Abstract
Several recent studies have reviewed the extent of fungal biodiversity, and have used these data as basis for revised estimates of species numbers based on known numbers of plants and insects. None of these studies, however, have focused on fungal biodiversity in South Africa. Coinciding with the 100th anniversary of the National Collection of Fungi (PREM) in South Africa in 2005, it is thus timely to reflect on the taxonomic research that has been conducted in South Africa over the past Century. Information is presented on the extent of fungal collections preserved at PREM, and the associated research publications that have largely resulted from this resource. These data are placed in context of the known plant and insect biodiversity, and used as basis to estimate the potential number of fungi that could be expected in South Africa. The conservative estimate is of approximately 200 000 species without taking into account those associated with a substantial insect biodiversity.
Keywords: Biodiversity, conservation, National Collection of Fungi, numbers of fungi, undescribed species
INTRODUCTION
Defining the number of fungi on earth has always been a point of discussion (Fries 1825, Bisby & Ainsworth 1943), but has gained prominence in scientific literature towards the latter part of the twentieth century. While this exercise might on the surface appear to be of peripheral importance, it is fundamental to understanding and protecting the world's biodiversity. Thus, a number of studies have in recent years focused on enumerating the world's fungal biodiversity (Pirozynski 1972, Pascoe 1990, Hawksworth 1991, Dreyfuss & Chapela 1994, Rossman 1994, Hyde 1996, Hyde et al. 1997, Hawksworth 1998, Fröhlich & Hyde 1999, Hawksworth 2001, 2004). They have provided the foundation for studies aimed at a better understanding of fungal biodiversity worldwide, and results have been used to motivate for bioconservation and fungal biodiversity studies.
It is widely recognised that fungi have been relatively poorly collected and studied from most countries, regions and habitats. This is at least in comparison to plants and larger animals that are considerably easier to collect and identify than fungi. One might then have expected that the predicted numbers of fungi on earth would have been considerably greater than the 1.5 M suggested by Hawksworth (1991). Clearly different authors that have considered the likely total number of fungi have had differing views of an appropriate answer, but the discrepancy between the results of most of these studies is not particularly great. It is currently accepted that the 1.5 M estimate is highly conservative. The 100 000 that have thus far been described, therefore represents no more than 7 % of the estimated total.
One hundred years of mycology in South Africa as celebrated in this volume is not a particularly long history for mycology as science. The country is widely recognised as one of the world's biodiversity “hotspots”, including areas such as the Cape Floral Kingdom, which is the smallest and most diverse biome presently known. This paper aims to summarize the major mycological developments that have happened in South Africa, as well as to provide an estimate of the number of fungi in this country. In a paper such as this it is never possible to comment on all activities and groups that have been active over the past 100 years, and for many of these there will only be a brief mention, chiefly because much of the information was not available to us at the time this paper was written. Nevertheless, it is hoped that this summary and estimate will not only be interesting, but also provide a foundation to promote and guide future mycological activity in South Africa and elsewhere.
UNIQUE SOUTHERN AFRICAN FLORA
Germishuizen & Meyer (2003) list just over 24 500 taxa (including those at infraspecific level) as occurring in the flora of the southern African region, which includes South Africa, Botswana, Lesotho, Swaziland and Namibia. They observe that this means that about 10 % of the world's flora occurs in less than 2.5 % of the total land area of the world. This represents an increase of about 500 taxa on the previous checklist of this flora (Arnold & de Wet 1993). By no means is all of this increase due to “cryptic” taxa appearing as a result of new revisions, or new invader plants appearing in our area. Plant collectors are finding previously unknown taxa in ever more inaccessible areas, sometimes embarrassingly close to major centres of population. As examples of these discoveries, one may cite recent papers by Edwards et al. (2005) detailing a new Drimia from within the Durban municipal boundaries, and any one of Van Jaarsveld's numerous recent discoveries (e.g. Van Jaarsveld et al. 2005; this issue of Aloe contains two other similar new discoveries) from further afield. As an example of a recent invader, one may point to Campuloclinium macrocephalum, which became noticeable a few years ago in Pretoria, and has already spread to Durban (mapped and described without historical data by Henderson 2001). In the South African National Biodiversity Institute (SANBI), much systematic research is directed to cataloguing plant diversity by means of regional floras; some (Goldblatt & Manning 2000, Retief & Herman 1997) already published and others still in various stages of preparation. Monographic studies tend to be the province of university departments. That both kinds of study are needed is shown by the fact that some groups, notably some genera of legumes, have not been critically examined since the pioneering work of W.H. Harvey, over a century ago (in Harvey & Sonder 1862).
Naturally, these plants are not evenly distributed over southern Africa, and there have been various studies of the vegetation of southern Africa from the historically classic studies of Drège (1843) and Bolus (1886), through Acocks (1953 and still the most often-cited work in South African botany) to the recent studies of Low & Rebelo (1996) and Rutherford & Mucina (in prep.). For the purposes of this contribution, the concepts of biomes and centres of diversity given by Van Wyk & Smith (2001) will be used (Figs 1, 2).
Fig. 1.
Unique South African flora. A–C. Cape Floristic Region. D. Beach vegetation. E. Coastal forest. F. Mangrove vegetation. G. Ceres Karoo. H–J. Succulent Karoo. K. Bushveld and grassland. L. Wolkberg centre. All photographs by H. Glen, except A by P.W. Crous.
Fig. 2.
Unique South African flora (continued). A. Wolkberg centre. B. Montane grassland. C. Degraded bushveld. D–F. Soutpansberg centre. Photographs by H. Glen.
Kaokoveld Centre
In the far north-west of Namibia and adjacent southern Angola, the vegetation of this centre is mainly desert and semi-desert of the Namib and the arid savannas to the immediate east of it. One of the most striking features of the semi-desert is the so-called fairy rings: circular patches completely devoid of plant cover, surrounded by grass. These are not permanent, and several theories have been put forward to explain their existence. One suggests fungal action (Eicker et al. 1982), but no convincing fungi have been found in them. The presently most-popular theory involves both termites and a biological factor inhibiting the resistance of grasses to desiccation (Albrecht et al. 2001). This area is very poorly known, and the estimate of the flora of 400 species (Viljoen 1980) is likely to be far too low.
Succulent Karoo
This is the arid area to the north and west of the Cape fynbos. Different workers have varying concepts of the boundaries of the area, giving rise to varying estimates of the number of species present. A current SANBI project to prepare a flora of the area is working on some 4 000 species (Paterson-Jones, pers. comm.), but other estimates with wider geographical limits put the plant diversity as high as 5 000 species. Many of the plants here are succulents, but another significant part of the flora is made up of ephemeral annuals. In both groups, it is known that not all seeds from a given year will germinate together; up to half the crop will remain dormant until at least the second rainy season after they have ripened.
Cape Floristic Region
The fynbos of the Western Cape has been studied for longer than any other part of sub-Saharan Africa, and so should be among the best-known floras in Africa. However, it is also among the world's most diverse. A recent synopsis of the flora of this region (Goldblatt & Manning 2000) lists some 9000 species for this area, approximately 80 % of which are endemic. Sadly much of the area has been replaced by, or is under threat from agriculture and urbanisation. Presently less than 5 % of the West Coast Renosterveld is still in pristine condition. The flora here is remarkable for reaching its greatest diversity on essentially nutrient-free soils.
Griqualand West Centre
This region occupies the dry area bounded roughly by a line connecting the towns of Prieska, Vryburg, Vorstershoop and Upington in the Northern Cape Province. The vegetation is mainly various kinds of bushveld, grassland and Karoo. There are many succulents endemic here, but the flora is not as well-known as one may suppose. The only checklist of the plants of the area is by Wilman (1946). Acocks (1953) recorded over 300 species from a single sampling point in the Asbestos Hills – the highest number from any of the thousands of points he sampled for this work.
Albany Centre
This area of endemism stretches westwards from the Kei River roughly to Middelburg, Aberdeen and the Baviaanskloof Mountains in the Eastern Cape. The climate is transitional between the summer-rainfall regime of the eastern part of southern Africa and the winter-rainfall climate of the Western Cape, and at first glance the vegetation seems transitional, too. The vegetation is highly varied, with as many as a third (21 of 70) of Acocks's (1953) veld types and five of White's (1983) main phytochoria being represented here. A flora of the Eastern Cape is in preparation, and is expected to treat some 8400 species (Bredenkamp, pers. comm.).
Drakensberg Alpine Centre
The highest mountain area in southern Africa includes most of Lesotho, a very mountainous area around Barkly East in the Eastern Cape, and a fringing area stretching along the eastern (seaward) slope of the Drakensberg from there through KwaZulu-Natal to the mountainous area southwest of Harrismith in the Free State. Although this area does not have an up-to-date flora, it is well supplied with comparatively recent field guides (Hilliard & Burtt 1987, Killick 1990, Pooley 2003). The last-cited guide, estimates the flora of this centre as comprising some 2200 species, 20 % of which are endemic. Van Rooy (2000) considers this to be one of the main centres of diversity of mosses in southern Africa.
Soutpansberg Centre
The mountains separating the Limpopo Valley from the rest of South Africa have long been known in botanical folklore as interesting, but have only recently been defined as a centre of endemism, running from the Blouberg in the west, along the Soutpansberg and including parts of the Limpopo valley into the north-western corner of the Kruger National Park and a tiny area of Zimbabwe. The area contains representatives of 41 % of all plant genera and 68 % of all plant families in the Flora of southern Africa area (South Africa, Botswana, Lesotho, Swaziland, Namibia), and is certainly a meeting place between the floras of east and west south-tropical Africa (Hahn 1998). The only recent account of the flora of this area specifically is Hahn's (1994) tree checklist, but the number of plant taxa here is estimated at about 3000.
Wolkberg Centre
Stretching from near Carolina, Mpumalanga, in the south to Haenertsburg, Limpopo Province in the north, with a westward extension to Zebediela south of Polokwane, this centre comprises the escarpment areas once known as the Transvaal Drakensberg. The flora numbers between 1500 and 2000 species, some 10 % of which are endemic.
Sekhukhuneland Centre
Inland of the Wolkberg Centre escarpment is a much drier area, underlain by basic and ultramafic rocks of the Bushveld Igneous Complex; as with the Barberton centre below, this geology may lead one to expect many endemic species. The flora of the area is severely undercollected, but is believed to number some 2000 species (Retief et al. 2001). The importance of the area as a centre of endemism was demonstrated by Siebert (1998), who has also done a still-unpublished phytosociological study of this Centre (Siebert 2001). The vegetation is conventionally considered to be Mixed Bushveld (e.g. Low & Rebelo 1996), but grassland and forest remnants are also to be found there. The flora of the Sekhukhuneland bushveld is unique. However, most of the area has been severely degraded by creeping urbanization and subsistence farming.
Barberton Centre
The mountains between Barberton, Mpumalanga Province, and the Swaziland border (stretching into a small part of north-western Swaziland) have a remarkable flora with many endemics. These mountains are geologically complex, and include some of the oldest fossil-bearing rocks on earth (MacRae 1999), as well as serpentinite (noted wherever it occurs for supporting unusual endemic species) and ancient volcanic rocks. Balkwill & Balkwill (1999) report 30 endemic species here, many of them as yet undescribed. However, the rugged terrain means that much of the area is effectively unexplored, and so many other taxa of limited occurrence are no doubt still awaiting discovery (Hurter & Van Wyk 2004).
Maputaland-Pondoland Region
This includes almost the whole of the KwaZulu-Natal Province, and a smaller or larger fringe in surrounding areas, most notably southern Mozambique and Eastern Cape (former Transkei). A newly-started project to generate an electronic Flora of KwaZulu-Natal is working on a checklist of some 6500 taxa, suggesting a flora for the whole area of about 7500 species. Vegetationally, the area may be divided into numerous grassland, thicket and forest types. Among the earliest specimens from KwaZulu-Natal are a series collected by Gerrard & McKen in 1860, but serious study of the flora of the area may be said to have started with Medley Wood, who founded the first herbarium in the then colony in 1882 (Schrire 1983), and also collected and wrote on some fungi, mostly associated with plant diseases. With the immense variety of plants and habitats in this area, it is hardly surprising that undescribed fungi are found here even more frequently than undescribed plants.
BEGINNING OF MYCOLOGY IN SOUTHERN AFRICA
Mycology in southern Africa was formally initiated by the appointment of I.B. Pole Evans in 1905. At that time, the only fungal collections in the country were those of MacOwan and Medley Wood, consisting of some 765 specimens (Pole Evans 1916). Pole Evans established a national collection of fungi in Pretoria. At the time of publication of Doidge's book (Doidge 1950), this collection included more than 35 000 fungal specimens. Other fungal collections were housed at Stellenbosch (collections of P.A. van der Bijl and L. Verwoerd), Cape Town (P. MacOwan collection and Bolus herbarium), and at several European herbaria, the most important of which are Kew and the International Mycological Institute (CABI Bioscience). Pole Evans also sent numerous collections to Europe, many to P. Hennings, P. Magnus and H. and P. Sydow. Several collections of larger fungi were also sent to C.G. Lloyd in the U.S.A., and many duplicates can be found in Vienna. Doidge (1950) summarised the content of her book in tabular form, listing (species) 93 Myxomycetes, 77 Phycomycetes, 835 Ascomycetes, 1159 lichens, 1704 Basidiomycetes, 880 fungi imperfecti, making a grand total of 4748 species. Many of these specimens were collected under extremely difficult circumstances and personal danger, which is reported on in detail by Doidge (1950).
Most mycological activity in the post-Doidge era focused on reports of fungi causing plant diseases, with some attention to saprobic fungi, and those found to be mycotoxigenic. Reference works such as the Gorter bulletins (1977, 1979, 1981, 1982), Van der Westhuizen & Eicker (1994), and Eicker & Baxter (1999) provide lists of various groups of fungi compiled after Doidge et al. (1953). Information on fungi subsequent to 1999 can be obtained via the Internet-based electronic system of CAB abstracts (CAB) (www.cabi-publishing.org). Several lists of plant diseases caused by fungi, bacteria and viruses in South Afirica have been published. The first of these was Doidge (1924) followed by Doidge & Bottomley (1931) and Doidge et al. (1953), which was chiefly based on Doidge (1950). These records were updated in a series of bulletins published by Gorter (1977, 1979, 1981, 1982). Later, Crous et al. (2000b) published the compilation “Phytopathogenic Fungi of South Africa”, which was made available online by the Systematic Botany & Mycology Laboratory in the U.S.A., and is searchable via <http://nt.ars-grin.gov/fungaldatabases/southafrica/>.
INTENSIVELY STUDIED GROUPS
Ascomycetes
The ascomycetes represent a group of fungi that have been relatively widely studied in South Africa. This group of fungi was intensively collected during the Doidge era (Doidge 1950). Subsequently, the emphasis changed (Baxter 1994) from broader-based data collection or taxonomic work and the description of new species to the study of fungi that are important as plant pathogens. This was closely linked to the fact that the responsibility for the National Collection of Fungi was placed with the Plant Protection Research Institute of the National Department of Agriculture. Examples include studies on the Xylariaceae (Martin 1970), Botryosphaeria Ces. & De Not. (Denman et al. 2000, 2003, Slippers et al. 2004a,b,c,d), Mycosphaerella Johanson (Crous 1998, Crous et al. 2000a, 2001, 2004c), powdery mildews (Erysiphaceae) (Gorter 1988a, b, 1989), Valsaceae (Adams et al. 2005) and Sclerotinia Fuckel (Thompson & Van der Westhuizen 1979, Phillips 1987, Van der Westhuizen & Eicker 1988), causing diseases on various crop plants. Numerous miscellaneous pathogenic species were newly described, such as Uncinula praeterita Marasas & I.H. Schum. (Marasas & Schumann 1966), a powdery mildew from the indigenous shrub Ehretia rigida, and Magnaporthe rhizophila D.B. Scott & Deacon (Anelich 1986) from roots of wheat. The teleomorph of Pithomyces chartarum (Berk. & M.A. Curtis) M.B. Ellis, studied for years as a contributing factor in the aetiology of “geeldikkop” and facial eczema – both of which are photosensitization syndromes – was found on Galenia procumbens in the Karoo, and described as Leptosphaerulina chartarum Cec. Roux (Roux 1986). The genus Togninia Berl. was recently linked to Phaeoacremonium W. Gams, Crous & M.J. Wingf., which was shown to be one of the causal organisms of Petri disease of grapevines (Mostert et al. 2003). Many reports were published as part of the New and Interesting Fungi series in Bothalia, which later moved to the South African Journal of Botany (Van der Linde & Van Warmelo 1989). Other work included a revision of the South African Hysteriaceae (Van der Linde 1992). Johannes P. van der Walt, who was associated with the Centre of Scientific and Industrial Research (CSIR) made an enormous international contribution to the knowledge of yeast taxonomy with numerous publications on the distribution and diversity of South African fungi. Now retired, J.P. van der Walt contributed to the description of 81 new ascomycetous teleomorphs and anamorphs (14 genera). Some of these species are still known only from South African isolates.
Subsequent to the late 1990's (Eicker & Baxter 1999), the relative dormancy in activities on ascomycetous fungi following Doidge (1950) became broken by a steady flow of contributions by plant- and forest pathologists (Agricultural News 1990), with the genera Ophiostoma Syd. & P. Syd., Ceratocystis Ellis & Halst. (Marais & Wingfield 2001, Roux et al. 2001a) and Mycosphaerella (Crous 1998, Crous & Braun 2003, Crous et al. 2004a, b) receiving particular attention. Plant-pathogenic ascomycetes occurring on Proteaceae and Restionaceae also received considerable attention, with the description of numerous new species and genera (Taylor & Crous 2000, Lee & Crous 2003a, c, d, Lee et al. 2003, 2004a). Saprobic ascomycetes occurring on Proteaceae litter, however, were less intensively studied (Lee & Crous 2003a, b, Lee et al. 2005). Contributions from other South African scientists included L. Korsten, F. Wehner, N. McClaren, B. Flett, N. Labuschagne and G. Thompson, who added valuable information pertaining to distribution records, host preferences and new disease reports.
Some ascomycete genera have received considerably more attention than others. One such genus is Mycosphaerella. Doidge (1950) listed 21 species of Mycosphaerella, and more than 100 cercosporoid anamorph species. A revision of species in this complex commenced in the early 90's, with the description of numerous new taxa from indigenous and exotic hosts (Crous & Braun 1994, Morris & Crous 1994, Crous & Braun 1995). The cercosporoids occurring in South Africa were treated by Crous & Braun (1996a), who listed 159 species from diverse hosts. Taxa occurring on Proteaceae were treated by Taylor and co-workers in a series of papers focusing on Mycosphaerella, and anamorph-genera such as Phaeophleospora Rangel, and Batcheloromyces Marasas, P.S. van Wyk & Knox-Dav. (Taylor & Crous 1999, Taylor et al. 1999). Other than Proteaceae, the Myrtaceae has also received some attention. Species of Mycosphaerella occurring on Eucalyptus were treated in several papers by Crous and co-workers (Crous & Wingfield 1996, Crous 1998, Crous et al. 2004a, Hunter et al. 2004a, b), listing 17 odd species on Eucalyptus in South Africa. The genus Syzygium, of which S. cordatum is indigenous to South Africa, was also investigated, revealing four species of Mycosphaerella on this host (Crous & Sutton 1997, Sutton & Crous 1997, Crous 1999). Given the exceptional Mycosphaerella species richness on hosts in the Proteaceae and Myrtaceae, it can be assumed that numerous species await description once leaves of other hosts are studied in more detail.
The genus Botryosphaeria and associated anamorph-genera have received considerable attention in recent years (Denman et al. 2000). Specific papers have addressed species occurring on hosts such as Eucalyptus (Smith et al. 2001, Slippers et al. 2004a,b,c,d), Proteaceae (Denman et al. 2003), Vitis vinifera (Van Niekerk et al. 2004a), Syzygium (Pavlic et al. 2004), Southern Hemisphere conifers (such as Widdringtonia) (Slippers et al. 2005b), various fruit trees (Slippers et al. 2006) and Mangifera indica (Slippers et al. 2005a), to name but a few. These studies once again indicate that there are numerous unknown species of the Botryosphaeriaceae in the Southern Hemisphere, and specifically in South Africa, awaiting description.
A suite of recent studies have focused on species that have traditionally been treated in the genus Cryphonectria (Sacc.) Sacc. & D. Sacc. (Myburg et al. 2004a, b, Gryzenhout et al. 2004, 2005a, b, 2006 – this volume). These investigations arose from the first discovery of the serious Eucalyptus stem pathogen Cryphonectria cubensis (Bruner) Hodges in South Africa (Wingfield et al. 1989). The various studies, strongly supported by DNA sequence comparisons, have shown that Cryphonectria is a genus restricted to the Northern Hemisphere (Myburg et al. 2004a, b). Related fungi occurring in Southern Hemisphere countries including South Africa, reside in various genera such as Chrysoporthe Gryzenh. & M.J. Wingf. (Gryzenhout et al. 2004), Microthia Gryzenh. & M.J. Wingf., Holocryphia Gryzenh. & M.J. Wingf. (Gryzenhout et al. 2006), Rostraureum Gryzenh. & M.J. Wingf. (Gryzenhout et al. 2005a) and Amphilogia Gryzenh., Glen & M.J. Wingf. (Gryzenhout et al. 2005b). New species and genera have also been discovered that are closely related to Cryphonectria, such as Celoporthe dispersa Nakab., Gryzenh., J. Roux and M. J. Wingf. (Nakabonge et al. 2006 – this volume).
Hyphomycetous fungi: A cursory look through Doidge (1950) reveals that none of the earlier collectors in South Africa took particular note of hyphomycetes. Of the total of 4748 species that she listed, a mere 18.5 % represented asexual forms. Subsequent studies comprised comprehensive inventories of the genera Fusarium Link (Marasas et al. 1988), Penicillium Link (Schutte 1992), Aspergillus Link (Schutte 1994), entomophagous fungi (Rong & Grobbelaar 1998), dematiaceous fungi (Sinclair & Eicker 1985, Sinclair et al. 1983, 1985, 1987, 1990, 1994), and nematode-trapping fungi (Gorter 1993b). A review of Cercospora Fresen. and similar fungi (Crous & Braun 1995), revision of the genus Cylindrocladium Morgan and related genera (Crous & Wingfield 1993, 1994, Crous 2002), and differentiation of species of Bipolaris Shoemaker, Exserohilum K.J. Leonard & Suggs and Curvularia Boedijn (Rong 2002) was compiled mostly from specimens in PREM.
The bulk of subsequent studies are miscellaneous reports of fungi from crops and animal feeds such as lucerne (Thompson 1985), Cenchrus ciliaris pastures (Bezuidenhout 1977), natural Karoo pastures (Roux 1985), as well as toxigenic representatives of Fusarium (Kellerman et al. 1972, Marasas et al. 1976) and Pithomyces Berk. & Broome (Marasas & Schumann 1972, Kellerman et al. 1980), and various synnematous and other hyphomycetes (Rong & Botha 1993, Roux et al. 1995, Jacobs et al. 2001). Other genera that received attention include Graphium Corda (Jacobs et al. 2003b), Leptographium Lagerb. & Melin (Wingfield & Marasas 1980, 1983, Wingfield 1985, Zhou et al. 2001), Cladosporium Link (Braun et al. 2003, Surridge et al. 2003), Phialocephala W.B. Kendr. (Jacobs et al. 2003a), and cercosporoid fungi (Crous & Braun 1996a, b, Pretorius et al. 2003). Several unique hyphomycetes were also reported from Proteaceae and Restionaceae in the fynbos (Wingfield et al. 1988, Mel'nik et al. 2004, Lee et al. 2004b).
Specific mention should be made of the work of Marasas on the taxonomy of the toxigenicity and phytopathologically important genus Fusarium, including soil surveys (Rheeder & Marasas 1998), and the description of numerous new species such as Fusarium andiyazi Marasas, Rheeder, Lampr., K.A. Zeller & J.F. Leslie (Marasas et al. 2001), and F. thapsinum Klittich, J.F. Leslie, P.E. Nelson & Marasas (Klittich et al. 1997). Similarly studies on the mycota of proteaceous fungi by Crous and co-workers (Crous et al. 2004a) have been very extensive.
Eyespot disease of wheat received considerable attention, with the causal organism originally being ascribed to Cercosporella Sacc., then Pseudocercosporella Deighton, and Ramulispora Miura (Robbertse et al. 1995). Crous et al. (2003) erected the genus Helgardia Crous & W. Gams to accommodate these cercosporoid anamorphs, while their discomycete teleomorphs were placed in the genus Oculimacula Crous & W. Gams. Schroers et al. (2005) recently characterised the hyphomycetes associated with guava wilt, and identified the causal organism as a species of Nalanthamala Subram.
Hyphomycetes from Vitis vinifera have been intensively studied. Halleen et al. (2004) treated the Cylindrocarpon Wollenw. complex associated with black foot disease, and introduced a new genus, Campylocarpon Halleen, Schroers & Crous. Crous et al. (1996a) erected the genus Phaeoacremonium for species associated with grapevine decline disease of grapevines and human infections, while Crous & Gams (2000) described the genus Phaeomoniella Crous & W. Gams as the main causal organism of Petri disease.
Species of Cylindrocladium Morgan (teleomorph: Calonectria De Not.) (Hypocreales) are common in tropical and subtropical regions of the world, and cause disease problems on a wide range of hosts. Schoch et al. (2000) placed teleomorphs of Cylindrocladiella Boesew. in Nectricladiella Crous & C.L. Schoch. In subsequent years Schoch & Crous (1999) reported Cylindrocladium spathiphylli Schoult., El-Gholl & Alfieri as causing Cylindrocladium root and petiole rot of Spathiphyllum, while Schoch et al. (1999) resolved the Cylindrocladium candelabrum Viégas species complex, describing the common soil-inhabiting species in South Africa as C. pauciramosum C.L. Schoch & Crous. In a recent monograph of Cylindrocladium and allied genera, Crous (2002) reported six Cylindrocladium species and five Cylindrocladiella species from South Africa.
Coelomycetous fungi: In the Doidge era, investigators often recorded coelomycetes only incidentally, usually alongside their sexual state. Because they were mainly found on plant-pathological specimens, those that were most often reported were commonly occurring members of the genera Colletotrichum Corda, Phyllosticta Pers. and Pestalotia De Not. (Doidge 1950). At this time there was a tendency to name taxa as new if they were found on new substrates. Many of these names would not hold up when judged with robust systematic techniques currently applied.
Many coelomycete reports were done from animal feed implicated in poisonings or other maladies in farm animals i.e. Phomopsis leptostromiformis (J.G. Kühn) Bubák (Van Warmelo et al. 1970) on lupins, Tiarosporella Höhn. (Sutton & Marasas 1976), Colletotrichum (Baxter et al. 1983, 1993), Urohendersonia platensis Speg. (Roux & Van Warmelo 1989), and Bartalinia robillardoides Tassi (Roux & Van Warmelo 1990).
Paraconiothyrium minitans (W.A. Campb.) Verkley was investigated as an antagonist of the devastating pathogen, Sclerotinia sclerotiorum. (Lib.) de Bary (Phillips 1985). Possibly the most notorious coelomycetes in South Africa are Phyllosticta citricarpa (McAlpine) Aa, the cause of black spot of oranges (Meyer et al. 2001), and Stenocarpella maydis (Berk.) B. Sutton (syn. Diplodia zeae van der Bijl) causing black rot of maize and intoxication of sheep (Rheeder et al. 1990). Other genera that received attention include Coniella Höhn. and Pilidiella Petr. & Syd. (Van Niekerk et al. 2004b), Colletotrichum (Lubbe et al. 2004), Harknessia Cooke (Crous et al. 1993, Lee et al. 2004a), Dothistroma Hulbary (Barnes et al. 2004), the Fusicoccum/Diplodia anamorphs of Botryosphaeria (Denman et al. 2000, Slippers et al. 2004a,b,c,d), and Phomopsis (Sacc.) Bubák (Smit et al. 1989a, b, 1996a, b, Mostert et al. 2001, Van Niekerk et al. 2005). Coelomycetous fungi occurring in the fynbos also received some attention, namely those occurring on Proteaceae (Crous et al. 2004a) and Restionaceae (Lee & Crous 2003b).
Basidiomycetes
In early years, basidiomycetes such as the Hymenomycetes (McNabb & Talbot 1973, Talbot 1951a, b, 1954, 1958, 1965) and Ustilaginales (Doidge 1950) received some attention. Approximately 36 % of the fungi listed by Doidge (1950) are basidiomycetous. Eicker & Baxter (1999) present a good overview of work done on basidiomycetes from 1977 to 1999. Their publication provides references to studies on the genera Phaeolus (Pat.) Pat., Pisolithus Alb. & Schwein., Termitomyces R. Heim, Amanita Pers., Chlorophyllum Massee, Clathrus P. Micheli ex L., Hymenagaricus Heinem., Lepiota (Pers.) Gray, Macrolepiota Singer, Leucoagaricus Locq. ex Singer, Leucocoprinus Pat., Montagnea Fr. and Hymenochaete Lév. Several new genera of heterobasidiomycetous yeasts were also newly described through the years by J.P. van der Walt and colleagues.
Some of the more extensive investigations were the monographic work on resupinate and stereoid Hymenomycetes and a series of papers between 1951 to 1958, dealing with Stereum Pers., Lopharia Kalchbr. & MacOwan, Cymatoderma Jungh. and the Thelephoraceae (Gorter 1979). Early investigations also included a series of papers on tree pathogens and wood-destroying Hymenomycetes (Van der Westhuizen 1972).
Some revisionary work has been done on collections of certain taxa at PREM in the course of monographic work at the University of Buenos Aires, Argentina, namely species of Hymenochaete Lév. (Hymenochaetaceae) (Job 1987). Limited information is available about the Geasteraceae (Coetzee & Eicker 1994, Coetzee & van Wyk 2003) but a revision of the South African Stereum species, and the Geasteraceae (earth stars), which will to a large part rely on material lodged in PREM, is in progress (J. Coetzee, pers. comm.).
Significant contributions following Doidge were made by D.A. Reid from the Royal Botanic Gardens Herbarium, Kew. Reid reappraised the type and authentic specimens of species of Basidiomycota described from South Africa in PREM (Van der Westhuizen & Eicker 1994), the van der Bijl Herbarium (now housed at PREM), and elsewhere (Reid 1975). This has been the foundation for his documentation of South African mushrooms in collaboration with Albert Eicker from the University of Pretoria. An equally productive partnership has provided us with a scientific guide to our edible and poisonous mushrooms and other large fungi. In 1994, G.C.A. van der Westhuizen's lifetime of mycological research and photography culminated in a field guide (Van der Westhuizen & Eicker 1994), with excellent colour photographs of some 160 species of local macrofungi. The most recent studies include DNA phylogenetic data, like that published on species of Termitomyces R. Heim (Botha & Eicker 1991a, b, De Fine Licht et al. 2005).
Studies during the early part of the last Century reported Armillaria mellea (Vahl: Fr.) P. Kumm. in South Africa (Pole Evans 1933, Kotzé 1935, Bottomley 1937). These were largely associated with an expanding plantation forestry industry and the fact that this fungus resulted in tree death. A series of recent studies have shown that the fungus killing trees in this country is A. fuscipes Petch (Coetzee et al. 2000) and that there are probably at least two other species occurring in neighbouring countries such as Zimbabwe (Mwenje et al. 2003). Intriguingly, it has also been shown that the Northern Hemisphere species, A. mellea was introduced into South Africa, probably by the early Dutch settlers (Coetzee et al. 2001). Likewise, the Northern Hemisphere species A. gallica Marxm. & Romagn. has recently been recorded from dying Protea plants in the Kirstenbosch botanical gardens (Coetzee et al. 2003).
G.L.I. Zundel of the U.S.A., who collaborated with Pole Evans and Doidge in describing and re-investigating material found by South African mycologists, extensively studied local smut fungi. K. Vánky in Germany radically changed the taxonomic study of this group of fungi by advocating the use of morphological characters obtained by germinating spores (Vánky 1997, 1999a, b, 2000a–b, 2001). It is problematic when we apply these methods to old herbarium specimens, however, because their spores have lost the ability to germinate. One of our earliest records and most striking smuts is maize boil smut, Ustilago maydis (DC.) Corda [= U. zeae (Link) Unger].
Rust fungi (Uredinales):Most of the rust fungi known from southern Africa were treated and described by Doidge (1927, 1928, 1939, 1940, 1948a, b). This suite of papers remain the basis for identification of these fungi in southern Africa, and are relevant to the whole of the African continent. All known species were listed in Doidge (1950). A total of 474 species are listed from southern Africa, including 145 anamorphs.
Since Doidge (1950), nine species have been transferred to other genera, and a further 30 species names have been reduced to synonymy. In addition, eight anamorph species of Aecidium Pers. have been connected to their teleomorphs (Kleinjan et al. 2004), and three have been demonstrated to be endocyclic (Wood 1998, 2004, Wood & Crous 2005), whilst 11 anamorph species of Uredo Pers. have been connected to their teleomorphs. Many of these changes were published in Jørstad (1956) and various publications summarised in Cummins (1971). It can be expected that the status of many more names will change as further taxonomic studies progress (Van Reenen 1995).
Since Doidge (1950), a number of new species have been described by G. B. Cummins and H. Gjærum in various papers, none dealing exclusively with species from the region (Cummins 1960, Gjærum & Reid 1983, 1998, Gjærum 1988a, b, 1999). More new species have been described recently (Shivas 1991, Wood 2002, Mennicken et al. 2003, Mennicken & Oberwinkler 2004, Mennicken et al. 2005a, b, Wood & Crous 2005, Wood & Scholler 2005). Taking these above-mentioned changes into account, there are currently 537 species of rust fungi, representing 40 genera and 10 families presently recorded from southern Africa (A. Wood, unpubl. data). This tally will increase, as several more new species and records await publication. Of the countries included, southern Angola, Botswana, Mozambique, Namibia and Swaziland have few species recorded, and Lesotho has none. Only South Africa and Zimbabwe are relatively well explored mycologically, though little or no collecting has been done in large areas of even these two countries. A total of 20 new species have been described from the Western and Northern Cape Provinces of South Africa, and Namibia, in just the last 5 years (Wood 2002, Mennicken et al. 2003, Mennicken & Oberwinkler 2004, Mennicken et al. 2005a, b, Wood & Crous 2005, Wood & Scholler 2005), demonstrating that many new species await discovery in the subregion where there has been little or no collecting in the past. However, the number of species known to occur is probably not a gross underestimation of the actual total present, as with the study of fresh specimens it is probable that a number of species will be reduced to synonyms, and many anamorphic species will be linked to their teleomorphs.
Hennen & McCain (1993) suggested that the number of rust species in any area would be at least 5 % of the number of vascular plants. In South Africa and Zimbabwe the figures are only 1.7 and 3.2 %, respectively (Table 1). As stated above, the number of species is not considered likely to rise considerably. Why then are there relatively few rust species recorded from the subcontinent in comparison to other countries (Table 1)? There are a number of possible explanations for this, the most likely including: (1) Much of South Africa, as well as Botswana and Namibia is semi-arid to desert. The low rainfall associated with these areas would restrict opportunities available to rust fungi to infect their hosts, which could result in the lower diversity of rust fungi. (2) There are very few rust species recorded from the fynbos and succulent Karoo biomes, despite the high plant diversity for which these biomes are well known. The high plant species diversity is largely produced by few but speciose genera and families with high turnover of species over short distances (Linder et al. 1992, Goldblatt 1997).
Table 1.
Number of recorded rust fungi (Uredinales) as a percentage of the number of vascular plants in various countries.
| Country | No. rust species | No. plant species | Rusts as % of plants | Reference |
|---|---|---|---|---|
| South Africa | 397 | 23420 | 1.7 % | A. Wood, unpubl. data |
| Zimbabwe | 143 | 4440 | 3.2 % | A. Wood, unpubl. data |
| Hawaii | 22 | 1897 | 1.1 % | Gardner (1994) |
| Great Britain | 238 | 1443 | 16.5 % | Wilson & Henderson (1966) |
| Norway | 265 | ca. 2000 | 13.3 % | Gjærum (1974) |
| Japan | 790 | 4022 | 19.6 % | Hiratsuka et al. (1992) |
| Canadian arctic | 53 | 325 | 16.3 % | Parmelee (1989) |
| Guatemala | 416 | ca. 8000 | 5.2 % | Hennen & McCain (1993) |
| El Salvador | 140 | ca. 2500 | 5.6 % | Hennen & McCain (1993) |
The rust fungi in South Africa tend to either occur on one or a few closely related plant species with a large distribution, or on numerous related plant species, many of which have limited distributions. Endophyllum osteospermi (Doidge) A.R. Wood, E. elytropappi (Henn.) A.R. Wood & Crous (Wood & Crous 2005) and Uromyces kentaniensis Doidge are examples of the former, whilst U. bolusii Massee, U. ixiae (Lév.) G. Winter and Puccinia byliana Dippen. are examples of the latter (A. Wood, unpubl. data). The greatest diversity of rust fungi in South Africa is in the eastern parts, associated with a greater amount of summer rain. There the ratio of rust fungi to plant species is probably approximately the same as for Zimbabwe, which is ecologically similar.
Zygomycota
Only seven genera of the Mucorales were noted by Doidge (1950), one of which, Haplosporangium Thaxt., has not since been found. They include the important saprotrophic species, identified early on, such as the ubiquitous Rhizopus stolonifer (Ehrenb.) Vuill. – listed as R. nigricans Ehrenb. – which causes post-harvest decay, particularly in sub-tropical fruit. A subsequently recorded species, R. oryzae Went & Prins. Geerl., has been associated with mycoses in humans. It is the most commonly found member of this group, particularly in fodder samples. During the course of several years, PREM has supplied numerous cultures of Mucorales, and participated in research focused on metabolite studies. Certain strains produce gamma linoleic acids (Botha et al. 1995) culminating in patenting of a fermentation process.
By 1999 the number of genera from this group known to South Africa had risen to 20 (Roux 1996). A study on Trichomycetes, parasites or commensals in the digestive tract of arthropods, yielded several new records and new species (Gorter 1993a).
Lichen-forming and lichenicolous fungi
In comparison with other fungal groups, the macrolichens of South Africa in particular are relatively well-studied, though much needs to be done on the microlichens and lichenicolous species. Early records were compiled in Stizenberger (1890–1895), but the major synthesis to date is that of Doidge (1950). Extensive collections were made by Elise Esterhuysen (1912–) in the late 1940s–1950s (material in the Bolus Herbarium, University of Cape Town), but the principal contributions have been by Ove Almborn (1914–1992) from Lund (Sweden) and Franklin A. Brusse (1951–) who was for some years on the staff of the National Herbarium in Pretoria. Almborn specialized on Teloschistes Norman (Almborn 1989) but travelled extensively in the country, for example spending 5 mo there in 1953 (Almborn 1966), analyzing distribution patterns (Almborn 1987, 1988), and also issued an important exsciccate, “Lichenes Africani”; this comprised six fascicles from 1956–1991 including 150 numbers. Brusse had his major interest in the Parmeliaceae and described many species new to science, especially effigurate-crustose species on rock later placed in the endemic genus Karoowia Hale (the species known from Australia appear to belong elsewhere). Numerous other lichenologists have visited and collected, amongst the most notable being Gunnar Degelius (1903–1993) who specialized in Collemataceae (Degelius 1974), Ingvar Kärnefelt (University of Lund) with a special interest in Teloschistaceae, Leif Tibell who specializes in caliciaceous groups (who visited with colleagues from the University of Uppsala in 1997), and Mason E. Hale jr (1928–1990) who described numerous species and several new genera in the Parmeliaceae (e.g. Hale 1986, 1988), although not all his new genera have yet been evaluated by molecular methods. Much collecting was also carried out during the International Association for Lichenology's field meeting in Namibia and South Africa in 1986, but the results from that excursion have not been synthesized. Some revisionary studies have been carried out based on the collections of these and other lichenologists, for example the monograph of Diploschistes Norman in South Africa by Guderley & Lumbsch (1996).
More recently, as a part of the Biota-So5 project funded by the German government, an extensive survey of lichen biodiversity from the Cape Province to northern Namibia is underway, the leading researcher involved being Tassilo Feuerer (University of Hamburg). In addition, an international expedition led by Ana Crespo (Universidad Complutense de Madrid) and focussing on the Parmeliaceae made about 750 collections in May–June 2005 from the Western Cape north to the Namibian border.
Sadly there is no systematic lichenologist resident and active in South Africa today. Further, there is no modern critical checklist available, but a preliminary list compiled from the literature (www.biologie.unihamburg.de/checklists/africa/southafrica) contained 1716 species as of 1 March 2005. This list is a valuable basis for future work but is very preliminary and includes both many early names copied from the literature whose position and status needs to be reassessed, and others that have been revised but have yet to be updated from other literature. A reasonable estimate of the total number of lichen-forming species to be expected, by comparison with checklists from other regions of the world with similarly diverse climatic regions and rock types (Will-Wolf et al. 2004) would be about 2000 (excluding lichenicolous fungi).
In comparison to the flowering plants, only a handful of genera appear to be endemic, notably Combea De Not. and Karoowia s. str., with others requiring reassessment, for example Almbornia Esslinger, Canomaculina Elix & Hale, Namakwa Hale, and Xanthomaculina Hale. In contrast, there are many endemic species, especially in the Parmeliaceae and the Teloschistaceae. The lichen assemblages are very distinctive in different parts of the country, as discussed by Almborn (1988), comprising at least eight phytogeographic categories: ubiquitous, steppe and desert (e.g. Namaqualand, Karoo), montane (over 1000 m alt.), oceanic (e.g. Table Mountain, Drakensberg Mountains), tropical-oceanic (e.g. northern KwaZulu-Natal, Mpumalanga), maritime (on coastal rocks), and endemic.
Lichenicolous fungi, fungi obligatory on lichens as pathogens, commensals or saprobes, have hardly been reported from South Africa. None are included in the 2005 preliminary checklist, not even representatives of such ubiquitous genera as Abrothallus De Not., Lichenoconium Petr. & Syd., Polycoccum Saut. ex Körb., and Stigmidium Trevis. There are, however, some scattered records, such as that of Lichenostigma cosmopolites Hafellner & Calat. (Hafellner & Calatayud 1999) which is very common on Xanthoparmelia (Vain.) Hale species in the country (D.L. Hawksworth, pers. obs.), and an undescribed Polycoccum on Karoowia adhaerens (Nyl.) Hale (Váczi & Hawksworth 2001). Many lichenicolous fungi were collected during the visit of Ana Crespo's group in 2005, but have yet to be fully identified. In comparison with what is known of the species richness of lichenicolous fungi compared with the number of potential host lichen species, for example 403 vs. 1677 lichen species in Great Britain and Ireland (Hawksworth 2003), around 475 species would seem to be a reasonable estimate of the number to be expected in South Africa.
Oomycetes
Fifteen species of Phytophthora de Bary from 74 different hosts have been recorded from eight southern African countries during the period 1941 to 2001. Typical disease symptoms were damping off, root rot, stem rot, stem canker, crown rot, tip blight, leaf rot and fruit rot. Records for Pythuim Pringsheim species date from 1931 to 2004. Twenty species of Pythium have been reported from 65 different hosts, and representative disease symptoms were damping off, seedling blight, root rot, stem rot (foot rot), crown rot (heart rot), fruit rot, tuber rot and soft rot.
Phytophthora: The first detailed study of Phytophthora species with associated hosts and distribution patterns in South Africa, was done by Wager (1935, 1941) describing P. cactorum (Lebert & Cohn) J. Schröter, P. cinnamomi Rands, P. citrophthora (R.E. Smith & E.H. Smith) Leonian, P. cryptogea Pethybridge & Lafferty, P. infestans (Montagne) de Bary, P. nicotianae Breda de Haan and P. syringae (Klebahn) Klebahn. Mes (1934) and Wijers (1937) reported P. cactorum on various hosts and included descriptions and disease symptoms. Other studies confirmed species identification (Wager 1931, van der Merwe et al. 1972). Descriptions of various Phytophthora species from different vegetable and ornamental crops were recorded by Thompson (1981), Thompson & Phillips (1988), Ferreira et al. (1991), Thompson & Naudé (1992) and Thompson et al. (1994). Morphological and molecular methods (RFLPs of total DNA) were used by Botha (1993) to confirm species identity of P. nicotianae on tree lucerne. Linde et al. (1997) applied isozymes to distinguish two separate populations of P. cinnamomi, determining genotypic variation within A1 and A2 isolates, and established that sexual reproduction was rare or absent in South African isolates. Linde et al. (1999) used both RAPDs and RFLPs to assess genotypic diversity and reveal DNA polymorphisms in South African and Australian isolates of P. cinnamomi. Von Maltitz & von Broembsen (1984) reported P. porri Foister from onions, followed by P. citricola Sawada causing shoot tip blight of lemons in propagation tunnels (Von Maltitz & von Broembsen 1985).
Comprehensive disease surveys, but with limited taxonomic treatment, were performed by Marais (1979) in the Western Cape Province, surveying grapevine rootstock diseases. Von Broembsen (1984) did an extensive survey of P. cinnamomi root rot in indigenous fynbos, and from the major catchment rivers in the Western Cape. Thompson (1987, 1988) surveyed alfalfa in various provinces for P. medicaginis E.M. Hansen & D.P. Maxwell and P. drechsleri Tucker. Linde et al. (1994a, b) surveyed the major commercial forestry areas in South Africa for root rot caused by oomycetes, while Thompson et al. (1995) surveyed citrus roots for Phytophthora and Pythium in the Mpumalanga and Limpopo Provinces. Labuschagne et al. (2000) reported P. capsici Leonian causing wilt of pumpkin, and stem and root rot of tomato (Labuschagne et al. 2003). McLeod et al. (2001) conducted an extensive survey of Late Blight (P. infestans) in the major potato production areas in South Africa, determining mating type and using various molecular markers to characterise isolates.
Pythium: The first major contribution by Wager (1931) consisted of several species descriptions and disease symptoms on hosts. Additional species descriptions, temperature-growth measurements and hosts, was also reported by Wager (1941). Doidge (1950) and Doidge et al. (1953) compiled the host range of numerous Pythium species, and this compilation was updated by Gorter (1977, 1982) and Crous et al. (2000b). Darvas et al. (1978) and Darvas (1979) added further records of Pythium species with host records. Scott et al. (1979) and Scott (1987) made a major contribution with regard to the occurrence and disease symptoms of Pythium species from various small grain crops. An extensive overview of all recorded Pythium species in South Africa from 1926 to 1989 was compiled by Denman & Knox-Davies (1992) and included distribution, host range and species diversity. A detailed taxonomic description of various Pythium species associated with vegetables was reported by Botha & Coetzer (1996) and Pythium prolatum W.A. Campbell & Hendrix causing wilt of Rhododendron sp. (Botha & Crous 1992). Pythium species have also been associated with grapevine (Marais 1979, 1980), medics (Lamprecht et al. 1988), lucerne seedlings (Denman & Knox-Davies 1992), pines and eucalypts (Linde et al. 1994a, b) and lettuce (Labuschagne et al. 2002). Reports of asexual, sporangial isolates of Pythium, viz. G-group, F-group, T-group and P-group as well as homothallic species, e.g. P. coloratum Vaartaja, P. irregulare Buisman, P. myriotylum Drechsler, P. perplexum Kouyeas & Theohari, P. dissotocum Drechsler and P. spinosum Sawada, have been reported from closed hydroponic systems in South Africa (Labuschagne et al. 2001, 2002, Gull et al. 2004, Tesfaendrias et al. 2004).
MISCELLANEOUS REPORTS FROM LITTLE-STUDIED NICHES
Reference is made by Eicker & Baxter (1999) to fungi recorded in particular ecological niches. These include a number of publications of fungi from aquatic habitats such as fast-flowing rivers, submerged woods and twigs in stagnant freshwater habitats, wood invading fungi in marine waters, estuaries and from leaves in a mangrove. Non-aquatic habitats included phylloplane fungi, foliicolous fungi, airspora over pastures, coprophilous fungi from domestic, captivated and wild animals, fungi associated with cultivated mushrooms, undisturbed soil populations, fungi for the control of invasive plants and from plant litter. From 1955 to 1984 approximately six publications addressed reports of fungi causing human and animal diseases. These include a study on mycoses, dermatophytes and fungi causing subcutaneous infections.
Fungi associated with mycorrhizal associations have also received some attention in South Africa. A significant contribution was the ecological survey of arbuscular mycorrhiza made by Wehner in 1976 (Eicker & Baxter 1999). Subsequent studies include recordings of Acaulospora laevis Gerd. & Trappe, and investigations on fynbos soils by Mitchell, Reid, Straker, van Greuning, Sinclair and later during a survey of the fungi associated with Pinus by Van der Westhuizen and Eicker (Eicker & Baxter 1999).
Several papers were published on fungal endophytes. Crous et al. (1995a) listed 55 endophytes from Triticum aestivum, while Serdani et al. (1998) treated the endophytic Alternaria Nees species associated with core rot of apples. Kriel et al. (2000) provided an overview of foliar endophytes in Gymnospermae, and Mostert et al. (2000) treated the endophytic Phomopsis spp. occurring in grapevines. Smith et al. (1996) treated endophytic Botryosphaeria species in Pinus and Eucalyptus. Swart et al. (2000) reported on the fungal endophytes in cultivated Protea, Leucospermum and Leucadendron, and Taylor et al. (2001) treated the endophytes occurring in Protea spp. grown in the wild.
Several papers focused on leaf-litter fungi in general (Sinclair & Eicker 1985, Sinclair et al. 1985, 1987, Crous 1990, Sinclair et al. 1990, 1994), or on specific hosts such as Syzygium cordatum (Crous et al. 1995b), Podocarpus (Crous et al. 1996b), and Eucalyptus (Crous et al. 1990a, Crous & van der Linde 1993, Crous et al. 1997).
Fungi on commercially important exotic hosts such as Eucalyptus, Pinus and Acacia have been studied in detail. Crous et al. (1990b) listed the shoot and needle diseases occurring on Pinus spp., while the leaf pathogens occurring on Eucalyptus were treated Crous et al. (1989). Viljoen et al. (1992) provided a summary of the fungal pathogens on Pinus and Eucalyptus seedlings in nurseries in South Africa. Crous (1998) treated the species of Mycosphaerella occurring on Eucalyptus, and listed 14 species from South Africa, while Slippers et al. (2004d) treated the Botryosphaeria spp. occurring on this host. Roux & Wingfield (1997) treated the fungi causing diseases of A. mearnsii, and found various species of fungi, including Fusarium graminearum Schwabe and Ceratocystis albifundus M.J. Wingf., De Beer & M.J. Morris to be associated with a wilt disease of this host (Roux & Wingfield 1997, Roux et al. 2001a, b).
FUNGI ON INDIGENOUS HOSTS: FABULOUS FYNBOS FUNGI AS A CASE STUDY
The dicotyledonous Proteaceae (proteas) and monocotyledonous Restionaceae (restios) are two of the most prominent plant families in the Southern Hemisphere. In South Africa the majority of the species are confined to the south-western corner of the country in 90 000 km2, the so-called “Cape Floristic Region” or “Cape Floral Kingdom”. Proteas are represented by 320 species, of which 96 % are endemic to the region (mostly members of the genus Protea) and restios by 330 species, of which 94 % are endemic to the region (Goldblatt & Manning 2000).
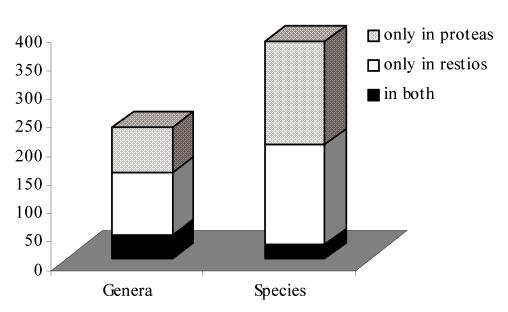
In a study aimed at exploring the saprobic (litter) microfungi inhabiting these two families, nature reserves and botanical gardens were visited over a 2-year period (2000–2001). About 580 fungal strains were isolated from 34 restio species (representing 15 genera), which consists of approx. 150 fungal genera and 180 species. Another 580 fungal strains were isolated from 43 protea species (representing five genera), which consists of approx. 120 fungal genera and 185 species. A total of about 230 fungal genera were isolated, of which 190 were confined to either the one or the other host family, while 40 fungal genera occurred on both families. A total of 380 were identified to species level, of which 355 were restricted to one or the other family, while 25 species occurred on both families (Fig. 4).
Fig. 4.
Numbers of fungal taxa associated with two plant groups: Proteaceae (proteas) and Restionaceae (restios).
Saprobic fungi are different from plant-pathogenic fungi, and do not show a strong host specificity, but rather have a broad range of host recurrence (or host preference) such as mono/dicotyledonous plants in the tropics or gymno/angiosperms in temperate forests (Lodge 1997, Yanna et al. 2002). A well-studied group in our collections, hyphomycetous fungi, showed a high degree of host-recurrence (Lee et al. 2004b). The differences in physical textures and chemical compositions of tissues between the two host groups, which reflect their taxonomic distance and different lineage, might be a cause for a higher degree of host-recurrence. In total only 7 % of the species occurred in both host groups. This again complies with the view of Polishook et al. (1996) that much of the host-recurrence in litter fungi is probably related to physical and chemical characteristics of leaves rather than host-specificity.
Pirozynski & Hawksworth (1988) argued that over 50 % of the fungi inhabiting microhabitats have possibly evolved in a very close relationship with their host. Inflorescences and infructescences are considered as miniature ecosystems, which accommodate different food chains and trophic levels (Zwölfer 1979). Eighteen Protea infructescences were collected during this study, which revealed a unique composition of fungi (Lee et al. 2005).
The number of fungal species isolated from each collection varied depending on host plant habitat. For example, 38 fungal species were isolated from Brabejum stellatifolium (Proteaceae), which has a riverine habitat and thick twigs and branches. In contrast, only four species were isolated from Elegia filacea (Restionaceae), which grows in dry areas, and has culms of approx. 1 mm diam.
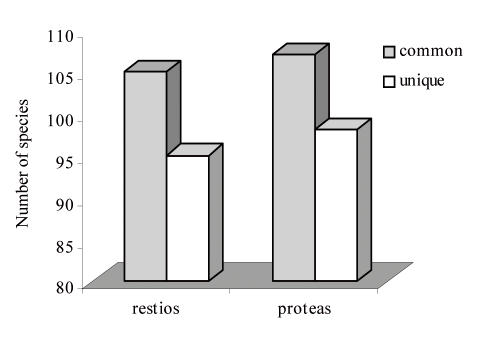
From these data we have found that there are at least three unique species of saprobic microfungi for each species of Proteaceae or Restionaceae thus far investigated (Fig. 5). However, we regard this as a minimal estimate because of the limitations of the damp-chamber isolation technique, and the undersampling of microhabitats such as infructescences.
Fig. 5.
Numbers of fungal species that are unique to each plant group (unique) and are found on other host plants (common).
FUNGI ASSOCIATED WITH INSECTS
Fungi associated with insects, ranging from parasitic to mutualistic associations, have been characterised in virtually all insect groups, and especially in families such as Isoptera, Hymenoptera, Homoptera, Coleoptera, Diptera and others (Vega & Blackwell 2005). Despite significant data pointing to the ubiquity and novelty of the fungi involved in these associations, they are left out of most estimates of fungal diversity due to insufficient data. This lack of information is due to the difficulty of characterising these fungi based on traditional criteria and, in some cases, ignorance of their existence and importance. For example, a recent paper by Suh et al. (2004) reported over 200 undescribed species of yeasts from the guts of 27 families of beetles; a novel niche. Species accumulation curves showed that they had probably not exhausted even half of the unique species in their samples. These findings provide a tempting example of the potential fungal biodiversity that could exist in South Africa. Southern Africa has an estimated 17 433 recognised beetle species in 104 families, but this species number could well be 2–3 times higher (Scholtz & Chown 1995). Extrapolating from the data of Suh et al. (2004), this niche alone most likely harbour hundreds, if not thousands, of undescribed yeast species in South Africa. The diverse array of specialised parasitic fungi of arthopods is equally poorly known from South Africa (see Vega & Blackwell 2005, as example of the potential diversity). Ignoring this niche thus overlooks a significant portion of the fungal biodiversity of the region. The fact that it will have to be ignored in the current paper, highlights the need for more specific research focus on this area in South Africa and elsewhere.
One group of insect-associated fungi that has been better characterised than most, especially in the Northern Hemisphere, is that of the ophiostomatoid fungi with bark and ambrosia beetles (Six 2003, Kirisits 2004). In South Africa, three Raffaelea Arx & Hennebert species have been described from two native ambrosia beetles (Scott & Du Toit 1970), while at least 11 ophiostomatoid species have been reported from introduced pine bark beetles (Zhou et al. 2001, 2006). Unfortunately the number of ambrosia and bark beetle species in South Africa has not been determined to date. Seven novel ophiostomatoid species have also been described from merely nine of the more than 300 Protea spp. in South Africa (Marais et al. 1998, Marais & Wingfield 2001, Roets et al. 2006). The morphology of the fungi and the wealth of insects present in protea infructescences suggest that the species from proteas are vectored by arthropods (Marais & Wingfield 2001). Five more, undescribed Sporothrix Hektoen & C.F. Perkins and Ophiostoma spp. were recently discovered (De Beer et al. 2005) growing on fungal combs in 13 termite mounds, representing only three of the at least 42 fungus-growing termite species (Macrotermitinae) in Southern Africa (Uys 2002). These studies on ophiostomatoid fungi associated with beetles, Protea spp. (and their insects), as well as termites, suggest that the species richness of the ophiostomatoid fungi, and the interaction between arthropods and fungi in these niches, are under-explored.
Termitomyces spp. associated with some termite species are arguably one of the best known fungi among non-specialists in South Africa, as they are rather obvious, numerous and a well-loved delicacy. A number of species have been described from South Africa (Eicker & Baxter 1999). However, not all species of Termitomyces R. Heim associated with the 42 South African fungus growing termite species have been characterised. Thus, these fabulous fungi might be more diverse than current numbers suggest. Neither have the Xylaria Hill ex Schrank species associated with termite nests been characterised.
The introduction of the woodwasp Sirex noctilio has recently added one species to the list of South African fungi, namely its mutualistic symbiont, Amylostereum areolatum (Fr.) Boidin (Slippers et al. 2003). These two organisms are currently causing significant damage to pine plantations throughout the country. This example illustrates the role of exotic insects introducing more fungi to South Africa, potentially with disastrous consequences.
FUNGAL ESTIMATES FOR SOUTH AFRICA
Hawksworth (1991) noted that the ratio between the number of vascular plants and fungi from all substrata in the British Isles, which is an intensively studied region on which he based his estimates, was around 1: 6 using several different data sets. Using this ratio, the conservative estimate of 270 000 vascular plant species resulted in an estimate of 1620 000 species of fungi. If we accept that the 1.5 M estimated number of fungal species exist, we currently only know around 7 % of these (Hawksworth 2004). Other estimates vary, namely Finland was estimated to have a plant to fungus ratio of 1: 4, while the U.S.A. was estimated at around 1: 1 (Hawksworth 1991). Although likely numbers of fungi occurring on insects were not taken into account due to insufficient data, estimates were as high as 13.5 M fungal species (Hawksworth 1991).
Pascoe (1990) estimated that there could be at least ten times as many fungi as vascular plants in Australia, with 2.7 M species of fungi occurring world-wide. Smith & Waller (1992) estimated that there could be 1 M on tropical plants alone. Dreyfuss & Chapela (1994) considered endophytic fungi, a group easily forgotten, and estimated that 1.3 M taxa might exist in this niche alone. By studying palm fungi, Fröhlich & Hyde (1999) concluded that on this group of plants there was most likely a ration of 1: 33 fungi per plant species. In Mexico, a study of macromycetes in pine-oak forests resulted in a ratio of 1: 3.5 species of macromycetes. Hyde (1996) considered that there were approx. three pathogens, 10 saprobes and 100 endophytes for each species of palm, suggesting that the fungus to plant ration could be as high as 1: 26. Hyde et al. (1997) also reported that 75 % of all fungi collected on palms were new to science.
Some individual examples have revealed a surprisingly large number of fungi. For example, 117 species have been described from Juncus roemerianus, of which 68 were apparently new taxa (Kohlmeyer & Volkmann-Kohlmeyer 2001). Hawksworth (1998) reported 92 species from Urtica dioica of which 17 appeared unique, and 55 on Lantana camara, of which 28 were host-specific. A further 893 fungi were reported from Pinus sylvestris (558 unique fungi if potential synonymies were taken into account), and 282 species on Eucalyptus globulus, of which 150 were not known from other eucalypts. Subsequently, Crous and co-workers have been describing more unique fungi from E. globulus, which suggests that the 282 figure reported by Hawksworth (1998) was an underestimate. So far only very few ecological niches and hosts have been thoroughly studied in South Africa.
The fungal biodiversity in southern Africa has been poorly studied to date, and no host has been thoroughly treated (e.g. all living plant parts: roots, stems, leaves, and litter, endophytes, epiphytes, and specific isolation techniques for specific fungal groups). Based on the lack of data, it is thus very difficult to estimate the number of unique fungi per host. However, by using moist-chamber incubation to culture saprobic fungi, Crous et al. (1996b) described four unique hyphomycetes from Podocarpus elongatus. Other fungal groups were seen, but not treated, thus from this one host, and one fraction of a niche, the ratio is 1: 4. By using the same technique to study hyphomycetes on leaf litter of Syzygium cordatum, Crous et al. (1995b) described five unique saprobic fungi, while in later studies a further five unique plant pathogenic fungi were described from this host (Sutton & Crous 1997, Pavlic et al. 2004), which increases the ratio to 1: 10, with several more host-specific fungi on this host awaiting description. In a study of the plant-pathogenic fungi occurring on Proteaceae, Crous et al. (2004a) reported six unique foliicolous species from Protea cynaroides, suggesting a ratio of 1: 6 as being an underestimate. Although these three hosts have not been fully studied, they provide a ratio of 4–10 unique fungi per host, suggesting that there could be an estimated seven unique species of fungi per indigenous host. Although it is highly unlikely that each host in each habitat would have seven unique species of fungi, many hosts might have some more, as this figure is an estimate based on a very incomplete examination of these hosts. Given that there are 24 500 species of plants in South Africa (Germishuizen & Meyer 2003), this estimate would mean that there could be 171 500 species of fungi, before taking numbers of insects into account. Based on this approach, however, the number of endemic fungi would be determined by the percentage endemic plants (and insects once taken into consideration). This ratio compares quite favourably to the ratio proposed by Hawksworth (1991), which was 1: 6, Pirozynski (1972) which was 1: 3–5, Pascoe (1990), which was 1: 10, and Rossman (1994) which was around 1: 4.
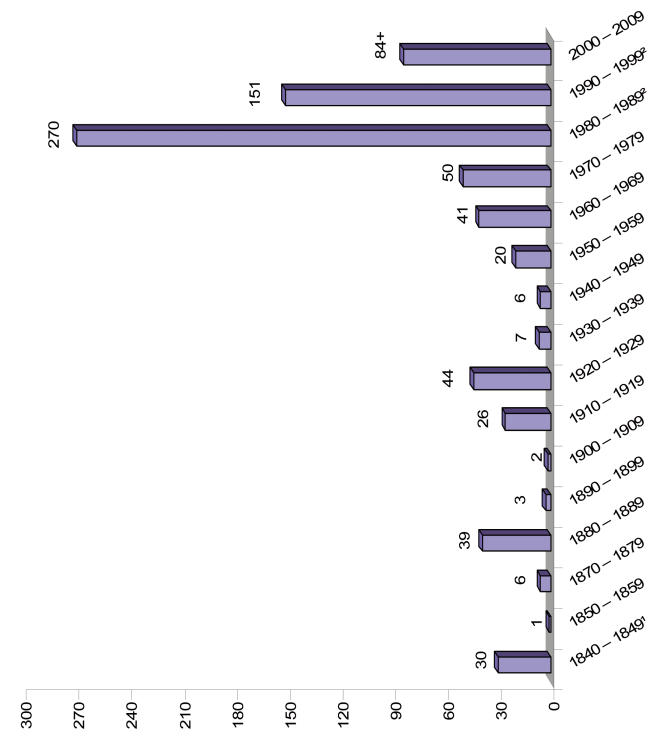
TO COLLECT, STUDY AND PRESERVE THE BASAL LINK
South Africa is well-known for its botanical beauty and diversity, but here we have attempted for the first time to use its botanical diversity to estimate its fungal biodiversity. Based on the 1: 7 ratio used above, we estimate that there could be as many as 171 500 species of fungi. This is definitely an underestimate, as no insect-associated fungi were taken into account, which alone makes a case that this estimate is inordinately conservative. Furthermore, given the high level of endemism found in the southern African flora, one would expect an equally high number of unique fungi. It is ironic, therefore, that only around 780 new species of fungi have thus far been described from South Africa (Figs 1, 2). It is obvious therefore, that the study of South Africa's unique, indigenous fungal biodiversity has never been regarded as a research priority. In the past almost no financial support has been allocated to it from the various southern African funding bodies. In our text, some attention was given to the study of saprobic and plant-pathogenic fungi. This is, however, a simplification of the reality as many diverse fungi exist that have unique ecological niches and roles yet to be studied. What level of attention has been given to, for instance, anthropophilic fungi (infectious to man), aquatic fungi (aquatic habitats), bryophilous fungi (on bryophytes), coprophilous fungi (on dung), dermatophytes (on skin, hair nails), endolithic fungi (on rocks), entomogenous fungi (on insects), halotolerant fungi (tolerant to salt), hypogeous fungi (growing below ground), keratinophilic fungi (on feathers, horns), lichens (some studied, see Fig. 3), marine fungi (in marine and estuarine habitats), mesophilic fungi (growing between 10–40 °C), mycorrhizal fungi (symbiotic with plant roots), mycoparasites (on other fungi), nematophagous fungi (parasitic on nematodes), osmotolerant fungi (growing at high osmotic pressure), psychrophilic fungi (at < 10 °C), pyroxylophilous fungi (on burnt areas and substrates), resinicolous fungi (on resin), rumen fungi (in anaerobic rumen environment), sewage fungi (polluted water), thermophilic fungi (at or above 45 °C), water moulds (in water), and xerotolerant fungi (at < 0.85 aw) (see Maheshwari 2005).
Fig. 3.
Approximate number of species described from South Africa1,2.
Given the current importance placed on ecotourism and the preservation of unique southern African flora and fauna, it is clearly timely that some thought, financial resources and research be focused on preserving the basal links of the ecosystem, which are the fungi. Clearly, South Africa's undescribed fungi represent a vast biological resource which has yet to be collected, cultured and studied. Undoubtedly the fungi of southern Africa contain numerous beneficial biological properties and other attributes that could be used to greatly improve the quality of life for all future generations of humanity.
Acknowledgments
The authors gratefully acknowledge Gerrit Stegehuis (CBS), who compiled the data of species numbers used in Fig. 3, and Johannes P. van der Walt, who provided a summary of the development and history of yeast taxonomy in South Africa.
Footnotes
Data derived from Index Fungorum <www.indexfungorum.org/Names/Names.asp>.
The increase in species during 1980–1999 can chiefly be attributed to the description of a large number of new lichens.
References
- Acocks JPH (1953). Veld types of South Africa. Memoirs of the Botanical Survey of South Africa 28: 1–192. [Google Scholar]
- Adams GC, Wingfield MJ, Common R, Roux J (2005). Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus. Studies in Mycology 52: 1–147. [Google Scholar]
- Agricultural News (1990). Numbers 23 (11 June), 29 (23 July), 37 (17 Sept.), 39 (1 Oct.) & 42 (22 Oct.) Directorate of Agricultural Information, Pretoria.
- Albrecht CF, Joubert JJ, Rycke PH de (2001). Origin of the enigmatic circular, barren patches (`fairy rings') of the pro-Namib. South African Journal of Science 97: 23–27. [Google Scholar]
- Almborn O (1966). Revision of some lichen genera in southern Africa I. Botaniska Notiser 119: 70–112. [Google Scholar]
- Almborn O (1987). Lichens at high altitudes in southern Africa. Bibliotheca Lichenologica 25: 401–417. [Google Scholar]
- Almborn O (1988). Some distribution patterns in the lichen flora of South Africa. Annals of the Missouri Botanical Garden 25: 429–432. [Google Scholar]
- Almborn O (1989). Revision of the lichen genus Teloschistes in central and southern Africa. Nordic Journal of Botany 8: 521–537. [Google Scholar]
- Anelich RY (1986). Two entomophagous Fusarium species from South Africa. M.Sc. thesis, University of the Orange Free State, Bloemfontein.
- Arnold TH, Wet BC De (eds) 1993. Plants of southern Africa: names and distribution. Memoirs of the Botanical Survey of South Africa 62: 1–825. [Google Scholar]
- Balkwill M-J, Balkwill K (1999). Characteristics, diversity, endemism and conservation of serpentine sites in the Barberton Greenstone Belt. Third International Congress on Serpentine Ecology, 28–30 March 1999, Post-congress tour guide. C.E. Moss Herbarium, University of the Witwatersrand, Johannesburg: 1–23.
- Barnes I, Crous PW, Wingfield BD, Wingfield MJ (2004). Multigene phylogenies reveal that red band needle blight of Pinus is caused by two distinct species of Dothistroma, D. septosporum and D. pini. Studies in Mycology 50: 551–565. [Google Scholar]
- Baxter AP (1994). Past, present and prospective activities: Mycology Unit, PPRI. In: Proceedings of the 13th Plenary Meeting of AETFAT, Malawi. (Seyani JH, Chikuni AC, eds): 687–695.
- Baxter AP, Dippenaar N, Eicker A (1993). Multivariate analysis of some characteristics of South African isolates of Colletotrichum. South African Journal of Botany 59: 557–565. [Google Scholar]
- Baxter AP, Westhuizen GC van der, Eicker A (1983). Morphology and taxonomy of South African isolates of Colletotrichum. South African Journal of Botany 2: 295–289. [Google Scholar]
- Beer ZW de, De Fine Licht HH, Aanen DK, Wingfield MJ (2005). Ophiostoma spp. associated with termites and termite combs in South Africa. Abstracts of the 43rd Annual Congress of the Southern African Society for Plant Pathology, 23–26 January 2005, Hartenbos, South Africa. South African Journal of Science 101: iii–iv. [Google Scholar]
- Bezuidenhout H (1977).'n Ondersoek van die Hyphomycetes geassosieer met Cenchrus ciliaris L. M.Sc Thesis, Rand Afrikaans University, Johannesburg, South Africa.
- Bisby GR, Ainsworth GC (1943). The numbers of fungi. Transactions of the British Mycological Society 26: 16–19. [Google Scholar]
- Bolus H (1886). Sketch of the Flora of South Africa. In: Official Handbook of the Cape of Good Hope. Richards, Cape Town: 286–317.
- Botha A, Kock JLF, Roux C, Coetzee DJ, Botes PJ (1995). An isolation medium for gamma-linoleic acid producing Mucoralean fungi. Systematic and Applied Microbiology 18: 448–454. [Google Scholar]
- Botha WJ (1993). Root-rot of tree lucerne caused by Phytophthora nicotianae. Plant Pathology 42: 824–826. [Google Scholar]
- Botha WJ, Coetzer RJL (1996). Species of Pythium associated with root-rot of vegetables in South Africa. South African Journal of Botany 62: 196–204. [Google Scholar]
- Botha WJ, Crous PW (1992). A wilt disease of Rhododendron caused by Pythium prolatum and Cylindrocladium scoparium. Phytophylactica 24: 75–78. [Google Scholar]
- Botha WJ, Eicker A (1991a). Cultural studies on the genus Termitomyces in South Africa. I. Macro- and microscopic characters of basidiome context cultures. Mycological Research 95: 435–443. [Google Scholar]
- Botha WJ, Eicker A (1991b). Cultural studies on the genus Termitomyces in South Africa. II. Macro- and micromorphology of comb sporodochia. Mycological Research 95: 444–451. [Google Scholar]
- Bottomley AM (1937). Some of the more important diseases affecting timber plantations in the Transvaal. South African Journal of Science 33: 373–376. [Google Scholar]
- Braun U, Crous PW, Dugan F, Groenewald JZ, Hoog SG de (2003). Phylogeny and taxonomy of Cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s.str. Mycological Progress 2: 3–18. [Google Scholar]
- Broembsen SL von (1984). Occurrence of Phytophthora cinnamomi on indigenous and exotic hosts in South Africa, with special reference to the south-western Cape Province. Phytophylactica 16: 221–225. [Google Scholar]
- Coetzee JC, Eicker A (1994). Battarreoides diguetii (Gasteromycetes, Tulostomatales) in southern Africa. Mycotaxon 50: 19–25. [Google Scholar]
- Coetzee JC, Wyk AE van (2003). Calvatia sect. Macrocalvatia redefined and a new combination in the genus Calvatia. Bothalia 33: 156–158. [Google Scholar]
- Coetzee MPA, Wingfield BD, Coutinho TA, Wingfield MJ (2000). Identification of the causal agent of Armillaria root rot of Pinus species in South Africa. Mycologia 92: 777–785. [Google Scholar]
- Coetzee MPA, Wingfield BD, Harrington TC, Steimel J, Coutinho TA, Wingfield MJ (2001). The root fungus Armillaria mellea introduced into South Africa by early Dutch settlers. Molecular Ecology 10: 387–396. [DOI] [PubMed] [Google Scholar]
- Coetzee MPA, Wingfield BD, Roux J, Crous PW, Denman S, Wingfield MJ (2003). Discovery of two northern hemisphere Armillaria species on Proteaceae in South Africa. Plant Pathology 52: 604–612. [Google Scholar]
- Crous PW (1990). Two newly reported leaf pathogens of Eucalyptus in South Africa. South African Forestry Journal 157: 12–15. [Google Scholar]
- Crous PW (1998). Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1–170. [Google Scholar]
- Crous PW (1999). Species of Mycosphaerella and related anamorphs occurring on Myrtaceae (excluding Eucalyptus). Mycological Research 103: 607–621. [Google Scholar]
- Crous PW (2002). Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera. APS Press, MN, U.S.A.
- Crous PW, Aptroot A, Kang J-C, Braun U, Wingfield MJ (2000a). The genus Mycosphaerella and its anamorphs. Studies in Mycology 45: 107–121. [Google Scholar]
- Crous PW, Braun U (1994). Cercospora species and similar fungi occurring in South Africa. Sydowia 46: 204–224. [Google Scholar]
- Crous PW, Braun U (1995). Cercospora species and similar fungi of South Africa. Mycological Research 99: 31–36. [Google Scholar]
- Crous PW, Braun U (1996a). Cercosporoid fungi from South Africa. Mycotaxon 57: 233–321. [Google Scholar]
- Crous PW, Braun U (1996b). Notes on cercosporoid fungi occurring on Dodonaea spp. South African Journal of Botany 62: 247–249. [Google Scholar]
- Crous PW, Braun U (2003). Mycosphaerella and its anamorphs. 1. Names published in Cercospora and Passalora. CBS Biodiversity Series 1: 1–571. [Google Scholar]
- Crous PW, Denman S, Taylor JE, Swart L, Palm ME (2004a). Cultivation and diseases of Proteaceae: Leucadendron, Leucospermum and Protea. CBS Biodiversity Series 2: 1–228. [Google Scholar]
- Crous PW, Gams W (2000). Phaeomoniella chlamydospora gen. et comb. nov., a causal organism of Petri grapevine decline and esca. Phytopathologia Mediterranea 39: 112–118. [Google Scholar]
- Crous PW, Gams W, Wingfield MJ, Wyk PS van (1996a). Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections. Mycologia 88: 786–796. [Google Scholar]
- Crous PW, Groenewald JZ, Gams W (2003). Eyespot of cereals revisited: ITS phylogeny reveals new species relationships. European Journal of Plant Pathology 109: 841–850. [Google Scholar]
- Crous PW, Groenewald JZ, Mansilla JP, Hunter GC, Wingfield MJ (2004b). Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. Studies in Mycology 50: 195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Pongpanich K, Himaman W, Arzanlou M, Wingfield MJ (2004c). Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Studies in Mycology 50: 457–469. [Google Scholar]
- Crous PW, Kang J-C, Braun U (2001). A phylogenetic redefinition of anamorph genera in Mycosphaerella based on ITS rDNA sequence and morphology. Mycologia 93: 1081–1101. [Google Scholar]
- Crous PW, Kendrick WB, Alfenas AC (1997). New species of hyphomycetes associated with Eucalyptus. South African Journal of Botany 63: 286–290. [Google Scholar]
- Crous PW, Knox-Davies PS, Wingfield MJ (1989). A list of Eucalyptus leaf fungi and their potential importance to South African forestry. South African Forestry Journal 149: 17–29. [Google Scholar]
- Crous PW, Linde EJ van der (1993). New and interesting fungi. 11. Eucalyptus leaf fungi. South African Journal of Botany 59: 300–304. [Google Scholar]
- Crous PW, Petrini O, Marais GF, Pretorius ZA, Rehder F (1995a). Occurrence of fungal endophytes in cultivars of Triticum aestivum L. in South Africa. Mycoscience 36: 105–111. [Google Scholar]
- Crous PW, Phillips AJL, Baxter AP (2000b). Phytopathogenic fungi from South Africa. University of Stellenbosch, Department of Plant Pathology Press, Stellenbosch, South Africa.
- Crous PW, Seifert KA, Castañeda Ruiz (1996b). Microfungi associated with Podocarpus leaf litter in South Africa. South African Journal of Botany 62: 89–98. [Google Scholar]
- Crous PW, Sutton BC (1997). New cercosporoid fungi from southern Africa. South African Journal of Botany 63: 280–285. [Google Scholar]
- Crous PW, Wingfield MJ (1993). A re-evaluation of Cylindrocladiella, and a comparison with allied genera. Mycological Research 97: 433–448. [Google Scholar]
- Crous PW, Wingfield MJ (1994). A monograph of Cylindrocladium, including anamorphs of Calonectria. Mycotaxon 51: 341–435. [Google Scholar]
- Crous PW, Wingfield MJ (1996). Species of Mycosphaerella and their anamorphs associated with leaf blotch disease of Eucalyptus in South Africa. Mycologia 88: 441–458. [Google Scholar]
- Crous PW, Wingfield MJ, Kendrick WB (1995b). Foliicolous dematiaceous hyphomycetes from Syzygium cordatum. Canadian Journal of Botany 73: 224–234. [Google Scholar]
- Crous PW, Wingfield MJ, Koch SH (1990a). New and interesting records of South African fungi. X. New records of Eucalyptus leaf fungi. South African Journal of Botany 56: 583–586. [Google Scholar]
- Crous PW, Wingfield MJ, Nag Raj TR (1993). Harknessia spp. occurring in South Africa. Mycologia 85: 108–118. [Google Scholar]
- Crous PW, Wingfield MJ, Swart WJ (1990b). Shoot and needle diseases of pines in South Africa. South African Forestry Journal 154: 60–66. [Google Scholar]
- Cummins GB (1960). Descriptions of tropical rusts–IX. Bulletin of the Torrey Botanical Club 87: 31–45. [Google Scholar]
- Cummins GB (1971). The rust fungi of cereals, grasses and bamboos. Springer-Verlag, New York.
- Darvas JM (1979). Ecology of avocado root pathogens. South African Avocado Grower's Association Research Report for 1979: 31–32.
- Darvas JM, Scott DB, Kotzé JM (1978). Fungi associated with damping-off in coniferous seedlings in South African nurseries. South African Forestry Journal 104: 15–19. [Google Scholar]
- Degelius G (1974). The lichen genus Collema with especial reference to the extra-european species. Symbolae Botanicae Upsalienses 20(2): 1–215. [Google Scholar]
- Denman S, Crous PW, Groenewald JG, Slippers B, Wingfield BD, Wingfield MJ (2003). Circumscription of Botryosphaeria species associated with Proteaceae based on morphology and DNA sequence data. Mycologia 95: 294–307. [PubMed] [Google Scholar]
- Denman S, Crous PW, Taylor JE, Kang J-C, Pascoe I, Wingfield MJ (2000). An overview of the taxonomic history of Botryosphaeria, and a re-evaluation of its anamorphs based on morphology and ITS rDNA phylogeny. Studies in Mycology 45: 129–140. [Google Scholar]
- Denman S, Knox-Davies PS (1992). Overview of Pythium species and diseases recorded in South Africa from 1926 to the end of 1989. Phytophylactica 24: 79–84. [Google Scholar]
- Doidge EM (1924). A preliminary checklist of plant diseases occurring in South Africa. Botanical Survey of South Africa, Memoir No. 6: 1–56. [Google Scholar]
- Doidge EM (1927). A preliminary study of the South African rust fungi. Bothalia 2: 1–228. [Google Scholar]
- Doidge EM (1928). South African rust fungi. II. Bothalia 2: 473–474. [Google Scholar]
- Doidge EM (1939). South African rust fungi. III. Bothalia 3: 487–512. [Google Scholar]
- Doidge EM (1940). South African rust fungi. IV. Bothalia 4: 229–236. [Google Scholar]
- Doidge EM (1948a). South African rust fungi. V. Bothalia 4: 895–918. [Google Scholar]
- Doidge EM (1948b). South African rust fungi. VI. The species of Uromyces on Iridaceae. Bothalia 4: 918–937. [Google Scholar]
- Doidge EM (1950). The South African fungi and lichens to the end of 1945. Bothalia 5: 1–1094. [Google Scholar]
- Doidge EM, Bottomley AM (1931). A revised list of diseases occurring in South Africa. Botanical Survey of South Africa, Memoir No. 11: 1–78. [Google Scholar]
- Doidge EM, Bottomley AM, Plank JE van der, Pauer GD (1953). A revised list of plant diseases in South Africa. Union of South Africa, Department of Agriculture, Science Bulletin 346: 1–122. [Google Scholar]
- Drège JF (1843). Zwei Pflanzengeographische Documente. Besondere Beigabe zur Flora 1843 Band II.
- Dreyfuss MM, Chapela IH (1994). Potential of fungi in the discovery of novel, low-molecular weight pharmaceuticals. In: The discovery of natural products with therapeutic potential. (Gullo VP, ed.). Butterworth-Heinemann, London: 49–80. [DOI] [PubMed]
- Edwards TJ, Crouch NR, Styles D (2005). Drimia flagellaris (Hyacinthaceae): a new discovery from KwaZulu-Natal. South African Journal of Botany 71: 122–126. [Google Scholar]
- Eicker A, Baxter AP (1999). An historical overview of southern African systematic mycology. Transactions of the Royal Society of South Africa 54: 5–19. [Google Scholar]
- Eicker A, Theron GK, Grobbelaar N (1982). `n Mikrobiologiese studie van `kaal kolle' in die Giribesvlakte van Kaokoland, S.W.A. South African Journal of Botany 1: 69–74. [Google Scholar]
- Ferreira JF, Naude SP, Thompson AH (1991). First report of Phytophthora nicotianae var. nicotianae in tomatoes in South Africa. Phytophylactica 23: 233–234. [Google Scholar]
- Fine Licht HH De, Andersen A, Aanen DK (2005). Termitomyces sp. associated with the termite Macrotermes natalensis has a heterothallic mating system and multinucleate cells. Mycological Research 109: 314–318. [DOI] [PubMed] [Google Scholar]
- Fries EM (1825). Systema Orbis Vegetabilis. Vol. 1. Typographia Academica, Lund.
- Fröhlich J, Hyde KD (1999). Biodiversity of palm fungi in the tropics: are global fungal diversity estimates realistic? Biodiversity and Conservation 8: 977–1004. [Google Scholar]
- Gardner DE (1994). The native rust fungi of Hawaii. Canadian Journal of Botany 72: 976–989. [Google Scholar]
- Germishuizen G, Meyer NL (eds) (2003). Plants of southern Africa: an annotated checklist. Strelitzia 14: 1–1231. [Google Scholar]
- Gjærum HB (1974). Nordens Rustsopper. Fungiflora, Oslo.
- Gjærum HB (1988a). Rust fungi (Uredinales) on Poaceae, mainly from Africa. Mycotaxon 31: 351–378. [Google Scholar]
- Gjærum HB (1988b). Rust fungi (Uredinales) on the genus Hyparrhenia (Poaceae). Mycotaxon 32: 143–160. [Google Scholar]
- Gjærum HB (1999). New African Carex rust species. Lidia 4: 133–137. [Google Scholar]
- Gjærum HB, Reid DA (1983). Three new species and a new combination in Uredinales. Transactions of the British Mycological Society 81: 650–654. [Google Scholar]
- Gjærum HB, Reid DA (1998). Pycnia and aecia of Uromyces hypoëstis (Uredinales) described from South Africa. South African Journal of Botany 64: 290–292. [Google Scholar]
- Goldblatt P (1997). Floristic diversity in the Cape Flora of South Africa. Biodiversity and Conservation 6: 359–377. [Google Scholar]
- Goldblatt P, Manning J (2000). Cape plants: a conspectus of the Cape Flora of South Africa. Strelitzia 9: 1–743. [Google Scholar]
- Gorter GJMA (1977). Index of plant pathogens and the diseases they cause in cultivated plants in South Africa. Republic of South Africa Department of Agricultural Technical Services Science Bulletin 392: 1–177. [Google Scholar]
- Gorter GJMA (1979). An annotated check list and selected bibliography of South African fungi for the period 1946–1977. Republic of South Africa Department of Agricultural Technical Services Science Bulletin 163: 1–34. [Google Scholar]
- Gorter GJMA (1981). Index of plant pathogens II and the diseases they cause in wild growing plants in South Africa. Republic of South Africa Department of Agricultural Technical Services Science Bulletin 398: 1–84. [Google Scholar]
- Gorter GJMA (1982). Supplement to Index of plant pathogens (I). Republic of South Africa Department of Agricultural Technical Services Science Bulletin 392: 1–14. [Google Scholar]
- Gorter GJMA (1988a). Die Suid-Afrikaanse Erysiphaceae (meeldouswamme). Annale van die Universiteit van Stellenbosch, A3 3: 1–64. [Google Scholar]
- Gorter GJMA (1988b). Identification of South African Erysiphaceae with a key to the species. Phytophylactica 20: 113–119. [Google Scholar]
- Gorter GJMA (1989). Supplementary list of host plants for powdery mildew fungi occurring in South Africa. Phytophylactica 21: 97. [Google Scholar]
- Gorter GJMA (1993a). First report of Enterobryus species (Trichomycetes: Eccrinales) in South Africa and the description of three new species. Bothalia 23: 85–90. [Google Scholar]
- Gorter GJMA (1993b). First report of nematode trapping by a fungus (Arthrobotrys oligospora) in South Africa. Phytophylactica 25: 279–281. [Google Scholar]
- Gryzenhout M, Glen HF, Wingfield BD, Wingfield MJ (2005b). Amphilogia gen. nov. for Cryphonectria-like fungi from Elaeocarpus spp. in New Zealand and Sri Lanka. Taxon 54: 1009–1021. [Google Scholar]
- Gryzenhout M, Myburg H, Hodges CS, Wingfield BD, Wingfield MJ (2006). Microthia and Holocryphia, two new genera of fungi from Eucalyptus representing the species previously known as Cryphonectria havanensis and Cryphonectria eucalypti. Studies in Mycology 55: 35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryzenhout M, Myburg H, Merwe NA van der, Wingfield BD, Wingfield MJ (2004). Chrysoporthe, a new genus to accommodate Cryphonectria cubensis. Studies in Mycology 50: 119–142. [Google Scholar]
- Gryzenhout M, Myburg H, Wingfield BD, Montenegro F, Wingfield MJ (2005a). Rostraureum tropicale gen. et sp. nov. (Diaporthales) associated with dying Terminalia ivorensis in Ecuador. Mycological Research 109: 1029–1044. [DOI] [PubMed] [Google Scholar]
- Guderley R, Lumbsch HT (1996). The lichen genus Diploschistes in South Africa (Thelotremataceae). Mycotaxon 58: 269–292. [Google Scholar]
- Gull C, Labuschagne N, Botha WJ (2004). Pythium species associated with wilt and root rot of hydroponically grown crops in South Africa. African Plant Protection 10: 109–116. [Google Scholar]
- Hafellner J, Calatayud V (1999). Lichenostigma cosmopolites, a common lichenicolous fungus on Xanthoparmelia species. Mycotaxon 72: 197–114. [Google Scholar]
- Hahn N (1994). Tree list of the Soutpansberg. Fantique Publishers, Pretoria.
- Hahn N (1998). Plant diversity statistics of the Soutpansberg. SABONET News 2: 106–108. [Google Scholar]
- Hale ME (1986). New species of the lichen genus Xanthoparmelia from South Africa (Ascomycotina, Parmeliaceae). Mycotaxon 27: 563–610. [Google Scholar]
- Hale ME (1988). Namakwa, a new lichen genus in the Parmeliaceae (Ascomycotina, Parmeliaceae). Mycotaxon 32: 169–174. [Google Scholar]
- Halleen F, Schroers H-J, Groenewald JZ, Crous PW (2004). Novel species of Cylindrocarpon (Neonectria) and Campylocarpon gen. nov. associated with black foot disease of grapevines (Vitis spp.). Studies in Mycology 50: 431–455. [Google Scholar]
- Harvey WH, Sonder OW (1862). Flora Capensis vol. 2. Hodges Smith, Dublin.
- Hawksworth DL (1991). The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycological Research 95: 641–655. [Google Scholar]
- Hawksworth DL (1998). The consequences of plant extinctions for their dependant biotas: an overlooked aspect of conservation science. In: Rare, threatened, and endangered floras of Asia and the Pacific Rim (Peng CI, Lowry PP, eds). Institute of Botany, Academia Sinica, Taipei: 1–15.
- Hawksworth DL (2001). The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycological Research 105: 1422–1432. [Google Scholar]
- Hawksworth DL (2003). The lichenicolous fungi of Great Britain and Ireland: an overview and annotated checklist. Lichenologist 35: 191–232. [Google Scholar]
- Hawksworth DL (2004). Fungal diversity and its implications for genetic resource collections. Studies in Mycology 50: 9–18. [Google Scholar]
- Henderson L (2001). Alien Weeds and invasive Plants. PlantProtection Research Institute Handbook 12. ARC, Pretoria.
- Hennen JF, McCain JW (1993). New species and records of Uredinales from the Neotropics. Mycologia 85: 970–986. [Google Scholar]
- Hilliard OM, Burtt BL (1987). The botany of the southern Natal Drakensberg. National Botanical Gardens, Kirstenbosch.
- Hiratsuka N, Sato S, Katsuya K, Kakishima M, Hiratsuka Y, Kaneko S, Ono Y, Sato T, Harada Y, Hiratsuka T, Nakayama K (1992). The rust flora of Japan. Tsukuba Shuppankai, Tsukuba.
- Hunter GC, Crous PW, Roux J, Wingfield BD, Wingfield MJ (2004a). Identification of Mycosphaerella species associated with Eucalyptus nitens leaf defoliation in South Africa. Australasian Plant Pathology 33: 349–355. [Google Scholar]
- Hunter GC, Roux J, Wingfield BD, Crous PW, Wingfield MJ (2004b). Mycosphaerella species causing leaf disease in South African Eucalyptus plantations. Mycological Research 108: 672–681. [DOI] [PubMed] [Google Scholar]
- Hurter PJH, Wyk AE van (2004). A new species of Acacia (Mimosoideae) from Mpumalanga, South Africa. Bothalia 34: 42–44. [Google Scholar]
- Hyde KD (1996). Measuring biodiversity: diversity of microfungi in north Queensland. In: Measuring and monitoring biodiversity in tropical and temperate forests (Boyle TJB, Boontawee B, eds). CIFOR, Bogor: 271–286.
- Hyde KD, Fröhlich J, Taylor JE (1997). Diversity of ascomycetes on palms in the tropics. In: Biodiversity of tropical microfungi (Hyde KD, ed.). Hong Kong University Press, Hong Kong: 141–156.
- Jaarsveld E van, Hammer S, Wyk AE van (2005). Bulbine retinens, a new cliff-dweller from the Eastern Cape. Aloe 42: 14–15. [Google Scholar]
- Jacobs A, Coetzee MPA, Wingfield BD, Jacobs K, Wingfield MJ (2003a). Phylogenetic relationships among Phialocephala species and other ascomycetes. Mycologia 95: 637–645. [DOI] [PubMed] [Google Scholar]
- Jacobs K, Kirisits T, Wingfield MJ (2003b). Taxonomic re-evaluation of three related species of Graphium, based on morphology, ecology and phylogeny. Mycologia 95: 714–727. [DOI] [PubMed] [Google Scholar]
- Jacobs K, Wingfield MJ, Uzunovic A, Frisullo S (2001). Three new species of Leptographium from pine. Mycological Research 105: 490–499. [Google Scholar]
- Job DJ (1987). South African species of Hymenochaete (Aphyllophorales). South African Journal of Botany 53: 293–299. [Google Scholar]
- Jørstad I (1956). Reliquiae Lagerheimianae, African Uredinales. Arkiv för Botanik ser. 2, b. 3 17: 563–598. [Google Scholar]
- Kellerman TS, Marasas WFO, Pienaar JG, Naudé TW (1972). A mycotoxicosis of Equidae caused by Fusarium moniliforme Sheldon, a preliminary communication. Onderstepoort Journal of Veterinary Research 39: 205–208. [PubMed] [Google Scholar]
- Kellerman TS, Westhuizen GCA Van Der, Coetzer JAW, Roux C, Marasas WFO, Minne JA, Bath GF, Basson PA (1980). Photosensitivity in South Africa. The experimental production of the ovine hepatogenous photosensitivity disease Geeldikkop (Tribulosis ovis) by the simultaneous ingestion of Tribulus terrestris plants and cultures of Pithomyces chartarum containing the mycotoxin sporidesmin. Onderstepoort Journal of Veterinary Research 47: 231–261. [PubMed] [Google Scholar]
- Killick DJB (1990). A field guide to the flora of the Natal Drakensberg. Jonathan Bell & Ad. Donker, Johannesburg.
- Kirisits T (2004). Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi. In: Bark and wood boring insects in living trees in Europe, a synthesis. (Lieutier F, ed.). Kluwer Academic Press, The Netherlands: 1–55.
- Kleinjan CA, Morin L, Edwards PB, Wood AR (2004). Distribution, host range and phenology of the rust fungus Puccinia myrsiphylli in South Africa. Australasian Plant Pathology 33: 263–271. [Google Scholar]
- Klittich CJR, Leslie JF, Nelson PE, Marasas WFO (1997). Fusarium thapsinum (Gibberella thapsina): A new species in Section Liseola from sorghum. Mycologia 89: 643–652. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B (2001). Biodiversity of fungi on Juncus roemerianus. Mycological Research 105: 1411–1412. [Google Scholar]
- Kotzé JJ (1935). Forest fungi: The position in South Africa. British Empire Forestry Conference, South Africa, 1935. The Government Printer, Pretoria, South Africa.
- Kriel W-M, Swart WJ, Crous PW (2000). Foliar endophytes and their interactions with host plants, with specific reference to Gymnospermae. Advances in Botanical Research 33: 1–29. [Google Scholar]
- Labuschagne N, Broekhuizen W van, Thompson AH (2000). First report of wilt and sudden death of pumpkin caused by Phytophthora capsici in South Africa. African Plant Protection 6: 61–63. [Google Scholar]
- Labuschagne N, Gull C, Wehner FC, Botha WJ (2001). Report of root rot caused by Pythium F-group on hydroponically-grown celery in South Africa. Plant Disease 86: 698. [DOI] [PubMed] [Google Scholar]
- Labuschagne N, Gull C, Wehner FC, Botha WJ (2002). Pythium spp. infecting hydroponically grown lettuce in South Africa. Plant Disease 86: 1175. [DOI] [PubMed] [Google Scholar]
- Labuschagne N, Thompson AH, Botha WJ (2003). First report of stem and root rot of tomato caused by Phytophthora capsici in South Africa. Plant Disease 87: 1540. [DOI] [PubMed] [Google Scholar]
- Lamprecht SC, Knox-Davies PS, Marasas WFO (1988). Fungi associated with root rot of annual Medicago spp. in South Africa. Phytophylactica 20: 281–286. [Google Scholar]
- Lee S, Crous PW (2003a). A new species of Helicogermslita from South Africa. Sydowia 55: 109–114. [Google Scholar]
- Lee S, Crous PW (2003b). New coelomycetes occurring on Restionaceae. Sydowia 55: 115–128. [Google Scholar]
- Lee S, Crous PW (2003c). New species of Anthostomella on fynbos, with a key to the genus in South Africa. Mycological Research 107: 360–370. [DOI] [PubMed] [Google Scholar]
- Lee S, Crous PW (2003d). Taxonomy and diversity of hysteriaceous ascomycetes in fynbos. South African Journal of Botany 69: 480–488. [Google Scholar]
- Lee S, Groenewald JZ, Crous PW (2004a). Phylogenetic reassessment of the coelomycete genus Harknessia and its teleomorph Wuestneia (Diaporthales), and the introduction of Apoharknessia gen. nov. Studies in Mycology 50: 235–252. [Google Scholar]
- Lee S, Groenewald JZ, Taylor JE, Roets F, Crous PW (2003). Rhynchostomatoid fungi occurring on Proteaceae. Mycologia 95: 902–910. [PubMed] [Google Scholar]
- Lee S, Mel'nik V, Taylor JE, Crous PW (2004b). Diversity of saprobic hyphomycetes on Proteaceae and Restionaceae from South Africa. Fungal Diversity 17: 91–114. [Google Scholar]
- Lee S, Roets F, Crous PW (2005). Biodiversity of saprobic microfungi associated with the infructescences of Protea species in South Africa. Fungal Diversity 19: 69–78. [Google Scholar]
- Linde C, Drenth A, Kemp GHJ, Wingfield MJ, Broembsen SL von (1997). Population structure of Phytophthora cinnamomi in South Africa. Phytopathology 87: 822–827. [DOI] [PubMed] [Google Scholar]
- Linde C, Drenth A, Wingfield MJ (1999). Gene and genotypic diversity of Phytophthora cinnamomi in South Africa and Australia revealed by DNA polymorphisms. European Journal of Plant Pathology 105: 667–680. [Google Scholar]
- Linde C, Kemp GHJ, Wingfield MJ (1994a). Diseases of pines and eucalypts in South Africa associated with Pythium and Phytophthora species. South African Forestry Journal 169: 25–32. [Google Scholar]
- Linde C, Kemp GHJ, Wingfield MJ (1994b). Pythium and Phytophthora species associated with eucalypts and pines in South Africa. European Journal of Forest Pathology 24: 345–356. [Google Scholar]
- Linde EJ van der (1992). Notes on the South African Hysteriaceae (Ascomycetes: Mycotina). South African Journal of Botany 58: 491–499. [Google Scholar]
- Linde EJ van der, Warmelo KT van (1989). New and interesting records of South African fungi. IX. New Ascomycete records. South African Journal of Botany 55: 536–538. [Google Scholar]
- Linder HP, Meadows ME, Cowling RM (1992). History of the Cape flora. In: The ecology of Fynbos: nutrients, fire and diversity. (Cowling RM, ed.). Oxford University Press, Cape Town: 113–134.
- Lodge DJ (1997). Factors related to diversity of decomposer fungi in tropical forests. Biodiversity and Conservation 6: 681–688. [Google Scholar]
- Low AB, Rebelo A (eds) (1996). Vegetation of South Africa, Lesotho and Swaziland. Department of Environmental Affairs and Tourism, Pretoria.
- Lubbe CM, Denman S, Cannon PF, Groenewald JZ, Lamprecht SC, Crous PW (2004). Characterization of Colletotrichum species associated with diseases of Proteaceae. Mycologia 96: 1268–1279. [PubMed] [Google Scholar]
- MacRae C (1999). Life etched in Stone: Fossils of South Africa. Geological Society of South Africa, Johannesburg.
- Maheshwari R (2005). Fungal biology in the 21st century. Current Science 88: 1406–1418. [Google Scholar]
- Maltitz PM von, Broembsen SL von (1984). Phytophthora porri on onions in South Africa. Plant Disease 68: 732. [Google Scholar]
- Maltitz PM von, Broembsen SL von (1985). Lemon shoot tip blight caused by Phytophthora citricola in propagation tunnels. Phytophylactica 17: 47–48. [Google Scholar]
- Marais GJ, Wingfield MJ (2001). Ophiostoma africanum sp. nov. and a key to other ophiostomatoid fungi associated with Protea infructescences. Mycological Research 105: 240–246. [Google Scholar]
- Marais GJ, Wingfield MJ, Viljoen CD, Wingfield BD (1998). A new ophiostomatoid genus from Protea infrutescences. Mycologia 90: 136–141. [Google Scholar]
- Marais PG (1979). Fungi associated with root rot in vineyards in the Western Cape. Phytophylactica 11: 65–68. [Google Scholar]
- Marais PG (1980). Fungi associated with decline and death of nursery grapevines in the Western Cape. Phytophylactica 12: 9–13. [Google Scholar]
- Marasas WFO, Kellerman TS, Pienaar JG, Naudé TW (1976). Leukoencephalomalacia: a mycotoxicosis of equidae caused by Fusarium moniliforme Sheldon. Onderstepoort Journal of Veterinary Research 43: 113–121. [PubMed] [Google Scholar]
- Marasas WFO, Lamprecht SC, Wyk PS van, Anelich RY (1988). Bibliography of Fusarium (Fungi: Hyphomycetes) in South Africa. Bothalia 17: 97–104. [Google Scholar]
- Marasas, WFO, Rheeder JP, Lamprecht S, Zeller KA, Leslie JF (2001). Fusarium andiyazi sp. nov., a new species from Sorghum. Mycologia 93: 1203–1210. [Google Scholar]
- Marasas WFO, Schumann IH (1966). Two species of Erysiphaceae from Pretoria. Bothalia 9: 245–249. [Google Scholar]
- Marasas WFO, Schumann IH (1972). The genus Pithomyces in South Africa. Bothalia 10: 509–516. [Google Scholar]
- Martin P (1970). Studies in the Xylariaceae: Viii. Xylaria and its allies. South African Journal of Botany 36: 73–138. [Google Scholar]
- McLeod A, Denman S, Sadie A, Denner FDN (2001). Characterization of South African isolates of Phytophthora infestans. Plant Disease 85: 287–291. [DOI] [PubMed] [Google Scholar]
- McNabb RFR, Talbot PHB (1973). Holobasidiomycetidae: Exobasidiales, Brachybasidiales, Dacrymycetales, Tulasnellales. In: The Fungi. An advanced Treatise. Vol IVB. (Ainsworth GC, Sparrow FK, Sussman AS, eds). Academic Press, New York: 317–326. [Google Scholar]
- Mel'nik V, Lee S, Groenewald JZ, Crous PW (2004). New hyphomycetes from Restionaceae in the fynbos: Parasarcopodium ceratocaryi gen. & sp. nov. and Rhexodenticula elegiae sp. nov. Mycological Progress 3: 19–28. [Google Scholar]
- Mennicken M, Berndt R, Oberwinkler F (2003). A new rust fungus (Uredinales) on Penaeaceae, Uredo sarcocollae on Salteria sarcocolla. Mycotaxon 85: 147–151. [Google Scholar]
- Mennicken M, Maier W, Crous PW, Oberwinkler F (2005a). A contribution to the rust flora (Uredinales) on Aizoaceae in southern Africa. Mycological Progress 4: 215–224. [Google Scholar]
- Mennicken M, Maier W, Oberwinkler F (2005b). A contribution to the rust flora (Uredinales) of southern Africa, with an emphasis on Namibia. Mycological Progress 4: 55–75. [Google Scholar]
- Mennicken M, Oberwinkler F (2004) A contribution to the rust flora (Uredinales) of southern Africa, with an emphasis on South Africa. Mycotaxon 90: 1–28. [Google Scholar]
- Merwe JJH van der, Joubert DJ, Matthee FN (1972). Phytophthora cinnamomi root rot of grapevine in the western Cape. Phytophylactica 4: 133–136. [Google Scholar]
- Mes MG (1934). A wilt of snapdragon (Antirrhinum majus) in South Africa. South African Journal of Science 31: 281–287. [Google Scholar]
- Meyer L, Slippers B, Korsten L, Kotzé JM, Wingfield JM (2001). Two distinct Guigardia species associated with Citrus in South Africa. South African Journal of Science 97: 191–194. [Google Scholar]
- Morris MJ, Crous PW (1994). New and Interesting records of South African fungi. XIV. Cercosporoid fungi from weeds. South African Journal of Botany 60: 325–332. [Google Scholar]
- Mostert L, Crous PW, Groenewald JZ, Gams W, Summerbell RC (2003). Togninia (Calosphaeriales) is confirmed as teleomorph of Phaeoacremonium by means of morphology, sexual compatibility, and DNA phylogeny. Mycologia 95: 646–659. [DOI] [PubMed] [Google Scholar]
- Mostert L, Crous PW, Kang C-J, Phillips AJL (2001). Species of Phomopsis and a Libertella sp. occurring on grapevines with specific reference to South Africa: morphological, cultural, molecular and pathological characterization. Mycologia 93: 145–166. [Google Scholar]
- Mostert L, Crous PW, Petrini O (2000). Endophytic fungi associated with shoots and leaves of Vitis vinifera, with specific reference to the Phomopsis viticola complex. Sydowia 52: 46–58. [Google Scholar]
- Mwenje E, Wingfield BD, Coetzee MPA, Wingfield MJ. (2003). Molecular characterisation of Armillaria species from Zimbabwe. Mycological Research 107, 291–296. [DOI] [PubMed] [Google Scholar]
- Myburg H, Gryzenhout M, Wingfield BD, Milgroom MG, Shigeru K, Wingfield MJ (2004a). DNA sequence data and morphology define Cryphonectria species in Europe, China, and Japan. Canadian Journal of Botany 82: 1730–1743. [Google Scholar]
- Myburg H, Gryzenhout M, Wingfield BD, Stipes RJ, Wingfield MJ (2004b). Phylogenetic relationships of Cryphonectria and Endothia species, based on DNA sequence data and morphology. Mycologia 96: 990–1001. [PubMed] [Google Scholar]
- Nakabonge G, Gryzenhout M, Roux J, Wingfield MJ (2006). Celoporthe dispersa gen. et sp. nov. from native Myrtales in South Africa.Studies in Mycology 55: 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niekerk JM van, Crous PW, Groenewald JZ, Fourie PH, Halleen F (2004a). DNA phylogeny and morphological characterization of Botryosphaeria species occurring on grapevines. Mycologia 96: 781–798. [PubMed] [Google Scholar]
- Niekerk JM van, Groenewald JZ, Farr DF, Fourie PH, Halleen F, Crous PW (2005). Reassessment of Phomopsis species on grapevines. Australasian Plant Pathology 34: 27–39. [Google Scholar]
- Niekerk JM van, Groenewald JZ, Verkley GJM, Fourie PH, Wingfield MJ, Crous PW (2004b). Systematic reappraisal of Coniella and Pilidiella, with specific reference to species occurring on Eucalyptus and Vitis in South Africa. Mycological Research 108: 283–303. [DOI] [PubMed] [Google Scholar]
- Parmelee JA (1989). The rusts (Uredinales) of arctic Canada. Canadian Journal of Botany 67: 3315–3365. [Google Scholar]
- Pascoe IG (1990). History of systematic mycology in Australia. In: History of systematic botany in Australia. (Short PS, ed.). Australian Systematic Botany Society, South Yarra: 259–264.
- Pavlic D, Slippers B, Coutinho TA, Venter M, Wingfield MJ (2004). Lasiodiplodia gonubiensis sp. nov., a new Botryosphaeria anamorph from native Syzygium cordatum in South Africa. Studies in Mycology 50: 313–322. [Google Scholar]
- Phillips AJL (1985). Coniothyrium minitans on sclerotia of Sclerotinia sclerotiorum in South Africa. Phytophylactica 17: 217–219. [Google Scholar]
- Phillips AJL (1987). Carpogenic germination of sclerotia of Sclerotinia sclerotiorum: a review. Phytophylactica 19: 279–283. [Google Scholar]
- Pirozynski KA (1972). Microfungi of Tanzania. Mycological Papers 129: 1–64. [Google Scholar]
- Pirozynski KA, Hawksworth DL (1988). Coevolution of Fungi with Plants and Animals. Academic Press, London.
- Pole Evans IB (1933). Safeguarding the soil products of the Union. Annual report of the Division of Plant Industry. Farming in South Africa 8 486–493. [Google Scholar]
- Pole Evans IB (1916). A sketch of the rise, growth and development of mycology in South Africa. South African Journal of Science 13: 97–116. [Google Scholar]
- Polishook JD, Bills GF, Lodge DJ (1996). Microfungi from decaying leaves of two rain forest trees in Puerto Rico. Journal of Industrial Microbiology 17: 284–294. [Google Scholar]
- Pooley ES (2003). Mountain Flowers: a field guide to the flora of the Drakensberg and Lesotho. The Flora Publications Trust, Durban.
- Pretorius MC, Crous PW, Groenewald JZ, Braun U (2003). Phylogeny of some cercosporoid fungi from Citrus. Sydowia 55: 286–305. [Google Scholar]
- Reenen M van (1995). An annotated list of Urediniomycetes (rust fungi) from South Africa 1: Melampsoraceae and Pucciniaceae, excluding Puccinia and Uromyces. Bothalia 25: 173–181. [Google Scholar]
- Reid DA (1975). Type studies of the larger Basidiomycetes described from southern Africa. Contributions from the Bolus Herbarium 7: 1–255. [Google Scholar]
- Retief E, Herman PPJ (1997). Plants of the northern provinces of South Africa: keys and diagnostic characters. Strelitzia 6: 1–681. [Google Scholar]
- Retief E, Siebert SJ, Wyk AE van (2001). A new species of Rhoicissus (Vitaceae) from Sekhukhuneland, South Africa. South African Journal of Botany 67: 230–234. [Google Scholar]
- Rheeder JP, Marasas WFO (1998). Fusarium species from plant debris associated with soils from maize production areas in the Transkei region of South Africa. Mycopathologia 143: 113–119. [DOI] [PubMed] [Google Scholar]
- Rheeder JP, Marasas WFO, Van Wyk PS, Du Toit W, Pretorius AJ, Van Schalkwyk DJ (1990). Incidence of Fusarium and Diplodia species and other fungi in naturally infected grain of South African maize cultivars. Phytophylactica 22: 97–102. [Google Scholar]
- Robbertse B, Campbell GF, Crous PW (1995). Revision of Pseudocercosporella-like species causing eyespot disease of wheat. South African Journal of Botany 61: 43–48. [Google Scholar]
- Roets F, De Beer ZW, Dreyer LL, Crous PW, Zipfel R, Wingfield MJ (2006) Multi-gene phylogeny of Ophiostoma spp. associated with Protea infructescenses including two new species. Studies in Mycology 55: 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong IH (2002). An integrated approach to the taxonomy of some mitosporic fungi of the Bipolaris-complex. Ph.D. Thesis. University of Pretoria, Pretoria, South Africa.
- Rong IH, Botha A (1993). New and interesting records of South African fungi. XII. Synnematous Hyphomycetes. South African Journal of Botany 59: 514–518. [Google Scholar]
- Rong IH, Grobbelaar E (1998). South African records of associations between fungi and arthropods. African Plant Protection 4: 43–63. [Google Scholar]
- Rooy J van (2000). Diversity and phytogeography of the moss flora of southern Africa. Ph.D. thesis, University of Pretoria, Pretoria.
- Rossman AY (1994). A strategy for an all-taxa inventory of fungal diversity. In: Biodiversity and terrestrial ecosystems. (Peng CI, Chen CH, eds). Institute of Botany, Academia Sinica, Taipei: 169–194.
- Roux C (1985). The morphology and taxonomy of some fungi selected from a survey of a natural Karoo pasture. Ph.D. Thesis, Botany Department, Rand Afrikaans University, Johannesburg, South Africa.
- Roux C (1986). Leptosphaerulina chartarum sp. nov., the teleomorph of Pithomyces chartarum. Transactions of the British Mycological Society 86: 319–323. [Google Scholar]
- Roux C (1996). A new species of Helicocephalum (Zygomycotina) from South Africa. South African Journal of Botany 62: 104–107. [Google Scholar]
- Roux C, Merwe van der CF, Warmelo KT van (1995). The ultrastructure of conidiogenesis in Stilbella annulata. South African Journal of Botany 61: 215–221. [Google Scholar]
- Roux C, Warmelo KT van (1989). Conidiomatal and conidial ontogeny in Urohendersonia platensis. Mycological Research 92: 223–229. [Google Scholar]
- Roux C, Warmelo KT van (1990). Conidiomata of Bartalinia robillardoides. Mycological Research 94: 109–116. [Google Scholar]
- Roux J, Harrington TC, Steimel JP, Wingfield MJ (2001a). Genetic variation in the wilt pathogen Ceratocystis albofundus. Mycoscience 42: 327–332. [Google Scholar]
- Roux J, Steenkamp ET, Marasas WFO, Wingfield MJ, Wingfield BD (2001b). Characterization of Fusarium graminearum from Acacia and Eucalyptus using beta tubulin and histone gene sequences. Mycologia 93: 704–711. [Google Scholar]
- Roux J, Wingfield MJ (1997). Survey and virulence of fungi occurring on diseased Acacia mearnsii in South Africa. Forest Ecology and Management 99: 327–336. [Google Scholar]
- Schoch CL, Crous PW (1999). First report of Cylindrocladium root and petiole rot on Spathiphyllum in South Africa. South African Journal of Botany 65: 208–211. [Google Scholar]
- Schoch CL, Crous PW, Wingfield BD, Wingfield MJ (1999). The Cylindrocladium candelabrum species complex includes four distinct mating populations. Mycologia 91: 286–298. [Google Scholar]
- Schoch CL, Crous PW, Wingfield MJ, Wingfield BD (2000). Phylogeny of Calonectria and selected hypocrealean genera with cylindrical macroconidia. Studies in Mycology 45: 45–62. [Google Scholar]
- Scholtz CH, Chown SL (1995). Insects in southern Africa: how many species are there? South African Journal of Science 91: 124–126. [Google Scholar]
- Schrire BD (1983). Centenary of the Natal Herbarium, Durban, 1882–1982. Bothalia 14: 223–236. [Google Scholar]
- Schroers H-J, Geldenhuis MM, Schoeman M, Wingfield BD, Wingfield MJ (2005). Classification of the guava wilt fungus Myxosporium psidii, the palm pathogen Gliocladium vermoesenii and the persimmon wilt fungus Acremonium diospyri in Nalanthamala. Mycologia 97: 375–395. [DOI] [PubMed] [Google Scholar]
- Schutte AL (1992). An overview of Penicillium (Hyphomycetes) and associated teleomorphs in southern Africa. Bothalia 22: 77–91. [Google Scholar]
- Schutte AL (1994). An overview of Aspergillus (Hyphomycetes) and associated teleomorphs in southern Africa. Bothalia 24: 171–185. [Google Scholar]
- Scott DB (1987). Identification and pathogenicity of Pythium isolates from wheat-field soils in South Africa. Phytophylactica 19: 499–504. [Google Scholar]
- Scott DB, Toit JW du (1970). Three new Raffaelea species. Transactions British Mycological Society 55: 181–186. [Google Scholar]
- Scott DB, Visser CPN, Rufenacht EMC (1979). Crater disease of summer wheat in African drylands. Plant Disease Reporter 63: 836–840. [Google Scholar]
- Serdani M, Crous PW, Holz G, Petrini O (1998). Endophytic fungi associated with core rot of apples in South Africa, with specific reference to Alternaria species. Sydowia 50: 257–271. [Google Scholar]
- Shivas RG (1991). Puccinia ursiniae sp. nov. on Ursinia anthemoides. Mycological Research 95: 379–381. [Google Scholar]
- Siebert SJ (1998). Ultramafic substrates and floristic patterns in Sekhukhuneland, South Africa. M.Sc. thesis, University of Pretoria, Pretoria.
- Siebert SJ (2001). Vegetation on the ultramafic soils of Sekhukhuneland. D.Sc. thesis, University of Pretoria, Pretoria.
- Sinclair R, Eicker A (1985). A new species of Chloridium from South Africa. Transactions of the British Mycological Society 84: 566–568. [Google Scholar]
- Sinclair RC, Eicker A, Bhat DJ (1985). Branching in Spadicoides. Transactions of the British Mycological Society 85: 736–738. [Google Scholar]
- Sinclair RC, Eicker A, Morgan-Jones G (1983). Conicomyces, a unique synnematous hyphomycete genus from South Africa. Mycologia 75: 1100–1103. [Google Scholar]
- Sinclair RC, Eicker A, Morgan-Jones G (1987). Notes on hyphomycetes. LVI. Ceratosporella cheiroidea, a new species. Mycotaxon 30: 351–355. [Google Scholar]
- Sinclair RC, Eicker A, Morgan-Jones G (1990). Dematiaceous hyphomycetes from South Africa. I. Some phragmosporous holoblastic and tretic species. South African Journal of Botany 56: 507–513. [Google Scholar]
- Sinclair RC, Eicker A, Morgan-Jones G (1994). A microfloristic survey of saphrophytic hyphomycetes of indigenous forest habitats in southern Africa. In: Proceedings of the Thirteenth Plenary Meeting of AETFAT, Zomba, Malawi. (Seyani JH, Chikuni AC, eds). 2–11 April 1991, National Herbarium and Botanic Gardens of Malawi: 613–617.
- Six DL (2003). Bark beetle-fungus symbiosis. In: Insect Symbiosis. (Bourtzis K, Miller TA, eds). CRC Press, New York: 97–114.
- Slippers B, Burgess T, Wingfield BD, Crous PW, Coutinho TA, Wingfield MJ (2004a). Development of simple sequence repeat markers for Botryosphaeria spp. with Fusicoccum anamorphs. Molecular Ecology Notes 4: 675–677. [Google Scholar]
- Slippers B, Coutinho TA, Wingfield BD, Wingfield MJ (2003). The genus Amylostereum and its association with woodwasps: a contemporary review. South African Journal of Science 99: 70–74. [Google Scholar]
- Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD, Wingfield MJ (2004b). Multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96: 83–101. [PubMed] [Google Scholar]
- Slippers B, Fourie G, Crous PW, Coutinho TA, Wingfield BD, Carnegie A, Wingfield MJ (2004c). Sympatric speciation and distribution of Botryosphaeria spp. on native and introduced Eucalyptus trees. Studies in Mycology 50: 343–358. [Google Scholar]
- Slippers B, Fourie G, Crous PW, Coutinho TA, Wingfield BD, Wingfield MJ (2004d). Multiple gene sequences delimit Botryosphaeria australis sp. nov. from B. lutea. Mycologia 96: 1028–1039. [PubMed] [Google Scholar]
- Slippers B, Johnson GI, Crous PW, Coutinho TA, Wingfield BD, Wingfield MJ (2005a). Phylogenetic and morphological re-evaluation of the Botryosphaeria species causing diseases of Mangifera indica. Mycologia 97: 102–113. [DOI] [PubMed] [Google Scholar]
- Slippers B, Smit WA, Crous PW, Coutinho TA, Wingfield BD, Wingfield MJ (2006). Taxonomy, phylogeny and identification of Botryosphaeriaceae causing diseases on pome and stone fruit trees in South Africa and other regions of the world. Plant Pathology: In Press.
- Slippers B, Summerell BA, Crous PW, Coutinho TA, Wingfield BD, Wingfield MJ (2005b). Preliminary studies on Botryosphaeria species from Wollemia nobilis and related southern hemisphere conifers in Australasia and South Africa. Australasian Plant Pathology 34: 213–220. [Google Scholar]
- Smit WA, Knox-Davies PS (1989a). Comparison of Diaporthe phaseolorum isolates from rooibos tea, Aspalathus linearis. Phytophylactica 21: 301–306. [Google Scholar]
- Smit WA, Knox-Davies PS (1989b). Die-back of rooibos tea caused by Diaporthe phaseolorum. Phytophylactica 21: 183–188. [Google Scholar]
- Smit WA, Viljoen CD, Wingfield BD, Wingfield MJ, Calitz FJ (1996a). A new canker disease of apple, pear, and plum rootstocks caused by Diaporthe ambigua in South Africa. Plant Disease 80: 1331–1335. [Google Scholar]
- Smit WA, Wingfield MJ, Wingfield BD (1996b). A new canker disease of apple, pear, and plum rootstocks caused by Diaporthe ambigua in South Africa. Plant Disease 80: 1331–1335. [Google Scholar]
- Smith D, Waller JM (1992). Culture collections and microorganisms: their importance in tropical plant pathology. Fitopathologia Brasileira 17: 1–8. [Google Scholar]
- Smith H, Crous PW, Wingfield MJ, Coutinho TA, Wingfield BD (2001). Botryosphaeria eucalyptorum sp. nov., a new species in the B. dothidea-complex on Eucalyptus in South Africa. Mycologia 93: 277–285. [Google Scholar]
- Smith H, Wingfield MJ, Crous PW, Coutinho TA (1996). Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. South African Journal of Botany 62: 86–88. [Google Scholar]
- Stizenberger E (1890–95). Lichenes africana. Bericht über die Tätigkeit der St. Gallischen Naturwissenschaftlichen Gesellschaft 1888/89: 105–249, 1889/90: 133–268, 1891/92: 86–96, 1893/94: 215–264. [Google Scholar]
- Suh S-O, McHugh JV, Pollock DD, Blackwell M (2004). The beetle gut: a hyperdiverse source of novel yeasts. Mycological Research 109: 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surridge AKJ, Viljoen A, Crous PW, Wehner FC (2003). Identification of the pathogen associated with Sigatoka disease of banana in South Africa. Australasian Plant Pathology 32: 27–31. [Google Scholar]
- Sutton BC, Crous PW (1997). Lecanostictopsis gen. nov. and similar fungi from Syzygium species. Mycological Research 101: 215–225. [Google Scholar]
- Sutton BC, Marasas WFO (1976). Observations on Neottiosporina and Tiarosporella. Transactions of the British Mycological Society 67: 69–76. [Google Scholar]
- Swart L, Crous PW, Petrini O, Taylor JE (2000). Fungal endophytes of Proteaceae, with particular emphasis on Botryosphaeria proteae. Mycoscience 41: 123–127. [Google Scholar]
- Talbot PHB (1951a). Studies of some South African resupinate Hymenomycetes. Bothalia 6: 1–116. [Google Scholar]
- Talbot PHB (1951b). New and interesting records of South African fungi. Bothalia 6: 183–193. [Google Scholar]
- Talbot PHB (1954). Micromorphology of the lower Hymenomycetes. Bothalia 6: 249–299. [Google Scholar]
- Talbot PHB (1958). Studies of some South African resupinate Hymenomycetes. Part II. Bothalia 7: 131–187. [Google Scholar]
- Talbot PHB (1965). Studies of `Pellicularia' and associated genera of Hymenomycetes. Persoonia 3: 371–406. [Google Scholar]
- Taylor JE, Crous PW (1999). Phaeophleospora faureae comb. nov. associated with leaf spots on Faurea saligna (Proteaceae), with a key to known species of Phaeophleospora. Fungal Diversity 3: 153–158. [Google Scholar]
- Taylor JE, Crous PW (2000). Fungi occurring on Proteaceae. New anamorphs for Teratosphaeria, Mycosphaerella and Lembosia, and other fungi associated with leaf spots and cankers of Proteaceous hosts. Mycological Research 104: 618–636. [Google Scholar]
- Taylor JE, Crous PW, Wingfield MJ (1999). The genus Batcheloromyces on Proteaceae. Mycological Research 103: 1478–1484. [Google Scholar]
- Taylor JE, Denman S, Crous PW (2001). Endophytes isolated from three species of Protea in a nature reserve in the Western Cape, South Africa. Sydowia 53: 247–260. [Google Scholar]
- Tesfaendrias MT, Swart WJ, Botha W (2004). The characterization of Pythium group G occurring on kenaf in South Africa. South African Journal of Plant and Soil 21: 25–30. [Google Scholar]
- Thompson AH (1981). Phytophthora finger-rot, a post harvest disease of banana in South Africa. Phytophylactica 13: 161–163. [Google Scholar]
- Thompson AH (1985). A preliminary survey of fungal diseases of lucerne in the Republic of South Africa. Department of Agriculture and Water Supply, Technical Communication No. 197.
- Thompson AH (1987). Phytophthora root rot of lucerne in the Transvaal, South Africa. Phytophylactica 19: 319–322. [Google Scholar]
- Thompson AH (1988). Phytophthora root rot of lucerne in the Cape Province, South Africa. Phytophylactica 20: 181–182. [Google Scholar]
- Thompson AH, Botha WJ, Uys MDR (1994). Phytophthora capsici (Oomycota: Fungi), a first report from South Africa. South African Journal of Botany 6: 257–260. [Google Scholar]
- Thompson AH, Naudé SP (1992). Report of Phytophthora wilt of baby's breath (Gypsophila paniculata) from South Africa. Phytophylactica 24: 349–350. [Google Scholar]
- Thompson AH, Phillips AJL (1988). Root rot of cabbage caused by Phytophthora drechsleri. Plant Pathology 37: 297–299. [Google Scholar]
- Thompson AH, Phillips AJL, Nel E (1995). Phytophthora and Pythium associated with feeder root rot of citrus in the Transvaal Province of South Africa. Journal of Phytopathology 143: 37–41. [Google Scholar]
- Thompson AH, Westhuizen GCA van der (1979). Sclerotinia sclerotiorum (Lib.) de Bary on soybean in South Africa. Phytophylactica 11: 145–148. [Google Scholar]
- Uys V (2002). A Guide to the Termite Genera of South Africa. Plant Protection Research Institute, Agricultural Research Council, Pretoria, South Africa.
- Váczi P, Hawksworth DL (2001). Polycoccum crespoae sp. nov., the first report of a lichenicolous fungus on Chondropsis semiviridis (Parmeliaceae). Lichenologist 33: 513–517. [Google Scholar]
- Vánky K (1997). Taxonomical studies on Ustilaginales. XVII. Mycotaxon 65: 159–182. [Google Scholar]
- Vánky K (1999a). New smut fungi from South Africa. Mycotaxon 70: 17–34. [Google Scholar]
- Vánky K (1999b). Taxonomical studies on Ustilaginales. XIX. Mycotaxon 73: 135–161. [Google Scholar]
- Vánky K (2000a). New taxa of Ustilaginomycetes. Mycotaxon 74: 343–356. [Google Scholar]
- Vánky K (2000b). The smut fungi on Saccharum and related grasses. Australasian Plant Pathology 29: 155–163. [Google Scholar]
- Vánky K (2001). Taxonomical studies on Ustilaginales. XXI. Mycotaxon 78: 265–326. [Google Scholar]
- Vega FE, Blackwell M (eds) (2005). Insect-fungal associations. Ecology and evolution. Oxford University Press Inc., New York.
- Viljoen A, Wingfield MJ, Crous PW (1992). Fungal pathogens in Pinus and Eucalyptus seedling nurseries in South Africa: a review. South African Forestry Journal 161: 45–51. [Google Scholar]
- Viljoen PJ (1980). Veldtipes, verspreiding van groter soogdiere, en enkele aspekte van die ekologie van Kaokoland. M.Sc. thesis, University of Pretoria, Pretoria.
- Wager VA (1931). Diseases of plants in South Africa due to members of the Pythiaceae. Union of South Africa, Department of Agriculture, Bulletin 105: 1–43. [Google Scholar]
- Wager VA (1935). Brown rot of tomato fruits due to Phytophthora parasitica Dast. South African Journal of Science 32: 235–237. [Google Scholar]
- Wager VA (1941). Descriptions of the South African Pythiaceae with records of their occurrence. Bothalia 4: 2–35. [Google Scholar]
- Warmelo KT van, Marasas WFO, Adelaar TF, Kellerman TS, Rensburg IBJ van, Minne JA (1970). Experimental evidence that lupinosis of sheep is caused by the fungus Phomopsis leptostromiformis (Kuhn) Bubak. Journal of the South African Veterinary Medicine Association 41: 235–247. [Google Scholar]
- Westhuizen GCA van der (1972). Studies of wood-rotting fungi II. Basidiomycetes of the wood-preservation field exposure test plot at Kruisfontein. Bothalia 10: 517–538. [Google Scholar]
- Westhuizen GCA van der, Eicker A (1988). The species of Sclerotinia Fuckel occurring in South Africa. Phytophylactica 20: 109–112. [Google Scholar]
- Westhuizen GCA van der, Eicker A (1994). Field guide: Mushrooms of southern Africa. Struik Publishers, Cape Town.
- White F (1983). The Vegetation of Africa: a descriptive memoir to accompany the Unesco/AETFAT/UNSO vegetation map of Africa. Natural Resources Research, Unesco, Paris.
- Wijers EE (1937). Phytophthora wilt in carnation plants. South African Journal of Science 34: 194–213. [Google Scholar]
- Will-Wolf S, Hawksworth DL, McCune B, Rosentreter R & Sipman HJM (2004). Lichenized fungi. In: Biodiversity of Fungi: Inventory and Monitoring Methods (Mueller GM, Bills GF & Foster MS, eds). Elsevier Academic Press, Amsterdam: 173–195.
- Wilman M (1946). Preliminary checklist of the flowering plants and ferns of Griqualand West (South Africa). Deighton Bell, Cambridge.
- Wilson M, Henderson DM (1966). British rust fungi. Cambridge University Press; London.
- Wingfield MJ (1985). Reclassification of Verticicladiella based on conidial development. Transactions of the British Mycological Society 85: 81–93. [Google Scholar]
- Wingfield MJ, Marasas WFO (1980). Verticicladiella alacris sp. nov., associated with a root disease of pines in South Africa. Transactions of the British Mycological Society 75: 21–28. [Google Scholar]
- Wingfield MJ, Marasas WFO (1983). Some Verticicladiella species including V. truncata sp. nov., associated with root diseases of pine in New Zealand and South Africa. Transactions of the British Mycological Society 80: 231–236. [Google Scholar]
- Wingfield MJ, Swart WJ, Abear B (1989). First record of Cryphonectria canker of Eucalyptus in South Africa. Phytophylactica 21: 311–313. [Google Scholar]
- Wingfield MJ, Wyk PS van, Marasas WFO (1988). Ceratocystiopsis proteae sp. nov. with a new anamorph genus. Mycologia 80: 23–30. [Google Scholar]
- Wood AR (1998). Endophyllum osteospermi, a new combination for Aecidium osteospermi (Basidiomycetes–Uredinales– Pucciniaceae). South African Journal of Botany 64: 146. [Google Scholar]
- Wood AR (2002). Infection of Chrysanthemoides monilifera ssp. monilifera by the rust fungus Endophyllum osteospermi is associated with a reduction in vegetative growth and reproduction. Australasian Plant Pathology 31: 409–415. [Google Scholar]
- Wood AR (2004). Endophyllum macowanianum, a new combination for Aecidium macowanianum (Uredinales– Pucciniaceae). South African Journal of Botany 70: 667–670. [Google Scholar]
- Wood AR, Crous PW (2005). Morphological and molecular characterization of Endophyllum species on perennial asteraceous plants in South Africa. Mycological Research 109: 387–400. [DOI] [PubMed] [Google Scholar]
- Wood AR, Scholler M (2005). Uromyces euryopsidicola sp. nov., a rust species that forms witches' brooms on Euryops (Asteraceae) in South Africa. Sydowia 57: 137–143. [Google Scholar]
- Wyk AE van, Smith GF (2001). Regions of floristic Endemism in southern Africa. Umdaus Press, Pretoria.
- Yanna, Ho WH, Hyde KD (2002). Fungal succession on fronds of Phoenix hanceana in Hong Kong. Fungal Diversity 10: 185–211. [Google Scholar]
- Zhou XD, De Beer ZW, Wingfield BD, Wingfield MJ (2001). Ophiostomatoid fungi associated with three pine-infesting bark beetles in South Africa. Sydowia 53: 290–300. [Google Scholar]
- Zhou XD, De Beer ZW, Wingfield MJ (2006). DNA sequence comparisons of Ophiostoma spp., including Ophiostoma aurorae sp. nov., associated with bark beetles in South Africa. Studies in Mycology 55: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwölfer H (1979). Strategies and counterstrategies in insect population systems competing for space and food in flowerheads and plant gall. Fortschritte der Zoologie 25: 331–353. [Google Scholar]