M.J. Wingf., comb. nov. MycoBank MB500798.
Basionym: Cryphonectria eucalypti M. Venter & M. J. Wingf., Sydowia 54: 113–115. 2002.
Specimens examined: South Africa, Northern Kwazulu-Natal, Mtubatuba, Nyalazi estate, bark of GC747 clone of Eucalyptus, 25 Feb. 1998, M. Venter, holotype, PREM 56211, ex-type culture CMW 7034; Dukuduku estate, bark of Eucalyptus grandis, Oct. 1998, M. Venter, PREM 56214, PREM 56216; KwaMbonambi, Amangwe estate, bark of E. grandis, Oct. 1998, M. Venter, epitype designated here PREM 56215, living culture CMW 7033 = CBS 115842; Mpumalanga, Sabie, bark of E. grandis, Aug. 1998, J. Roux, PREM 56212; Limpopo, Tzaneen, bark of Eucalyptus saligna, 6 Feb. 1999, M. Venter, PREM 56305, living culture CMW 7035. Australia, Western Australia, Perth, Eucalyptus globulus, 1997, M.J. Wingfield, PREM 56217, living culture CMW 7038 = CBS 119475.
DISCUSSION
In this study, we describe three new genera that are closely related to Cryphonectria. Microthia includes the fungi previously known as C. havanensis and C. coccolobae, while Holocryphia represents the Eucalyptus pathogen previously known as C. eucalypti. Ursicollum is a new genus that was discovered on Co. uvifera in Florida while attempting to locate fresh specimens of Mi. coccolobae. The description of these new genera is justified based primarily on the phylogenetic grouping of the isolates, which are distinct from Cryphonectria and other closely related genera such as Endothia, Chrysoporthe and Rostraureum.
Microthia, Holocryphia and Ursicollum are defined by the following morphological characteristics. The pulvinate and semi-immersed stromata of Microthia and Holocryphia are similar to those of Cryphonectria but are much smaller. Stromata of Microthia also tend to be more superficial on the substrate than those found in Cryphonectria. Another interesting and unique feature, shared by Microthia and Holocryphia, is that the conidiomata of both fungi contain exceptionally long cells between the conidiophores. These cells, previously referred to as paraphyses (Venter et al. 2002), do not produce conidia. Microthia and Holocryphia are thus morphologically quite similar but can be distinguished from each other based on ascospore morphology. Microthia has single-septate ascospores, while those of Holocryphia are aseptate. Ursicollum is morphologically distinct from the anamorphs of Microthia, Holocryphia and other related genera because of its unique orange, pyriform to globose conidiomata with cylindrical to attenuated necks.
Holocryphia eucalypti was previously known as Endothia gyrosa (Schwein.: Fr.) Fr. (Venter et al. 2002). The fungus was described as a species of Cryphonectria because phylogenetic analyses indicated that isolates of this fungus grouped more closely with Cryphonectria than with Endothia, the only two genera that it resembled at that time (Venter et al. 2001, 2002). This phylogenetic grouping was supported morphologically by the semi-immersed stromata similar to those of Cryphonectria. Consequently, the new species was placed in Cryphonectria, despite the fact that its single-celled ascospores were different from the two-celled ascospores characteristic of all other Cryphonectria species. Phylogenetic studies (Myburg et al. 2004b) including more genera and species than those considered by Venter et al. (2002) did not provide convincing evidence to separate H. eucalypti from other Cryphonectria species. It was necessary to include the isolates of additional taxa presented in this study and that of Gryzenhout et al. (2006), which are morphologically similar to those of H. eucalypti, to reveal the distinction between H. eucalypti and species in the Cryphonectria sensu stricto clade. The unusual and contradictory fact that H. eucalypti (as C. eucalypti) had single-celled ascospores different from all species in Cryphonectria s. str. with two-celled ascospores, could thus be resolved.
The newly recognised taxonomic position of Microthia is well defined because numerous isolates of Mi. havanensis could be subjected to DNA sequence comparisons in this study. Although careful examination of the herbarium specimens of Mi. coccolobae have led us to suspect that this fungus is a synonym of Mi. havanensis, the taxonomic position of the former fungus has yet to be defined precisely. In the past, morphological characteristics such as spore size (Hodges & Gardner 1992), constriction at the ascospore septa and stromatal size (Roane 1986), the length of the perithecial necks (Vizioli 1923, Hodges & Gardner 1992), and the small number of perithecia in the stromata (Vizioli 1923) have been used to distinguish C. coccolobae from other species in Cryphonectria. These features are, however, quite variable in specimens. For example, constricted ascospores were seen in specimens of both Mi. havanensis and Mi. coccolobae, and stromatal morphology varied greatly. Size variation of these characteristics between samples was also observed. For example, asci in specimen PREM 57518 were larger than those in other specimens of Mi. havanensis. This was despite the fact that isolate CMW 11298, linked to PREM 57518, grouped with isolates linked to the other specimens of the same species based on DNA sequence data. Another feature that may have convinced previous authors that Mi. coccolobae represents a distinct taxon is the superficial fruiting structures on Co. uvifera seeds. We believe that this is related to the substrate, since stromatal morphology on the seeds (Vizioli 1923) was superficial, while on bark it is semi-immersed (Micales & Stipes 1987, Gardner & Hodges 1990).
While the morphology of Mi. coccolobae and Mi. havanensis is very similar, the pathogenicity and ecology of these two species have been reported to be different. In studies to determine the identity of the Cryphonectria sp. on M. faya (Hodges & Gardner 1992), an isolate of Mi. coccolobae from Bermuda failed to colonise freshly-cut branch sections of M. faya as successfully as isolates obtained from M. faya, which have been shown in this study to represent Mi. havanensis. Likewise, the fungus from M. faya did not grow in freshly-cut branch sections of Co. uvifera, although the Mi. coccolobae isolate was able to colonise this substrate. No inoculations were made on living trees of either host (Hodges & Gardner 1992). Reciprocal inoculations on various hosts such as Co. uvifera, Quercus spp. and Eucalyptus spp. with several isolates including Mi. havanensis from Eucalyptus and Mi. coccolobae, showed that the Mi. coccolobae isolates alone were able to infect Co. uvifera resulting in cankers (Barnard et al. 1993). These differences in pathogenicity to Co. uvifera may indicate that the two species are distinct, despite their similar morphology. Another unusual characteristic that distinguishes Mi. coccolobae from other closely related fungi is its prolific colonization of fruits of Co. uvifera, often while they are still green. In contrast, other species of Microthia, Cryphonectria and allied genera have been found only on bark. It is for these reasons that we have chosen not to synonymise these species before isolates of Mi. coccolobae can be obtained for DNA sequence comparisons.
While searching for fresh material of C. coccolobae (now Mi. coccolobae) on sea grape in Florida, another morphologically similar fungus, U. fallax, was found on this host. This fungus represents a new genus and species, which is closely related to Cryphonectria and allied genera, although no teleomorph structures were found for the fungus. Morphological comparisons with Mi. coccolobae showed that U. fallax is distinctly different from Mi. coccolobae. Two closely related and morphologically similar fungi thus occur on Co. uvifera, although it could also be possible that previous reports of Mi. coccolobae in Florida actually represent U. fallax. This will complicate continuing surveys searching for Mi. coccolobae on this host in order to obtain isolates for later phylogenetic comparisons.
It has previously been suggested that the fungus referred to as C. havanensis in Japan, represents C. nitschkei (Myburg et al. 2004a). At the time of that study, it was not possible to determine whether C. nitschkei was the same as C. havanensis in Cuba (Myburg et al. 2004a). For the present study, we had at our disposal a substantial collection of isolates linked to additional specimens that we feel confident to have the fungus previously known as C. havanensis. We were thus able to conduct morphological and phylogenetic comparisons to show clearly that the type of Mi. havanensis represents a fungus different from that of C. nitschkei from Japan. The fungus now known as Mi. havanensis thus does not occur in Japan.
Microthia havanensis appears to occur saprotrophically on Eucalyptus and other hosts. Bruner (1916) described the fungus on dead branches and twigs. Barnard et al. (1987) also reported it as a saprotroph on E. grandis in Florida, while Chr. cubensis was the cause of canker disease in the same plantations. In Mexico and Kauai the fungus was found only on dead, suppressed trees of Eucalyptus, and was not associated with cankers. Similarly, although Mi. havanensis was associated with cankers on M. faya trees in the Azores (Gardner & Hodges 1990), it also occurs on dead trees, and may only play a saprotrophic role on cankers (Hodges & Gardner 1992).
Microthia havanensis frequently occurs on Eucalyptus in the same locality as trees infected with Chr. cubensis. This is consistent with the fact that both Chr. cubensis and Mi. havanensis were first described from Cuba in the same locality (Bruner 1916, 1917) and both occurred in the same plantations in Florida (Barnard et al. 1987) and Kauai. Clearly the pathogenicity of Mi. havanensis, factors that influence its pathogenicity and the ecological relationship between Mi. havanensis and Chr. cubensis, deserves further consideration.
This study emphasizes the fact that several closely related and morphologically similar fungi, all with orange stromatic tissue, occur on Eucalyptus trees worldwide. These fungi previously resided in the single genus Cryphonectria, but most have now been transferred to new genera. Microthia havanensis and H. eucalypti have been newly described in this study. Cryphonectria nitschkei occurs on Eucalyptus spp. in Japan, and C. parasitica and an unknown Cryphonectria sp. have also been reported from Eucalyptus spp. in Japan (Old & Kobayashi 1988). Lastly, Chrysoporthe species, previously treated as the single species Cryphonectria cubensis, also occur on Eucalyptus spp. and have been observed in the same geographic regions as H. eucalypti and Mi. havanensis (Gryzenhout et al. 2004).
The various Cryphonectria spp. and related fungi occur on Eucalyptus spp. in different parts of the world (Fig. 1). Thus C. nitschkei, C. parasitica and the undescribed Cryphonectria sp. on Eucalyptus are known from the Far East, H. eucalypti occurs in Australia and South Africa, and Mi. havanensis is now known from Mexico, Cuba, Puerto Rico, Florida, Hawaii, Azores and Madeira. Furthermore, the different species of Chrysoporthe occur in different tropical and sub-tropical countries of the world (Gryzenhout et al. 2004). For example, Chr. austroafricana occurs specifically in South Africa and Chr. cubensis occurs in Hawaii, Central and South America, Central Africa, South East Asia and Australia (Gryzenhout et al. 2004).
Cryphonectria, Chrysoporthe, Microthia and Holocryphia differ significantly in their pathogenicity to Eucalyptus spp., which is an ecologically important tree that also forms the basis of large forestry industries. Chrysoporthe spp. and H. eucalypti are considered the most important pathogens in this group. Mi. havanensis and the different Cryphonectria spp. are mild pathogens or saprophytes. Although the geographical range of C. nitschkei, Mi. havanensis and H. eucalypti is not currently known to overlap (Fig. 1), it is possible that these fungi could be introduced into new areas. It has been hypothesized that H. eucalypti has already moved from Australia, where it is presumed to be native due to the widespread occurrence of H. eucalypti in native Eucalyptus forests in Australia (Walker et al. 1985, Old et al. 1986), into Eucalyptus plantation areas of South Africa (Nakabonge et al. 2005). Because of the importance of some of these fungi as pathogens, every effort must be made to identify collections accurately. This underpins efforts to monitor the spread of diseases and to manage their impact.
The following key is provided to facilitate the distinction between different diaporthalean genera with orange stromatic tissue, some of which occur on Eucalyptus:
1a. Conidiomata pyriform to clavate; ascostromata with reduced stromatic tissue................................................. 2
1b. Conidiomata pulvinate; ascostromata well-developed....................................................................................... 4
2a. Conidiomata black; orange ascostroma with black perithecial necks............................................Chrysoporthe
2b. Conidiomata orange.......................................................................................................................................... 3
3a. Conidiomata rostrate with tapered necks; orange stroma with orange perithecial necks..............Rostraureum
3b. Conidiomata pyriform or rostrate or globose with more cylindrical necks; teleomorph unknown...........................................................................................................................Ursicollum
4a. Ascospores septate............................................................................................................................................ 5
4b. Ascospores aseptate.......................................................................................................................................... 6
5a. Ascostromata large, well-developed, semi-immersed; paraphyses absent in conidial locules..............................................................................................................................Cryphonectria
5b. Ascostromata small to medium size, usually superficial; conidial locules containing paraphyses......................................................................................................................... Microthia
6a. Ascostromata large, well-developed, superficial.................................................................................. Endothia
6b. Ascostromata small to medium size, semi-immersed.................................................................... Holocryphia
Acknowledgments
We thank Dr E.L. Barnard (Florida Division of Forestry, FDACS, Gainesville, Florida) for donating an isolate of Mi. havanensis from Mexico and his advice regarding Mi. coccolobae. Collaboration with Dr J. Mena Portales (Institute of Ecology and Systematics, Carretera Varona, Ciudad de La Habano, Cuba) made it possible to survey for Mi. havanensis in Cuba. We are grateful for assistance provided by Dr Timothy Schubert (Florida Department of Agriculture and Consumer Services, Gainesville, FL) and Dr Randy Ploetz (University of Florida's Tropical Research & Education Center, Homestead, FL) in collecting the samples from Co. uvifera. We thank Dr H.F. Glen (KwZulu-Natal Herbarium, SANBI, Durban, South Africa) for providing the Latin descriptions and assisting us in chosing names for the new taxa. We also thank Raksha Bhoora and Joyce Jakavula for their assistance with DNA sequencing. We are deeply grateful to the curators of the herbaria listed in this study for loans of specimens. Financial support was provided by the National Research Foundation (NRF), members of the Tree Pathology Co-operative Programme (TPCP), and the THRIP support programme of the Department of Trade and Industry, South Africa.
Taxonomic novelties: Microthia Gryzenh. & M.J. Wingf. gen. nov., Microthia havanensis (Bruner) Gryzenh. & M.J. Wingf. comb. nov., Microthia coccolobae (Vizioli) Gryzenh. & M.J. Wingf. comb. nov., Holocryphia Gryzenh. & M.J. Wingf. gen. nov., Holocryphia eucalypti (M. Venter & M.J. Wingf.) Gryzenh. & M.J. Wingf. comb. nov., Ursicollum Gryzenh. & M.J. Wingf. gen. nov., Ursicollum fallax Gryzenh. & M.J. Wingf. sp. nov.
References
- Barnard EL, Barnard MR, El-Gholl NE, Ash EC (1993). Preliminary investigations of some Florida isolates of Cryphonectria and/or Endothia spp. from Castanea, Coccoloba, Eucalyptus and Quercus spp. Southwide Forest Disease Workshop, Auburn, Alabama, U.S.A., 13–15 January 1993.
- Barnard EL, Geary T, English JT, Gilly SP (1987). Basal cankers and coppice failure of Eucalyptus grandis in Florida. Plant Disease 71: 358–361. [Google Scholar]
- Bruner SC (1916). A new species of Endothia. Mycologia 8: 239–242. [Google Scholar]
- Bruner SC (1917). Una enfermedad gangrenosa de los eucaliptos. Estacion Experimental Agronomica, Santiago de las Vegas, Cuba, Bulletin 37: 1–33. [Google Scholar]
- Castlebury LA, Rossman AY, Jaklitsch WJ, Vasilyeva LN (2002). A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94: 1017–1031. [PubMed] [Google Scholar]
- Earle FS (1901). Some fungi from Porto Rico. Muehlenbergia 1: 10–17. [Google Scholar]
- Farris JS, Källersjö M, Kluge AG, Bult C (1994). Testing significance of incongruence. Cladistics 10: 315–319. [Google Scholar]
- Gardner DE, Hodges CS (1990). Diseases of Myrica faya (firetree, Myricaceae) in the Azores, Madeira and the Canary Islands. Plant Pathology 39: 326–330. [Google Scholar]
- Glass NL, Donaldson GC (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greuter W, McNeill J, Barrie FR, Burdet HM, Demoulin V, Filgueiras TS, Nicolson DH, Silva PC, Skog JE, Trehane P, Turland NJ, Hawksworth DL (2000). International Code of Botanical Nomenclature (Saint Louis Code). Koeltz Scientific Books Königstein, Germany.
- Gryzenhout M, Eisenberg BE, Coutinho TA, Wingfield BD, Wingfield MJ (2003). Pathogenicity of Cryphonectria eucalypti to Eucalyptus clones in South Africa. Forest Ecology and Management 176: 427–437. [Google Scholar]
- Gryzenhout M, Glen HF, Wingfield BD, Wingfield MJ (2005b). Amphilogia gen. nov. for Cryphonectria-like fungi from Elaeocarpus spp. in New Zealand and Sri Lanka. Taxon 54: 1009–1021. [Google Scholar]
- Gryzenhout M, Myburg H, Van der Merwe NA, Wingfield BD, Wingfield MJ (2004). Chrysoporthe, a new genus to accommodate Cryphonectria cubensis. Studies in Mycology 50: 119–142. [Google Scholar]
- Gryzenhout M, Myburg H, Wingfield BD, Montenegro F, Wingfield MJ (2005a). Rostraureum tropicale gen. sp. nov. (Diaporthales) associated with dying Terminalia ivorensis in Ecuador. Mycological Research 109: 1029–1044. [DOI] [PubMed] [Google Scholar]
- Gryzenhout M, Wingfield BD, Wingfield MJ (2006). New taxonomic concepts for the important forest pathogen Cryphonectria parasitica and related fungi. FEMS Microbiology Letters 258: 161–172. [DOI] [PubMed] [Google Scholar]
- Hodges CS (1980). The taxonomy of Diaporthe cubensis. Mycologia 72: 542–548. [Google Scholar]
- Hodges CS, Gardner DE (1992). Survey for potential biological control agents for Myrica faya in the Azores and Madeira islands. Report submitted to the Cooperative National Park Resources Unit, University of Hawaii at Manoa, Dept. of Botany, Honolulu, Hawaii.
- Hodges CS, Geary TF, Cordell CE (1979). The occurrence of Diaporthe cubensis on Eucalyptus in Florida, Hawaii and Puerto Rico. Plant Disease Reporter 63: 216–220. [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acid Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T (1970). Taxonomic studies of Japanese Diaporthaceae with special reference to their life histories. Bulletin of the Government Forest Experiment Station 226: 132–147. [Google Scholar]
- Kobayashi T, Itô K (1956). Notes on the genus Endothia in Japan I. Species of Endothia collected in Japan. Japan Government Forest Experiment Station 92: 81–98. [Google Scholar]
- Liu Y-C, Linder-Basso D, Hillman BI, Kaneko S, Milgroom MG (2003). Evidence for interspecies transmission of viruses in natural populations of filamentous fungi in the genus Cryphonectria. Molecular Ecology 12: 1619–1628. [DOI] [PubMed] [Google Scholar]
- Micales JA, Stipes RJ (1987). A reexamination of the fungal genera Cryphonectria and Endothia. Phytopathology 77: 650–654. [Google Scholar]
- Myburg H, Gryzenhout M, Wingfield BD, Milgroom MG, Shigeru K, Wingfield MJ (2004a). DNA sequence data and morphology define Cryphonectria species in Europe, China, and Japan. Canadian Journal of Botany 82: 1730–1743. [Google Scholar]
- Myburg H, Gryzenhout M, Wingfield BD, Stipes RJ, Wingfield MJ (2004b). Phylogenetic relationships of Cryphonectria and Endothia species, based on DNA sequence data and morphology. Mycologia 96: 990–1001. [PubMed] [Google Scholar]
- Myburg H, Gryzenhout M, Wingfield BD, Wingfield MJ (2002). β-tubulin and Histone H3 gene sequences distinguish Cryphonectria cubensis from South Africa, Asia and South America. Canadian Journal of Botany 80: 590–596. [Google Scholar]
- Myburg H, Gryzenhout M, Wingfield BD, Wingfield MJ (2003). Conspecificity of Endothia eugeniae and Cryphonectria cubensis: A re-evaluation based on morphology and DNA sequence data. Mycoscience 104: 187–196. [Google Scholar]
- Myburg H, Wingfield BD, Wingfield MJ (1999). Phylogeny of Cryphonectria cubensis and allied species inferred from DNA analysis. Mycologia 91: 243–250. [Google Scholar]
- Nakabonge G, Cortinas MN, Roux J, Gryzenhout M, Wingfield BD, Wingfield MJ (2005). Development of polymorphic microsatellite markers for the fungal tree pathogen Cryphonectria eucalypti. Molecular Ecology Notes 5: 558–561. [Google Scholar]
- Old KM, Kobayashi T (1988). Eucalypts are susceptible to the chestnut blight fungus, Cryphonectria parasitica. Australian Journal of Botany 36: 599–603. [Google Scholar]
- Old KM, Murray DIL, Kile GA, Simpson J, Malafant KWJ (1986). The pathology of fungi isolated from eucalypt cankers in south-eastern Australia. Australian Forestry Research 16: 21–36. [Google Scholar]
- Posada D, Crandall KA (1998). MODELTEST: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- Rayner RW (1970). A Mycological Colour Chart. Commonwealth Mycological Institute and British Mycological Society, Kew, Surrey, U.K.
- Roane MK (1986). Taxonomy of the genus Endothia. In: Chestnut blight, other Endothia diseases, and the genus Endothia (Roane MK, Griffin GJ, Elkins JR, eds). APS Press, St. Paul, Minnesota, U.S.A.: 28–39.
- Rodas CA, Gryzenhout M, Myburg H, Wingfield BD, Wingfield MJ (2005). Discovery of the Eucalyptus canker pathogen Chrysoporthe cubensis on native Miconia (Melastomataceae) in Colombia. Plant Pathology 54: 460–470. [Google Scholar]
- Seaver FJ, Waterston JM (1940). Contributions towards the mycoflora in Bermuda–I. Mycologia 32: 388–407. [Google Scholar]
- Swofford DL (2002). PAUP. Phylogenetic Analysis Using Parsimony. Version 4.0b10. Sinauer Associates, Sunderland, Massachusetts.
- Tamura K, Nei M (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology & Evolution 10: 512–526. [DOI] [PubMed] [Google Scholar]
- Van der Westhuizen IP, Wingfield MJ, Kemp GHJ, Swart WJ (1993). First report of the canker pathogen Endothia gyrosa on Eucalyptus in South Africa. Plant Pathology 42: 661–663. [Google Scholar]
- Venter M, Myburg H, Wingfield BD, Coutinho TA, Wingfield MJ (2002). A new species of Cryphonectria from South Africa and Australia, pathogenic to Eucalyptus. Sydowia 54: 98–117. [Google Scholar]
- Venter M, Wingfield MJ, Coutinho TA, Wingfield BD (2001). Molecular characterization of Endothia gyrosa isolates from Eucalyptus in South Africa and Australia. Plant Pathology 50: 211–217. [Google Scholar]
- Vizioli J (1923). Some Pyrenomycetes of Bermuda. Mycologia 15: 107–119. [Google Scholar]
- Walker J, Old KM, Murray DIL (1985). Endothia gyrosa on Eucalyptus in Australia with notes on some other species of Endothia and Cryphonectria. Mycotaxon 23: 353–370. [Google Scholar]
- Waterston JM (1947). The fungi of Bermuda. Bulletin of the Department of Agriculture Bermuda 23: 108–110 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, San Diego, CA, U.S.A.: 315–322.
- Wingfield MJ (2003). Daniel McAlpine Memorial Lecture. Increasing threat of diseases to exotic plantation forests in the Southern Hemisphere: lessons from Cryphonectria canker. Australasian Plant Pathology 23: 133–139. [Google Scholar]
- Yuan ZQ, Mohammed C (2000). The pathogenicity of isolates of Endothia gyrosa to Eucalyptus nitens and E. globulus. Australasian Plant Pathology 29: 29–35. [Google Scholar]