Abstract
Morphological studies and phylogenetic analyses of DNA sequences from three genomic regions – the internal transcribed spacer (ITS) regions of the nuclear ribosomal gene repeat, a partial sequence of RNA polymerase II subunit (rpb2), and a partial sequence of translation elongation factor (tef1) – were used to investigate the systematics of Hypocrea citrina and related species. A neotype specimen is designated for H. citrina that conforms to Persoon's description of a yellow effuse fungus occurring on leaf litter. Historical information and results obtained in this study provide the foundation for selection of a lectotype specimen from Fries's herbarium for H. lactea. The results indicate that (1) Hypocrea citrina and H. pulvinata are distinct species; (2) H. lactea sensu Fries is a synonym of the older name H. citrina; (3) H. pulvinata, H. protopulvinata, and H. americana are phylogenetically distinct species that form a well-supported polyporicolous clade; (4) H. citrina is situated in a clade closely related to H. pulvinata; and (5) H. microcitrina and H. pseudostraminea reside in a highly supported clade phylogenetically distinct from H. citrina. Hypocrea protopulvinata, H. microcitrina, H. megalocitrina, H. pseudostraminea, and a new species, H. aurantiistroma, are reported and described from North America. Variation in rpb2 and tef1 gene sequences suggests geographical subgroupings between European and North American isolates of H. pulvinata. The phylogenies inferred from ITS, rpb2, and tef1 gene sequences are concordant. Hypocrea citrina var. americana is elevated to species status, Hypocrea americana.
Keywords: Ascomycetes, Hypocrea citrina, Hypocrea lactea, Hypocrea pulvinata, Hypocreales, Hypocreanum, ITS, systematics, rpb2 gene sequences, tef1 gene sequences, Subsection Citrinae, Subsection Pulvinatae, Trichoderma
INTRODUCTION
Among the most common and conspicuous members of the genus Hypocrea (Hypocreales, Hypocreaceae) are species that form extensively effused, lightly or brightly coloured stromata on leaf litter, soil, wood, and bracket fungi. Names that have been applied to these fungi include H. citrina (Pers.: Fr.) Fr., H. lactea (Fr.: Fr.) Fr., H. pulvinata Fuckel, H. fungicola P. Karst., and H. karsteniana Niessl. Confusion surrounds the application of the name H. citrina (Pers.: Fr.) Fr., and phylogenetically related species have not been determined. The stroma of H. citrina is extensively effuse, typically yellow and asci contain eight 2-celled uniseriate ascospores.
Doi (1972) subdivided Hypocrea into subgenera, sections, subsections, and series based on stroma characters. He recognized H. pulvinata, H. citrina, and H. lactea as distinct species and placed them in Hypocrea subgen. Hypocrea sect. Homalocrea Yoshim. Doi. He included H. citrina and H. lactea in subsect. Citrinae Yoshim. Doi, while H. pulvinata was placed in subsect Pulvinatae Yoshim. Doi. The anamorph of H. citrina has acremonium- or verticillium-like conidiophores with hyaline conidia. Bissett (1991a, b) assigned acremonium- or verticillium-like condiophores to Trichoderma sect. Hypocreanum Bissett. Fourteen species of Hypocrea Fr., with stromata similar to that of H. citrina, or considered as its synonym, and anamorphs assignable to Trichoderma sect. Hypocreanum are newly described or redescribed, type specimens examined, and, where possible, phylogenetic relationships to H. citrina established.
Nomenclature and typification
The name H. citrina has been applied to two different species, one polyporicolous and one lignicolous or terrestrial. Rifai & Webster (1966) considered the terrestrial/lignicolous form of H. citrina to be a synonym of another terrestrial species, H. lactea. Canham (1969) used the name H. citrina, with H. pulvinata as a synonym, for the polyporicolous form. Perspectives on these differing conclusions can be found in Rifai & Webster (1966) and Canham (1969). Application of the current International Code of Botanical Nomenclature (ICBN: Greuter et al. 2000) for the selection of type material provides the mechanism for stabilizing the application of the names, H. citrina, H. lactea, and H. pulvinata.
Persoon (1796) described a yellow terrestrial fungus on leaf litter as Sphaeria citrina Pers. Fries (1816) described Sphaeria lactea Fr. as a white effuse fungus with dark ostioles that occurs on bark and soil. Fries (1823) expanded Persoon's concept of Sphaeria citrina Pers.: Fr. to include a polyporicolous element, listing Scleromyceti Sueciae 31 (SS 31) as representative material. Fries (1849) described the genus Hypocrea (formerly a section of Sphaeria) and transferred S. citrina and S. lactea into Hypocrea. Persoon's (1796) original protologue clearly emphasized a yellow effuse terrestrial species that occurs on leaf litter. Fries's expanded species concept caused confusion. He explicitly ascribed the species, however, to Persoon in the Index to the same volume and to volume 3 of his Systema mycologicum. Dingley (1955) and Canham (1969) followed Fries's (1823) concept of H. citrina to be a fungus that occurs on polypores, even though, historically, others such as Karsten (1873), Niessl (1883) and Saccardo (1883) had recognized the fungicolous element included by Fries as taxonomically distinct from S. citrina. To account for the polyporicolous element that Fries (1823) ascribed to S. citrina, Karsten (1873) published the new name, Hypocrea citrina * fungicola P. Karst., without designating a formal rank. Niessl (1883) raised Karsten's taxon to species rank, H. karsteniana Niessl. Only months later, Saccardo (1883), apparently unaware of Niessl's work, also elevated Karsten's taxon to species rank as H. fungicola (P. Karst.) Sacc. Karsten, Niessl, and Saccardo did not recognize H. pulvinata as a synonym, although it had previously been described from a polypore (Fuckel 1870). Seaver (1910) listed H. karsteniana and H. fungicola as questionable synonyms of H. citrina. Weese (1927) synonymized H. karsteniana and H. fungicola with the oldest available name, H. pulvinata. The synonymy proposed by Weese (1927), which has never been disputed, serves as the basis for the application of the name H. pulvinata to the polyporicolous species.
There is no specimen of S. citrina in Persoon's herbarium (L), and collections that can be directly linked to Persoon cannot be located. Dingley (1955) took Fries's SS 31 (UPS), on a polypore, to be the type collection but did not formally designate the specimen as lectotype. Canham (1969) formally designated SS 31 (UPS) to be the lectotype of S. citrina. Canham (1969) described H. citrina var. americana Canham, with globose part-ascospores from polypores in North America. Fries (1823) adopted Persoon's epithet, thereby sanctioning it, and the name is therefore now cited as Hypocrea citrina (Pers.: Fr.) Fr. Article 7.8 ICBN allows the selection of neotypes among material cited by the sanctioning authority. In this case, the taxonomic circumscription of the name and its type specimen clearly comes from Persoon and Fries's species concept was confused. Fries's interpreation deviates from the protologue and thus we reject Canham's (1969) lectotypification.
Rifai & Webster (1966) rejected Dingley's (1955) [and Canham's (1969)] concept of H. citrina as being fungicolous, because it did not agree with the protologue of S. citrina. Based on the argument proposed by Rifai & Webster (1966), it is evident that they wished to preserve the distinction between the fungicolous and terrestrial/lignicolous species in order to retain the name H. pulvinata for the fungicolous species. Their argument emphasized the original protologues of H. citrina and H. pulvinata. Rifai & Webster (1966) designated Persoon's original description of S. citrina as the lectotype in contravention to Arts. 8.1 and 9.2 of the ICBN that state a lectotype must be a specimen, or, at least, an illustration. By designating a litter-inhabiting specimen as neotype, we intend to stabilize the application of the name H. citrina.
Fries (1816) described Sphaeria lactea as “... latissime effusa subcarnosa lactea nuda, ostiolis punctiformibus immersis prominulisque”. He reported that the new species grows on fallen logs and on naked soil in moist places and that the white colour does not change over time. He did not cite a specific collection or collecting locality. There are two specimens in Fries's herbarium (UPS) that can possibly be considered for neotypifying S. lactea, both of which were collected by Fries in “Småland, Femsjö”; one is identified as H. lactea and the other as Sphaeria lactea, but neither specimen has a date of collection. The specimen of H. lactea comprises an effused, yellow Hypocrea stroma that completely covers the leaf of a dicotyledonous plant. Ostiolar openings are visible throughout the specimen but mature ascospores were found only in one region. The specimen of S. lactea comprises an effused Hypocrea on the surface of what appears to be a piece of decorticated wood. The specimen is glued to a piece of stiff paper. The single stroma in this collection is mostly immature with no outward evidence of ostiolar openings or perithecia; most of it is cream or off-white in colour. Perithecial openings are visible in one small part of the stroma; this part of the stroma is darker in colour, more yellow, and the ostioles appear as minute papillae. Perithecia in this region contain asci with developing ascospores, and a few asci were found with apparently mature ascospores. It is unlikely that the specimen identified as H. lactea is part of the original gathering of this species simply because the genus Hypocrea dates from 1849.
The protologues of H. citrina and H. lactea are distinctly different, yet these taxa are now considered synonymous. According to the protologues, H. citrina is yellow and effuse and occurs on leaf litter, while H. lactea is white and effuse and occurs on soil and bark. As early as 1818, Fries's concept of S. lactea began to change as he illustrated S. lactea as a goldish white to greyish orange effuse fungus and suggested that it was closely related to H. citrina. Further confusing the issue, Doi's (1972) concept of H. lactea, which is based on Japanese material, differs from Rifai & Webster's concept of H. lactea. Doi's (1972) description and illustration of H. lactea featuring a yellow-brown stroma with distinct hyphal elements near the surface, likely represents an undescribed species from Japan. The importance that Fries (1816) and Persoon (1796) ascribed to stroma colour in the protologues of H. lactea and H. citrina must be reexamined using current methods including molecular phylogenetics. By designating the specimen in Fries's herbarium labeled S. lactea as neotype, we intend to stabilize the application of the name H. lactea and provide the basis for comparing H. lactea and H. citrina.
Species recognition
Species limits in Hypocrea have been historically based on morphology. Phylogenetic data and genealogical concordance phylogenetic species recognition (GCPSR: Taylor et al. 2000) are now being employed to define species and for systematic studies of Hypocrea (Chaverri et al. 2001, 2003a). GCPSR utilizes the concordance of multiple gene genealogies and the transition from concordance among branches to incongruity among branches to establish species limits (Taylor et al. 2000). Molecular data from RNA polymerase II subunit (rpb2), translation elongation factor 1-alpha (tef1), and ITS1-5.8S-ITS2 (ITS) rDNA, have been used to make crucial anamorph–teleomorph connections in Hypocrea, to establish anamorph relationships at the sectional level in Trichoderma, and to place genera previously described in other pyrenomycete orders within the Hypocreales (Chaverri et al. 2003b, Dodd et al. 2002, Kullnig-Gradinger et al. 2002, Zhang & Blackwell 2002). Data from these gene regions and morphological analyses will be used to address the following objectives: (1) the typification and redescription of H. citrina sensu Persoon and H. lactea sensu Fries using strict interpretations of the protologues for typification; (2) the determination of species related to Hypocrea citrina using GCPSR; (3) the morphological description of phylogenetic species identified in this study; and (4) a discussion of several species treated by Doi (1972) in Hypocrea subsect. Citrinae and subsect. Pulvinatae.
MATERIALS AND METHODS
Collections and isolates
Species concepts, illustrations, and descriptions from Doi (1972) were used in making identifications except for H. lactea and H. fulva. Table 1 lists the isolates used in this study. Frequently cited collectors are abbreviated, B.E. Overton (B.E.O.) and G. J. Samuels (G.J.S.). All isolates with G.J.S. designations were obtained by isolating single ascospores on CMD with the aid of a micromanipulator. All isolates with B.E.O. designations were obtained from plating the entire contents of individual perithecia. Unless otherwise annotated, host and substrate information were obtained from herbarium labels, and data were entered in specimens examined as it appears on the labels.
Table 1.
Isolates used in molecular phylogenetic analyses. (* = ex-type strain)
| Name | Isolate accession number | Origin |
GenBank accession number
|
||
|---|---|---|---|---|---|
| ITS | tef1 | rpb2 | |||
| H. pulvinata Fuckel | G.J.S. 94-20 | Tushar Mountains, Utah, U.S.A. | DQ835409 | DQ835433 | DQ835451 |
| G.J.S. 92-127 | Olympia National Park, Washington, U.S.A. | AF487666 | DQ835429 | DQ835461 | |
| G.J.S. 98-104 | Naturpark Saar-Hundsrück, Germany | AF487665 | DQ835430 | AF545559 | |
| G.J.S. 95-220 | Waldviertel, Lower Austria | DQ835407 | DQ835431 | DQ835452 | |
| H. americana (Canham) Overton | G.J.S. 92-93 | New Mexico, U.S.A. | DQ835410 | DQ835434 | DQ835455 |
| G.J.S. 94-79 | White Mountains, Arizona, U.S.A. | DQ835408 | DQ835432 | DQ835456 | |
| H. protopulvinata Yoshim. Doi | K. Põldmaa 00-56 | Unknown, U.S.A. | DQ835406 | DQ835428 | DQ835453 |
| CBS 739.83* | Chiba Pref., Kiyosumi, Fudagou, Japan | DQ835405 | DQ835427 | DQ835463 | |
| H. citrina (Pers. : Fr.) Fr. | G.J.S. 95-183 | Daniel Boone National Forest, Kentucky, U.S.A. | DQ835413 | DQ835437 | DQ835458 |
| B.E.O. 99-29 | Oswego County, New York, U.S.A. | DQ835412 | DQ835436 | Dq835464 | |
| G.J.S. 89-145 | Devon, Budleigh, Saltaton, England, U.K. | DQ835414 | DQ835438 | DQ835457 | |
| G.J.S. 96-275 | Ascutung, Vermont, U.S.A. | DQ835418 | DQ835442 | - | |
| CBS 708.73 | Baarn, Zandheuvelweg, Netherlands | DQ835415 | DQ835439 | - | |
| CBS 853.70 | Pelmer Wald near Gerolstein, Germany | DQ835416 | DQ835440 | - | |
| CBS 894.85* | Hestreux near Eupen, Belgium | DQ835417 | DQ835441 | AF545561 | |
| H. pseudostraminea Yoshim. Doi | G.J.S. 91-135 | Prince Georges County, Maryland, U.S.A. | DQ835419 | DQ835443 | - |
| G.J.S. 90-74 | Dutches County, New York, U.S.A. | DQ835420 | DQ835444 | DQ835454 | |
| G.J.S. 95-169 | Daniel Boon National Forest, Kentucky, U.S.A. | DQ835421 | DQ835445 | - | |
| G.J.S. 95-189 | Brown County, Indiana, U.S.A. | DQ835422 | DQ835446 | DQ835459 | |
| B.E.O. 99-36 | Patapsco State Park, Maryland, U.S.A. | DQ835423 | DQ835447 | DQ835465 | |
| H. megalocitrina Yoshim. Doi | B.E.O. 00-09 | North Carolina, U.S.A. | DQ835511 | - | AF545563 |
| H. microcitrina Yoshim. Doi | G.J.S. 97-248 | Chattahoochee National Forest, Georgia, U.S.A. | DQ835424 | DQ835449 | DQ835462 |
| G.J.S. 91-61 | Mt. Lake Biological Station, Virginia, U.S.A. | DQ835426 | DQ835450 | DQ835460 | |
| H. sulphurea (Schw.) Sacc. | G.J.S. 95-190 | Brown County, Indiana, U.S.A. | DQ835425 | DQ835448 | AF545560 |
Molecular phylogenetic analysis
DNA sequence analysis of the isolates was conducted using three gene sequences: ITS1-5.8S-ITS2 (ITS), a partial sequence of translation elongation factor (tef1), delimited by the primer pair ef1/2, and a partial sequence of RNA polymerase II subunit (rpb2) delimited by the primer pair frpb2-5f/-7cr. Mycelia were lyophilized prior to DNA extraction. The lyophilization protocol is described in Stewart et al. (1999). Extraction of genomic DNA was carried out using the phenol and chloroform extraction outlined in Stewart et al. (1999). A 50 μL polymerase chain reaction (PCR) for ITS and tef1 was performed following the conditions outlined by Chaverri et al. (2001) using the following primer pairs: for ITS, the primers utilized were ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') (White et al. 1990); for tef1, the primers were ef-1 (5'-ATGGGTAAGGA(A/G)GACAAGAC-3') and ef-2 (5'-GGA(G/A)GTACCAGT(G/C)ATCATGTT-3') (O'Donnell et al. 1998). A 50 μL polymerase chain reaction (PCR) following the conditions outlined by Chaverri et al. (2003b) was utilized for generating a partial rpb2 product. The following two fungus-specific primers were employed: frpb2-5f (5'-GA(T/C)GA(T/C)(A/C)G(A/T)GATCA(T/C)TT(T/C)GG -3') and frpb2-7cr(5'-CCCAT(A/G)GCTTG(T/C)TT(A/G)CCCAT-3') (Liu et al. 1999). Two percent dimethyl sulfoxide (DMSO) from AMRESCO® was added to each 50 μL PCR reaction. A PCR purification was carried out using a QIAquick® PCR purification kit. The ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction Kit with Amplitaq DNA Polymerase FS (PE Applied Biosystems) was utilized for sequencing cleaned PCR products for each primer direction. Performa® DTR gel filtration cartridges from Edge BioSystems were used for cleaning cycle sequencing products. Sequencing was either conducted on an ABI 377 located in the Plant Pathology Department at The Pennsylvania State University or sent to the Nucleic Acid Facility (Life Science Consortium, The Pennsylvania State University). Sequence data were trimmed at the 5' and 3' ends. The ITS region was sequenced for all isolates and used to screen for replicate isolates. Replicate isolates were removed from subsequent analyses if there were no obvious morphological or locality differences noted from the herbarium specimens. Sequences were assembled using SeqMan® II option and aligned using Clustal W in DNA Star (DNA Star Inc., Madison, Wisconsin), and a phylogenetic analysis was performed using PAUP* v. 4.0b8 (Sinauer Associates, Sunderland, MA). Alignments were manually adjusted in PAUP*. Overton et al. (1999) determined H. sulphurea to be a phylogenetically appropriate outgroup taxon for H. citrina and allies and this species was used as outgroup in all data sets. Maximum parsimony (MP) analyses were completed using the heuristic search option with starting trees obtained via random addition sequence (10 replicates) and TBR branch swapping. Gaps (insertions/deletions) were treated as missing data. Bootstrap analysis was performed in 500 replicates with random sequence addition (10 replicates). The sequences and alignments were deposited in GenBank (Table 1).
Determination of optimal growth temperature
A growth trial using multiple isolates from each phylogenetic species established in the species tree (Fig. 1) was carried out using 20 mL of three media: potato-dextrose agar (PDA, Difco), cornmeal-dextrose agar (CMD, Difco), and SNA with filter paper (Nirenberg 1976). Inoculum was prepared on CMD. A 1-cm-diam disk from the actively growing inoculum colony was placed near the edge of the plate. One plate of each medium for each culture was incubated at 20, 25, 30, and 35 °C in total darkness. The radius of the colony was measured from the edge of the inoculum at 24-h intervals for 4 d. The experiment was replicated three times during subsequent weeks. Optimal growth temperatures are summarized in the species descriptions.
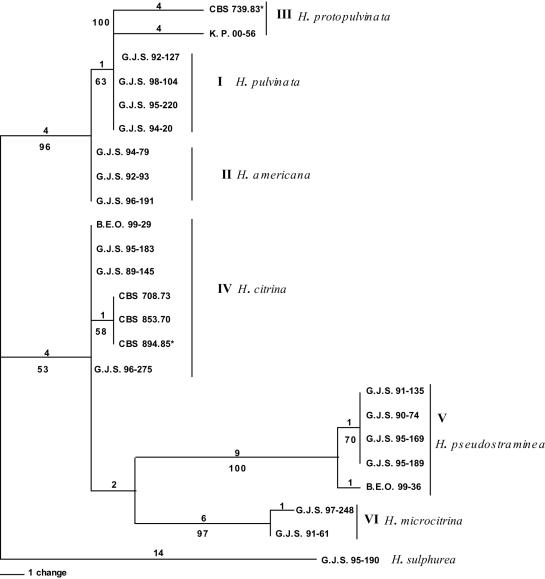
Fig. 1.
Parsimony analysis of ITS1-5.8S-ITS2. One phylogram of two most parsimonious trees; 48 steps; consistency index: 0.854; retention index; 0.989; homoplasy index: 0.146; numerical values of branch lengths are given above and bootstrap values (500 replicates with 10 random addition replications) are indicated below branches. Outgroup taxon: H. sulphurea.
Morphological descriptions
Anamorph and teleomorph characteristics were measured from isolates and specimens representative of each phylogenetic species distinguished in Fig. 1. Cultures of Hypocrea were grown on PDA, CMD and SNA at 20 °C, with 12 h fluorescent light and 12 h darkness to observe and measure microscopic characters of the anamorph. Observations were made at ∼7–10 d. Observations were discontinued for cultures that did not produce conidiophores after 10 d on each respective medium. Anamorph characters measured were phialide length, width of the phialides at the middle, and conidium length and width. Phialide arrangement, number of phialides in a whorl and colony characteristics were recorded. Herbarium specimens were rehydrated in 3 % KOH for sectioning. Rehydrated stromata were supported by Tissue-Tek O.C.T. Compound 4583 (Miles Inc., Elkhart, Indiana) and sectioned at a thickness of approximately 15 μm with a freezing microtome. Teleomorph characters measured were ascus length, ascus width, ascospore proximal length and width, ascospore distal length and width, perithecial length and width, and stroma length and width. Stroma colour, including colour reactions of stroma tissue in response to 3 % KOH and lactic acid, were also recorded. Anatomical characters of the stroma were described and photographed. Measurements of continuous characters were made using the image-capturing software Scion Image beta 4.0.2 (Scion Corporation, Frederick, Maryland). Descriptive statistics of micromorphological characters were made based on 30 measurements per specimen, except where noted, with confidence intervals (a = 0.05), and minimum and maximum values reported. Colour terminology was obtained from Kornerup & Wanscher (1981). Anamorph and teleomorph characters are included in the species descriptions. Important morphological characters used in species recognition are discussed in the comments section immediately following each species description.
RESULTS
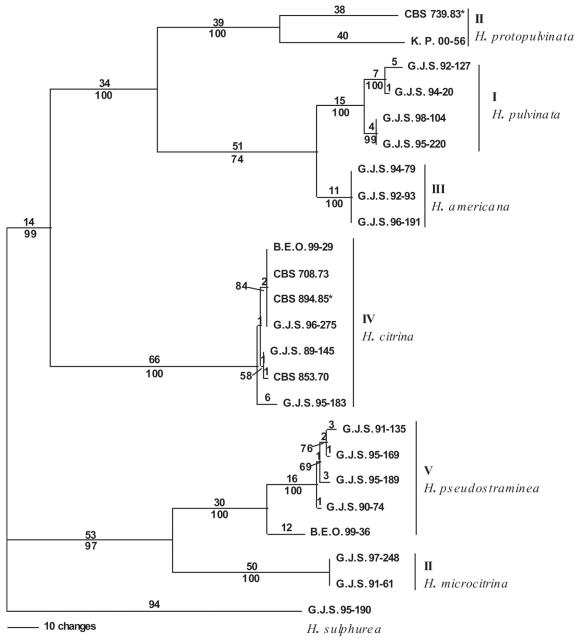
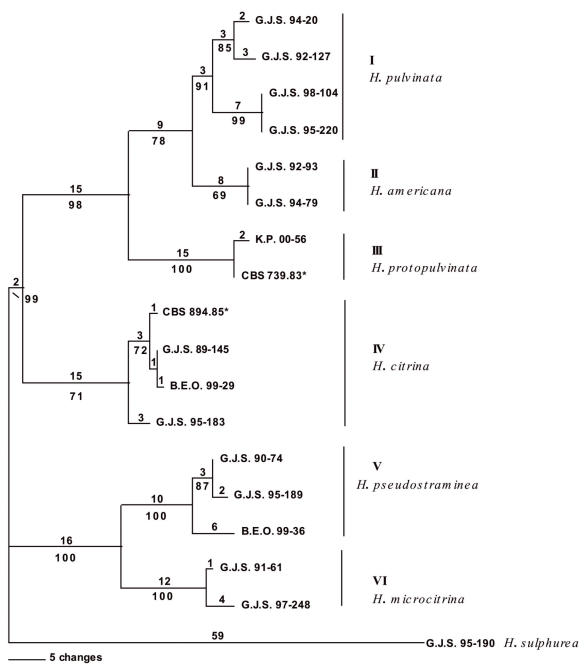
The trees generated from ITS are slightly different from those obtained from tef1 and rpb2 (Figs 1, 2, 3). In the ITS gene tree, the proposed neotype of H. citrina from Europe (CBS 894.85) differed from North American isolates of H. citrina in a single nucleotide. The H. citrina isolate G.J.S. 95-183 is identical to other isolates from North America in the ITS tree. The ITS result differed from the tef1 gene tree in which the European CBS 894.85 was identical with the North American isolate B.E.O. 99-29 (Fig. 2) and from the tef1 and rpb2 gene trees, in which isolate G.J.S. 95-183 had a longer branch length than other isolates of H. citrina (Figs 2, 3). Parsimony analysis of the ITS sequences showed a single nucleotide polymorphism separating isolates of H. pulvinata from H. americana supported by a bootstrap score of 63 % (Fig. 1). The tef1 and rpb2 gene trees were more pronounced in placing H. citrina in a highly supported monophyletic group with H. pulvinata, H. protopulvinata, and H. americana with bootstrap scores of 99 % for both trees.
Fig. 2.
Parsimony analysis of partial sequences of tef1. One phylogram of six most parsimonious trees; 476 steps; consistency index: 0.826; retention index: 0.769; homoplasy index: 0.174. Rest as in Fig. 1.
Fig. 3.
Parsimony analysis of partial sequences of rpb2. One phylogram of two most parsimonious trees; 172 steps; consistency index: 0.872; retention index: 0.914; homoplasy index: 0.128. Rest as in Fig. 1.
All three data sets are concordant with similar homoplasy indices. The heuristic search of the most parsimonious tree for the ITS dataset yielded two trees with 48 steps. The minimal possible tree length is 41 (Fig. 1). From a total of 523 characters, 484 characters are constant, 11 variable characters are parsimony-uninformative and 28 characters are parsimony-informative. The heuristic search of the most parsimonious trees for the tef1 dataset yielded six trees with 476 steps with the minimum possible tree length of 393 (Fig. 2). From 695 total characters, 392 characters are constant, 61 variable characters are parsimony-uninformative, and 242 characters are parsimony-informative. The heuristic search of the most parsimonious trees for the rpb2 dataset yielded two trees with 172 steps with the minimum possible tree length of 150 (Fig. 3). From 969 total characters, 833 characters are constant, 56 variable characters are parsimony-uninformative, and 80 characters are parsimony-informative.
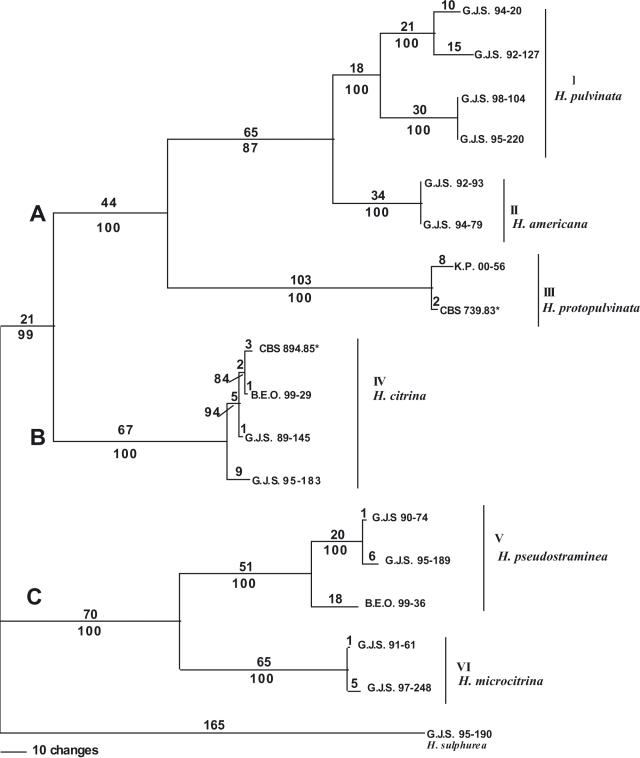
The combined phylogenetic analysis using ITS, partial sequences of tef1 and rpb2 distinguished H. americana from H. pulvinata with a bootstrap score of 100 % (Fig. 4). European and North American isolates of H. pulvinata formed two distinct subclades, each supported by a bootstrap score of 100 %. Hypocrea pulvinata and H. americana formed a monophyletic group supported by a bootstrap score of 87 % (I, II). Hypocrea americana, H. pulvinata, and H. protopulvinata (III) formed a strongly supported monophyletic group with a bootstrap score of 100 % (Clade A). Isolates of H. citrina (including the ex-neotype (IV)) formed a strongly supported monophyletic group with a bootstrap score of 100 % (Clade B). Hypocrea microcitrina (VI) and H. pseudostraminea (V) are situated in an unresolved sister clade to H. citrina supported by a bootstrap score of 100 % (Clade C). Isolate B.E.O. 99-36 is divergent from other isolates of H. pseudostraminea; nevertheless, all isolates of H. pseudostraminea formed a strongly supported monophyletic group (V) with a bootstrap score of 100 %. Isolates of H. microcitrina (VI) formed a strongly supported clade with a bootstrap score of 100 %. The heuristic search of the most parsimonious trees yielded one tree with 678 steps with the minimum possible tree length of 575 (Fig. 4). From 2189 total characters, 1710 characters are constant, 133 variable characters are parsimony-uninformative, and 346 characters are parsimony-informative.
Fig. 4.
Combined parsimony analysis of ITS, tef1, rpb2. Phylogram of single most parsimonious tree; 678 steps; consistency index: 0.848; retention index: 0.915; homoplasy index: 0.152. Rest as in Fig. 1.
Overall, ITS sequences are less variable than the tef1 and rpb2 sequences. The tef1 and rpb2 regions distinguished between North American and European isolates of H. pulvinata, whereas ITS did not. None of the gene regions consistently resolved North American and European isolates of H. citrina. Hypocrea citrina isolate G.J.S. 95-183 grouped with North American and European isolates of H. citrina in the ITS tree, but had a significantly longer branch in the tef1 and rpb2 trees. This represents a point of discordance that establishes a phylogenetic species boundary for H. citrina. The highest bootstrap scores for isolates of H. citrina were obtained in all three datasets at the base of Clade B.
DISCUSSION
Phylogenetic analysis and establishment of major clades
Despite some variation between the ITSl, tef1, and rpb2 gene trees, it can be concluded that H. citrina is phylogenetically distinct from H. pulvinata. In the combined analysis, Clade A, consisting of H. pulvinata, H. americana, and H. protopulvinata, is monophyletic; Clade B consists of all H. citrina isolates; and Clade C consists of H. pseudostraminea and H. microcitrina (Fig. 4). The H. microcitrina and H. pseudostraminea subclades are separated by long branches (Fig. 4). It is possible, given the large branch length differences, that long-branch attraction may be artificially inflating the bootstrap score uniting these taxa. Future phylogenetic studies that should include H. protocitrina, H. albocitrina, and H. lactea sensu Doi may help to more clearly elucidate the relationship of H. pseudostraminea and H. microcitrina to H. citrina.
Sequences from H. pulvinata and H. americana produced highly concordant gene trees, consistently generating the same subgroupings. ITS data from 3 out of 4 isolates of H. citrina from Europe differed from North American isolates in a single nucleotide. The ex-neotype culture of H. citrina (CBS 894.85) was identical with B.E.O. 99-29 in the tef1 gene tree, but they differed in the rpb2 tree. Furthermore, H. citrina isolate G.J.S. 95-183, which is invariant in the ITS tree from other North American isolates, had significant branch length differences in the tef1 and rpb2 gene trees from all other H. citrina isolates. The differences between isolates of H. citrina can best be explained by sexual recombination. Therefore, the species boundary for H. citrina was determined to be at the point of discordance separating clade B in the gene trees (Fig. 4). Isolates of H. pulvinata show large numbers of nucleotide changes between North American and European isolates in the rpb2 and tef1 data sets. The differences between North American and European isolates in the tef1 and rpb2 may represent cryptic species or simply variations in population structure. North American and European isolates of H. pulvinata are morphologically indistinguishable, and the ITS sequences are identical. Additional sequence analysis and morphological examination of European isolates will be required to determine whether European isolates represent a distinct species. In the ITS gene tree for H. americana, a single nucleotide change separates isolates of H. americana from H. pulvinata, while there are larger differences in the tef1 and rpb2 gene trees between these species.
The optimal temperature for growth was determined for each phylogenetic species. Optimal temperature for growth is reported only when multiple strains with branch length differences within a phylogenetic species could be sampled in order to determine the intra-species variation. Growth temperature optima could not adequately distinguish between any of the phylogenetic species nor were they variable within a phylogenetic species in which strain differences had been established based on branch length differences.
Phylogenetics and classification
The monophyly of many of the infrageneric groupings proposed by Doi (1972) have yet to be tested by DNA sequence analysis. In this study, it was found that subsections Citrinae and Pulvinatae as defined by Doi are not monophyletic assemblages of species. Hypocrea microcitrina and H. megalocitrina (Chaverri et al. 2003b) are not situated in the H. citrina clade. It is more difficult to determine the phylogenetic boundaries of Subsection Pulvinatae because Doi broke it up into several series from which isolates could not be sequenced.
Hypocrea microcitrina, H. pseudostraminea and H. lactea sensu Doi have similar morphological characters in the form of hyphal protrusions and textura intricata near the stroma surface. It is difficult to distinguish between hyphal protuberances and textura intricata. It is unclear at this time if the hyphal protrusions near the surface of the stromata and the textura intricata found in H. pseudostraminea and H. microcitrina are homologous characters, but both of these taxa have similar irregular verticillium-like anamorphs. Doi (1972) classified H. pseudostraminea in subsect. Pulvinatae, series Splendentes, and H. microcitrina in subsect. Citrinae. The results generated here suggest that the subgroupings proposed by Doi (1972) are artificial (Table 2).
Table 2.
Phylogenetic clades and morphological characters.
| Species | Clade | Ascospore ornamentation | Stroma anatomy (textura) | Hyphal protuberances above stroma surface | Anamorph and conidial shape | Infrageneric classification (Doi 1972) in Hypocrea subgenus Hypocrea Section Homalocrea |
|---|---|---|---|---|---|---|
| H. pulvinata | A | smooth to minutely spinulose | t. globulosa to t. angularis with vertically parallel t. intricata (compact) | warted hyphae | acremonium-like subglobose—subellipsoidal | Subsection Pulvinatae series Pulvinatae |
| H. americana | A | smooth | t. globulosa to t. angularis with vertically parallel t. intricata (compact) | warted hyphae | acremonium-like subglobose—subellipsoidal | Subsection Pulvinatae series Pulvinatae |
| H. protopulvinata | A | smooth | t. globulosa with vertically parallel t. intricata (not compact) | roughened hyphae | acremonium-like subglobose—subellipsoidal | Subsection Pulvinatae series Pulvinatae |
| H. platypulvinata | likely clade A | smooth to minutely spinulose | t. globulosa to t. angularis with vertically parallel t. intricata (compact) | warted hyphae | acremonium-like subglobose—cylindrical | Subsection Pulvinatae series Pulvinatae |
| H. citrina | B | minutely spinulose | t. globulosa to t. angularis with some vertically parallel t. intricata (compact) | none | verticillium-like subglobose—subellipsoidal | Subsection Citrinae |
| H. pseudostraminea | C | minutely spinulose | vertically parallel t. intricata (compact) with thin layer of t. globulosa to t. angularis near stroma surface | roughened hyphae | verticillium-like subellipsoidal—elongate ellipsoidal | Subsection Pulvinatae Series Splendentes |
| H. microcitrina | C | minutely spinulose | vertically parallel t. intricata (compact to loosely woven) | t. intricata2 | verticillium-like subellipsoidal—elongate ellipsoidal | Subsection Citrinae |
| H. lactea sensu Doi | likely clade C | spinulose | vertically parallel t. intricata (compact to loosely woven) | t. intricata roughened near stroma surface | — | Subsection Citrinae |
| H. megalocitrina | Psychrophila1 clade | nodulose-warted | vertically parallel t. intricata (compact to loosely woven) | t. intricata2 | verticillium-like subglobose—ellipsoidal, sometimes elongate ellipsoidal | Subsection Citrinae |
| H. psychrophila | psychrophila1 clade | nodulose-warted | thick-walled cells (compact), t. angularis | none | gliocladium-like ovoid to subellipsoidal | Not included by Doi |
| H. fulva | likely psychrophila clade | nodulose-warted | vertically parallel t. intricata (compact) | none (thin layer of compact cells at surface) | — | Subsection Citrinae |
| H. mikurajimensis | likely psychrophila clade | nodulose-warted | thick-walled cells (compact) | none | verticillium-like ovoid to subellipsoidal | Subsection Pulvinatae series Splendentes |
| H. aurantiistroma | likely psychrophila clade | minutely nodulose | vertically parallel t. intricata (loose) | t. intricata2 | — | — |
The psychrophila clade is supported by a bootstrap score of 93 % based on combined parsimony analysis of rpb2 and tef1 (Chaverri et al. 2003b, fig. 7).
It is difficult to distinguish between hyphal protuberances and t. intricata near the stroma surface because t. intricata does not form a well-defined margin at the stroma surface resulting in hyphal protuberances of varying lengths.
At this time, it is unclear how H. albocitrina and H. protocitrina are related to H. citrina, as cultures are not available for sequencing. The overall macromorphology of H. albocitrina and the stroma tissue of H. protocitrina consisting of textura intricata suggest that these taxa are more similar to H. microcitrina and H. pseudostraminea. Hypocrea megalocitrina has been sequenced and is not situated in the same clade as H. citrina (Chaverri et al. 2003b). Molecular data generated by Chaverri et al. (2003b) place H. megalocitrina near a cold-loving species described by Müller et al. (1972) as H. psychrophila. Doi (2001) described a species from Japan, H. mikurajimensis Yoshim. Doi that has characters similar to those of H. fulva, H. megalocitrina, H. aurantiistroma, and H. psychrophila. The strongly warted ascospores, stroma anatomy, and yellow pulvinate stromata of H. mikurajimensis are similar to the characteristics described by Müller et al. (1972) for H. psychrophila, which has a white gliocladium-like anamorph. The nodulose ascospores of H. mikurajimensis, longitudinal elongate ostiolar canals, and regularly verticillate conidiophores are similar to characters found in H. megalocitrina, H. aurantiistroma, and H. fulva. The warted, thick-walled ascospores and long ostiolar canals of Hypocrea psychrophila and H. megalocitrina are the morphological characters uniting these taxa.
Doi (2001) classified H. mikurajimensis in the H. patella group of subsection Pulvinatae, series Splendentes, which has been shown to be paraphyletic (Dodd et al. 2002). The exact phylogenetic position of this species in relation to all other taxa included by Doi (1972) in Series Splendentes cannot be determined at this time. However, Chaverri et al. (2003b) have shown that H. pezizoides Berk. & Broome, also series Splendentes, is situated in the H. rufa clade. These authors also showed that H. megalocitrina and H. psychrophila are phylogenetically related. Based on characters of the teleomorph and anamorph mentioned above, it is possible that H. megalocitrina, H. fulva, H. mikurajimensis, H. aurantiistroma, and H. psychrophila are phylogenetically related, but more taxa have to be sequenced to establish the limits of the “psychrophila” clade.
Typification and nomenclatural conclusions
With the selection of a specimen that agrees with the protologue of S. citrina (Gams 4031, CBS 894.85) as neotype (herein designated), the concept of the species can be firmly attached to a litter-inhabiting fungus. It is clear from the molecular and morphological data that H. pulvinata (= H. citrina var. citrina sensu Canham) and H. citrina, as neotypified here, are not synonyms and that Fries (1823) and later Canham (1969) made a taxonomic error in expanding the species concept of S. citrina to include the polyporicolous element. Hypocrea citrina var. americana is elevated to species status, H. americana, based on morphological characters and a well-delimited phylogenetic species boundary.
Molecular data were compared from many different specimens of H. citrina collected in different geographic locations with variations in stroma colour and substrate. It was determined that the protologue was too narrowly defined for H. citrina. Specimens BPI 1107145, G.J.S. 89-145 and CBS 853.70 (Fig. 5C), which are white to light yellow and occur on soil, were found to be phylogenetically the same as the neotype of H. citrina, which is bright yellow and occurs on leaf litter (Fig. 6). A lemon-yellow specimen, B.E.O. 99-29, on the moss Drepanocladus uncinatus (Hedw.) Warnst., was also found to be the same as the neotype based on molecular results. These findings show that stroma colour and substratum are variable characters, a finding not suggested by the original protologue of S. citrina. Hypocrea citrina was never observed growing on polypores in this study.
Fig. 5.
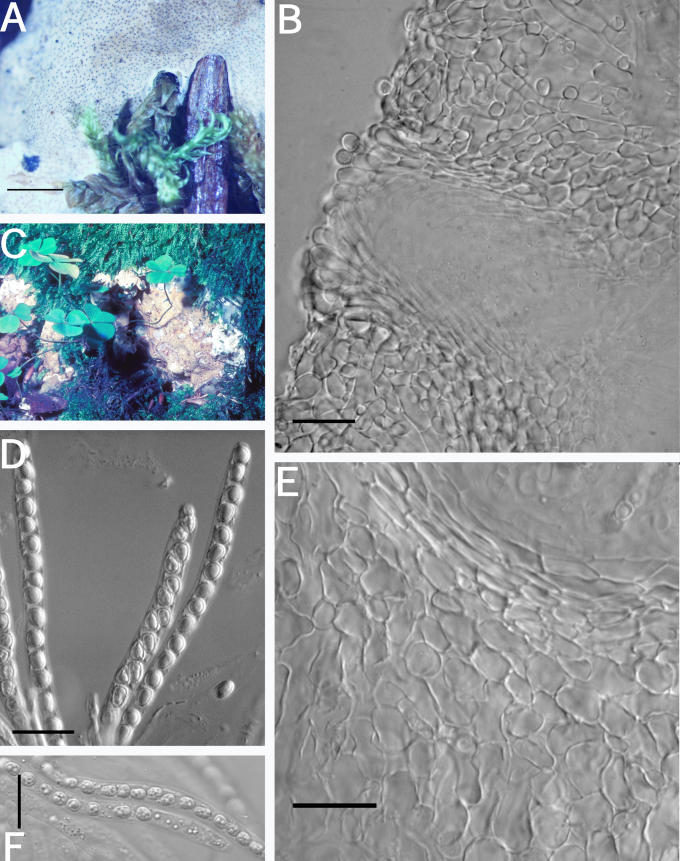
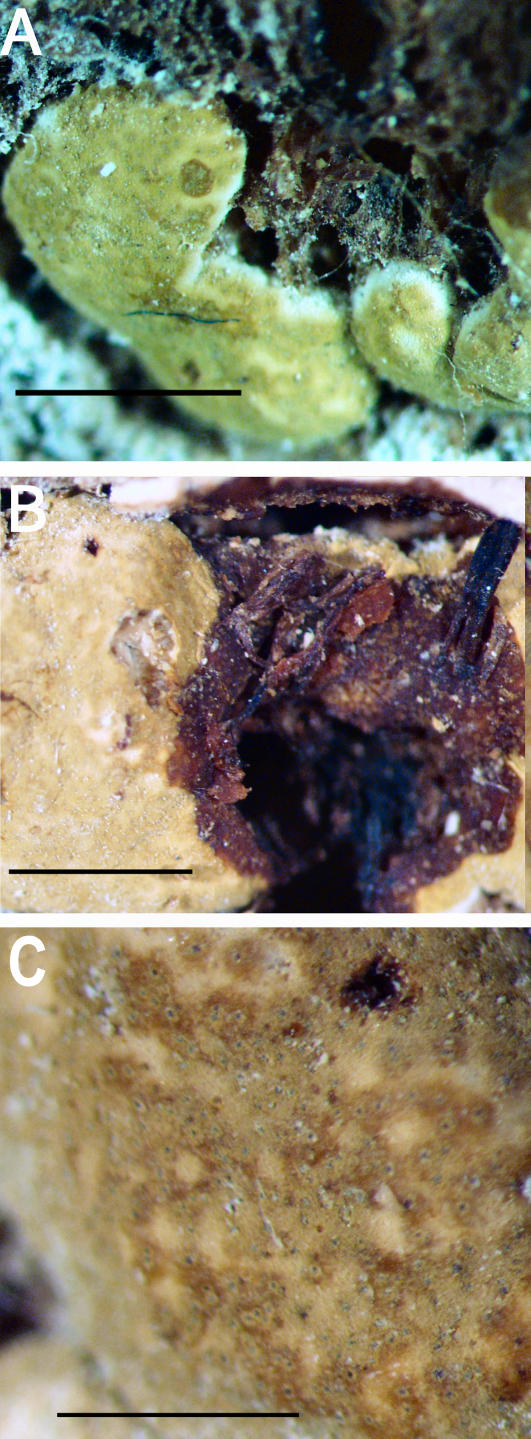
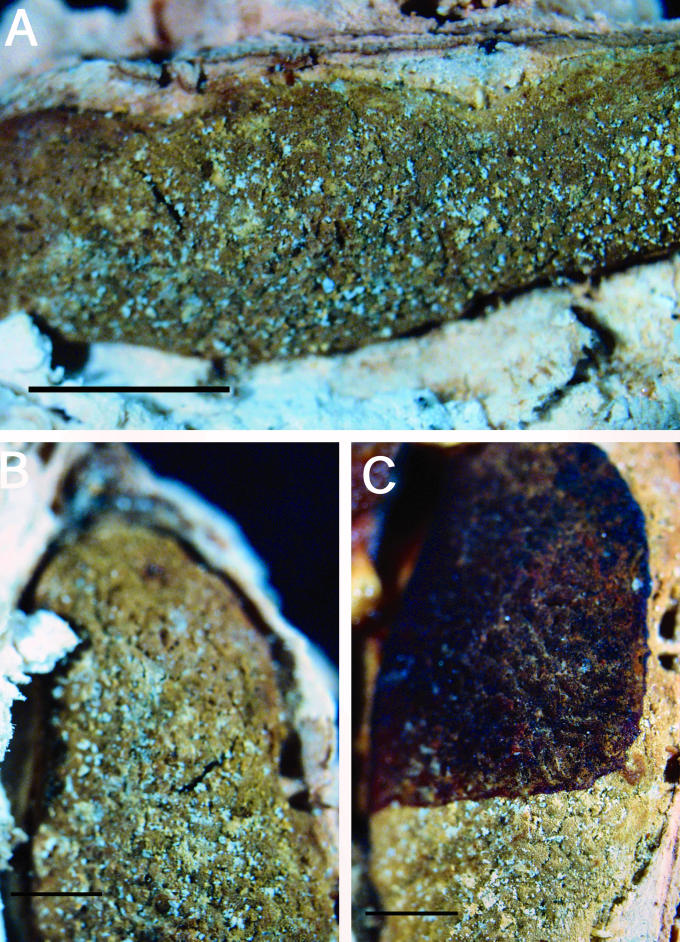
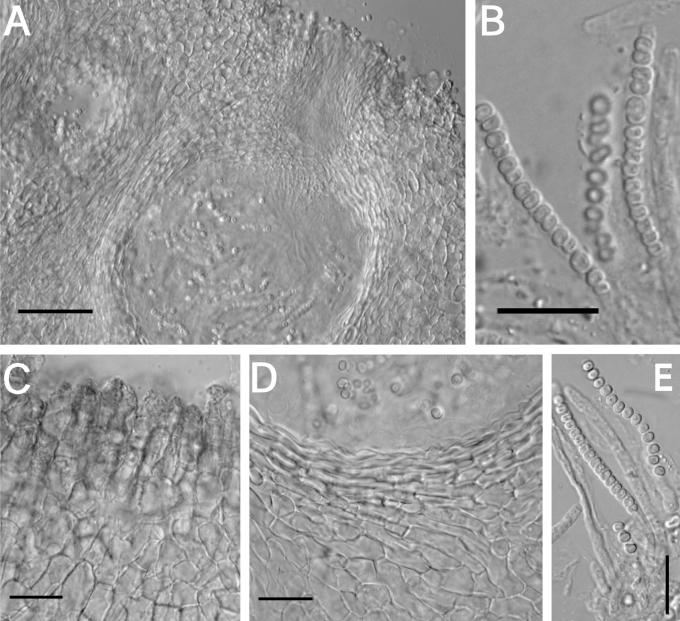
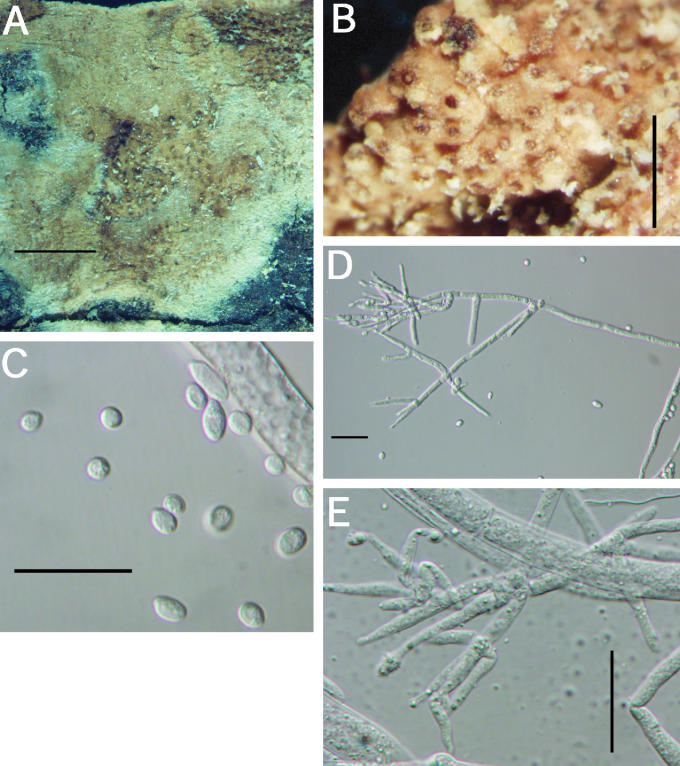
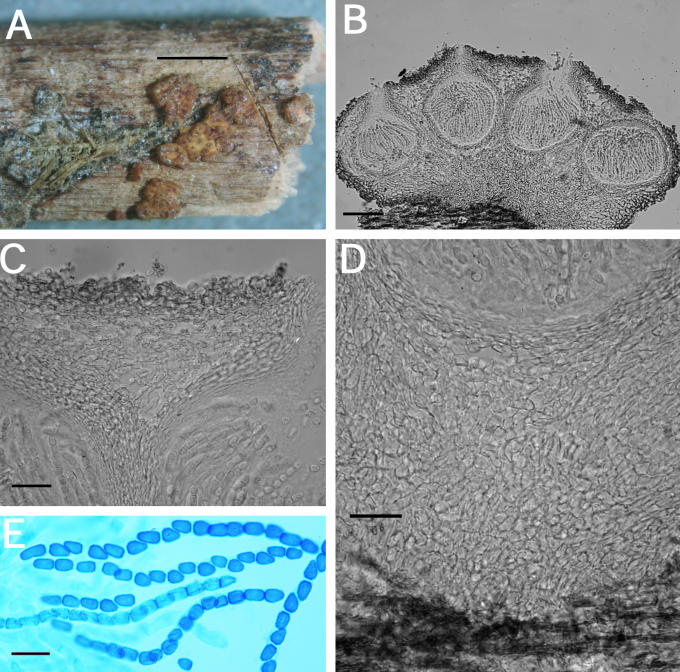
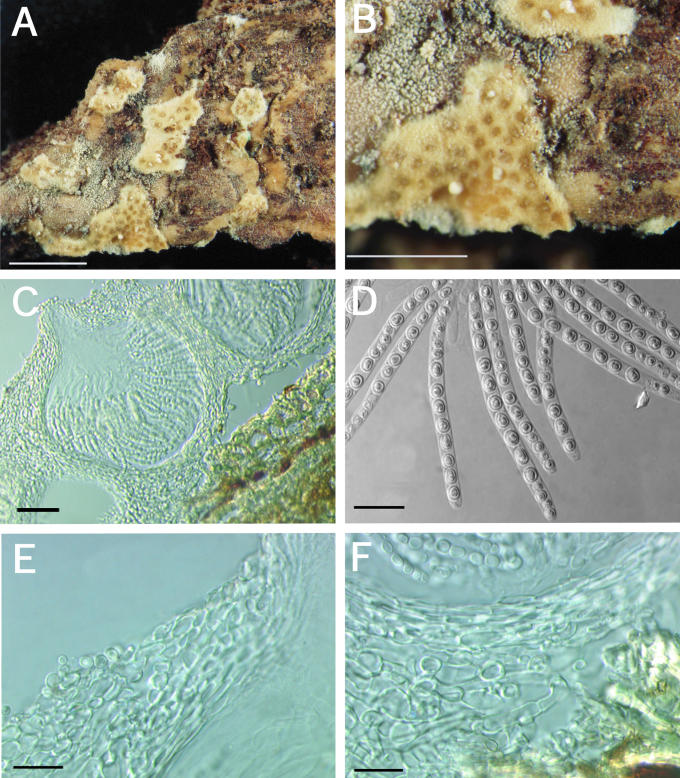
A–F. Hypocrea citrina. A. Macromorphology of B.E.O. 99-29; bar = 2 mm. B. Stromatal section showing t. globulosa to t. angularis tissues intermixed with t. intricata in BPI 737759; bar = 20 μm. C. Stroma variable in colour, white to yellow, CBS 853.70 isolated from specimen (photo courtesy of W. Gams). D. Asci with ascospores of neotype CBS 4031; bar = 20 μm. E. Stromatal cross section showing t. globulosa with angular edges due to mutual compaction in BPI 748251; bar = 20 μm. F. Immature asci and ascospores of BPI 748251; bar = 20 μm.
Fig. 6.
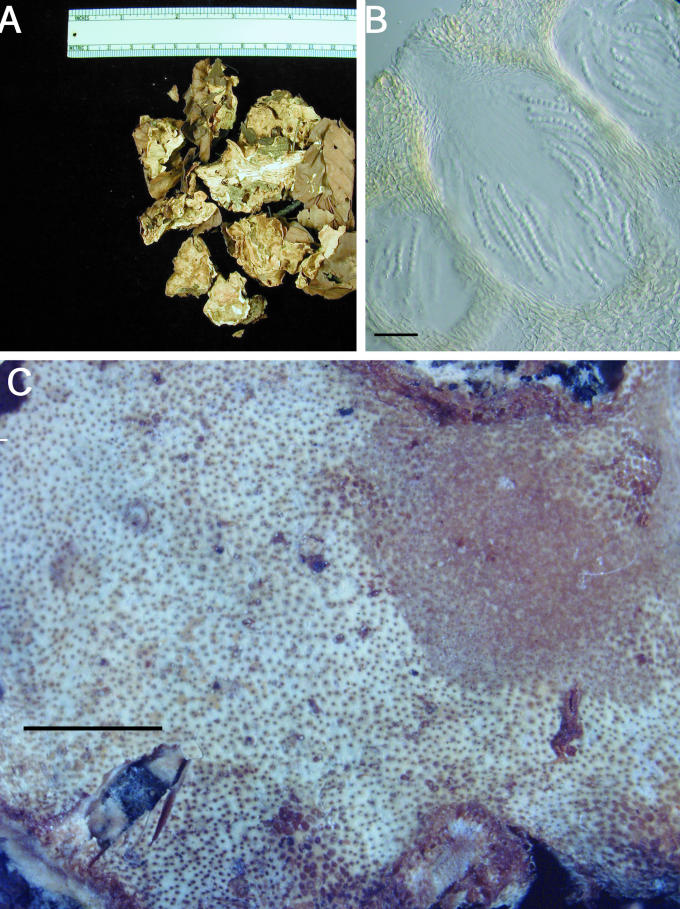
A–C. Hypocrea citrina. A. Neotype specimen Gams 4031, CBS 894.85 on leaf litter. B. Stromatal section of BPI 744475 showing textura angularis below the perithecium and near the stroma surface; bar = 40 μm. C. Variable KOH reaction usually not greater than observed here in BPI 744475; bar = 2 mm.
The only two available historical specimens in Fries's herbarium (UPS) for H. lactea are not consistent with the protologue in that they are not white, as emphasized in the original protologue of H. lactea. Recommendations 9A.2 and 9A.3 of the ICBN clearly indicate that historical specimens should not automatically be taken as type material if they are discordant with the protologue. However, Fries's species concept of H. lactea changed from the white form described in his protologue (1816) to include a whitish gold to greyish orange form that Fries illustrated (1818) with a colour lithograph two years after the original description and sanctioned (Fries 1823). The illustrated specimen has almost exactly the same shape and colour as the specimen of S. lactea considered here as the lectotype specimen (Fig. 7). Taxonomically, both specimens of H. lactea in Fries's herbarium can be identified as the yellow form of H. citrina based on morphological characters. Molecular data have shown that H. citrina could in fact be white and could occur on soil just as Fries described in the protologue for H. lactea. The neotype of H. citrina is consistent in characteristics defined by Fries for H. lactea and is consistent with Rifai & Webster's (1966) conclusion that H. citrina and H. lactea are synonymous.
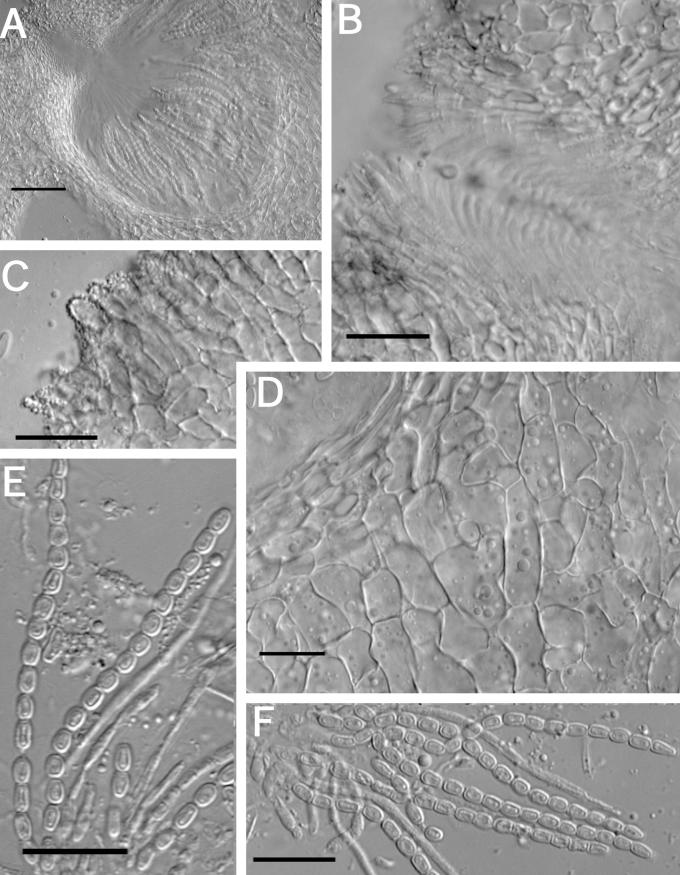
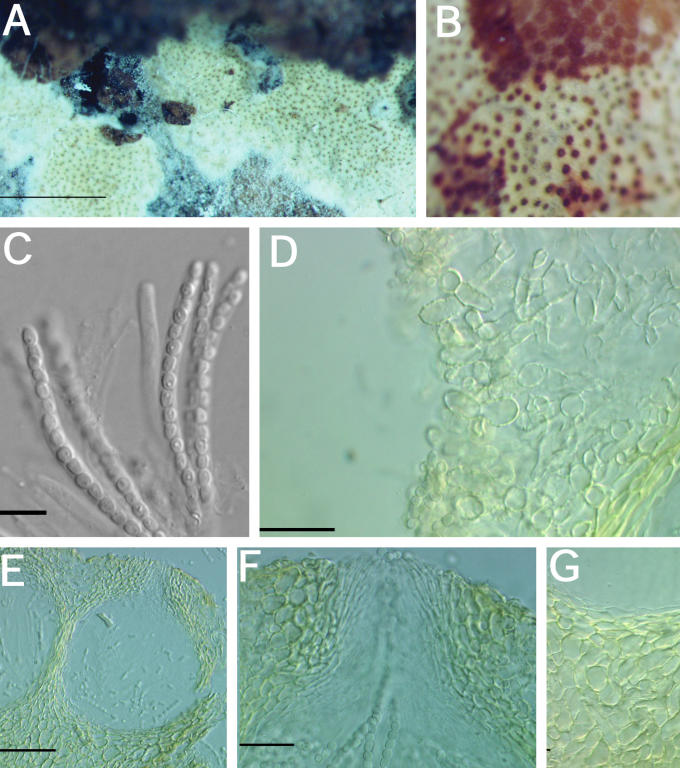
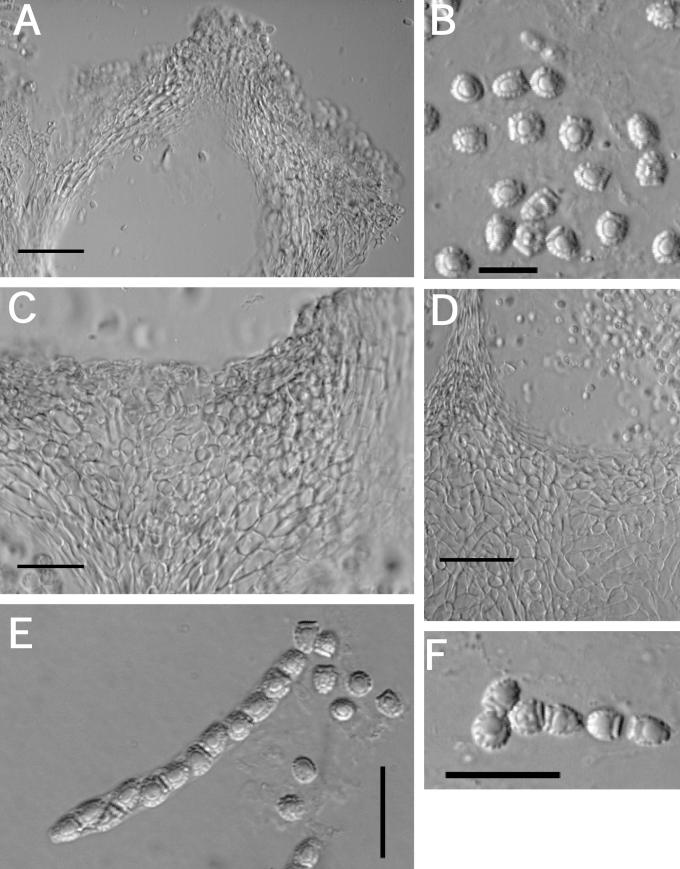
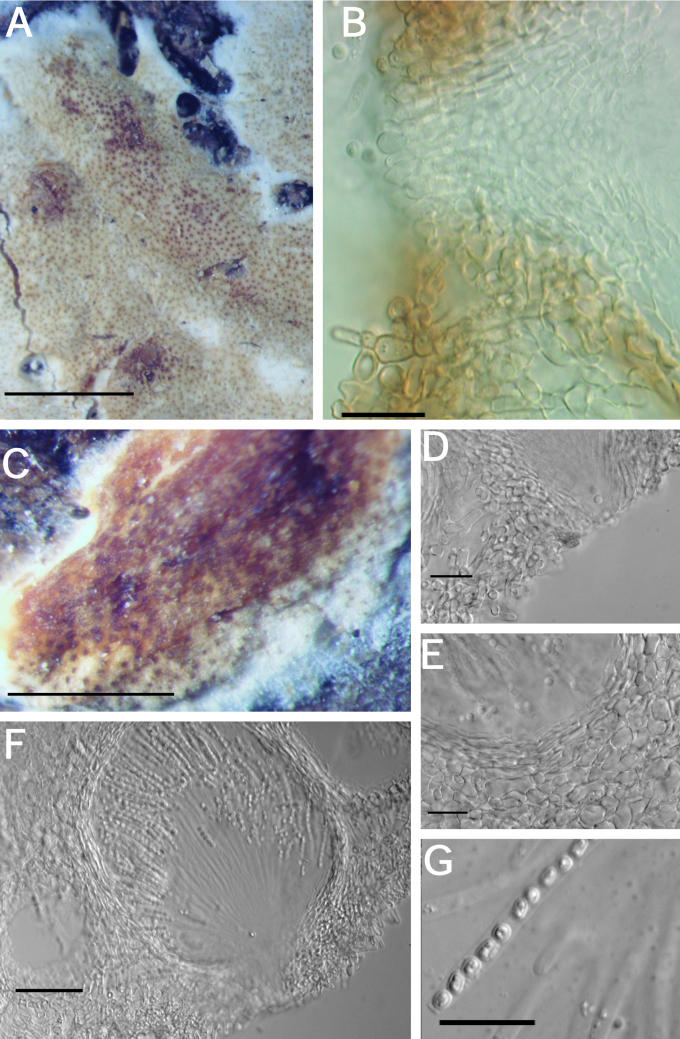
Fig. 7.
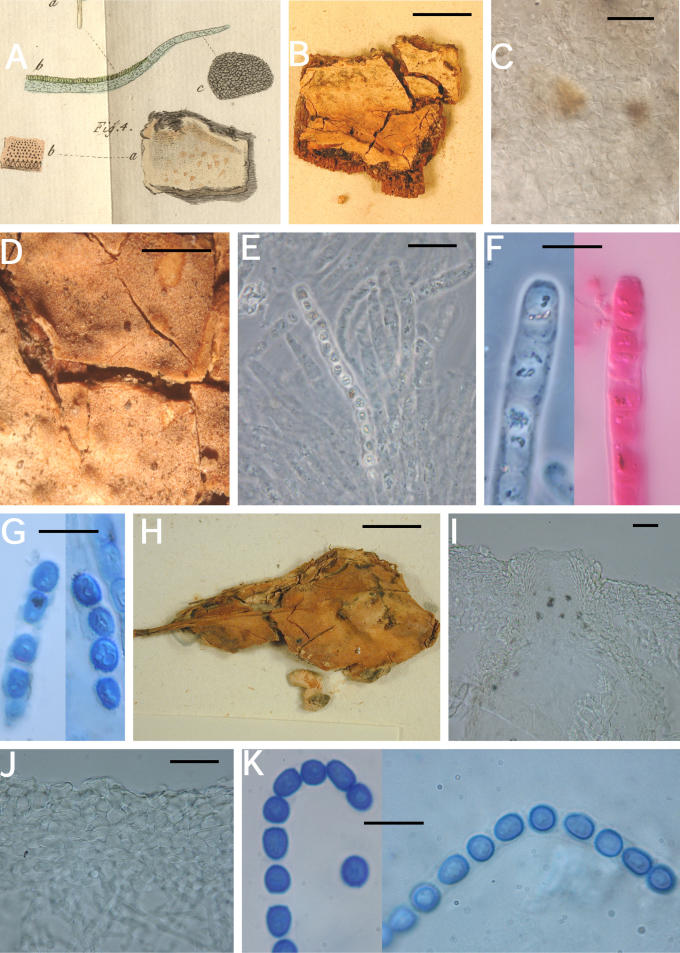
A–K. Hypocrea citrina (specimens of Hypocrea lactea). A. Illustration of Sphaeria lactea from Fries, Obs. Taf. 8, fig. 4. Compare to B. B–G. Sphaeria lactea, neotype specimen, coll. E.M. Fries, UPS; B. specimen glued to a piece of stiff paper. C. Surface of stroma seen in face view. D. Macroscopic view of stroma surface; bar = 1 mm. E. Ascus with developing ascospores; bar = 20 μm. F. Immature asci showing pore in ascus apex (left, cotton blue in lactic acid, right in 1 % aq. phloxine); bar = 10 μm. G. Mature ascospores in asci (cotton blue in lactic acid); bar = 10 μm. H–K. Hypocrea lactea specimen collected by Fries, Femsjö, UPS. H. Specimen; bar = 1 cm. I. Perithecial papilla, ostiolar opening and stroma surface; bar = 20 μm. J. Stroma surface showing pseudoparenchymatous cells at the surface and loosely disposed hyphae below; bar = 20 μm. K. Mature ascospores in asci (cotton blue in lactic acid); bar = 10 μm.
Rifai & Webster's selection of the name H. lactea over that of H. citrina is not consistent with the present Articles 11.4 and 15.4 of the ICBN, which govern the priority when two sanctioned names compete, giving priority to the older name. Consequently, terrestrial collections lodged in herbaria after 1966 were generally labeled H. lactea. Specimens previously identified under the name H. lactea should therefore be reidentified as H. citrina, the older name, which has priority over H. lactea.
Some confusion exists around the selection of type material associated with the name H. pulvinata. When Fuckel (1870) described H. pulvinata, he did not cite a specific specimen, but indicated Polyporus sulphureus as the substratum. Rifai & Webster (1966) indicated that the substratum for Fuckel, Fungi Rhenani 2467, preserved at K was “Polyporus resinosus”, which recently was identified as Laetiporus sulphureus. This specimen is over-mature, and no asci remain. Two specimens of F. Rhenani 2467 on L. sulphureus in G are also overmature. A specimen at FH, #876, was part of Fuckel's herbarium; the collecting data match those given in the protologue and the substratum also is identified as being Polyporus (= Laetiporus) sulphureus. Moreover, this collection is in good condition and is designated here as the lectotype for H. pulvinata. Measurements obtained from all historical specimens included in this study were consistent with measurements from more recent collections of H. pulvinata used in the molecular analysis. The species concept presented here for the polyporicolous form and the application of the name H. pulvinata is consistent with Weese (1927), Rifai & Webster (1966), and Doi (1972).
TAXONOMY
-
Hypocrea citrina (Pers.: Fr.) Fr., Summa Veg. Scand.: 383.1849. Figs 5, 6, 7, 8.
- ≡ Sphaeria citrina Pers., Obs. Mycol. 1: 68. 1796: Fr., Syst. Mycol. 2: 337. 1823.
- = Sphaeria lactea Fr., K. Svenska VetenskAkad. Handl. II, 37: 141. 1816: Fr., Syst. Mycol. 2: 337. 1823.
- ≡ Hypocrea lactea (Fr.: Fr.) Fr., Summa Veg. Scand.: 383. 1849.
Anamorph: Trichoderma lacteum Bissett [sect. Hypocreanum Bissett], Canad. J. Bot. 69: 2367. 1991.
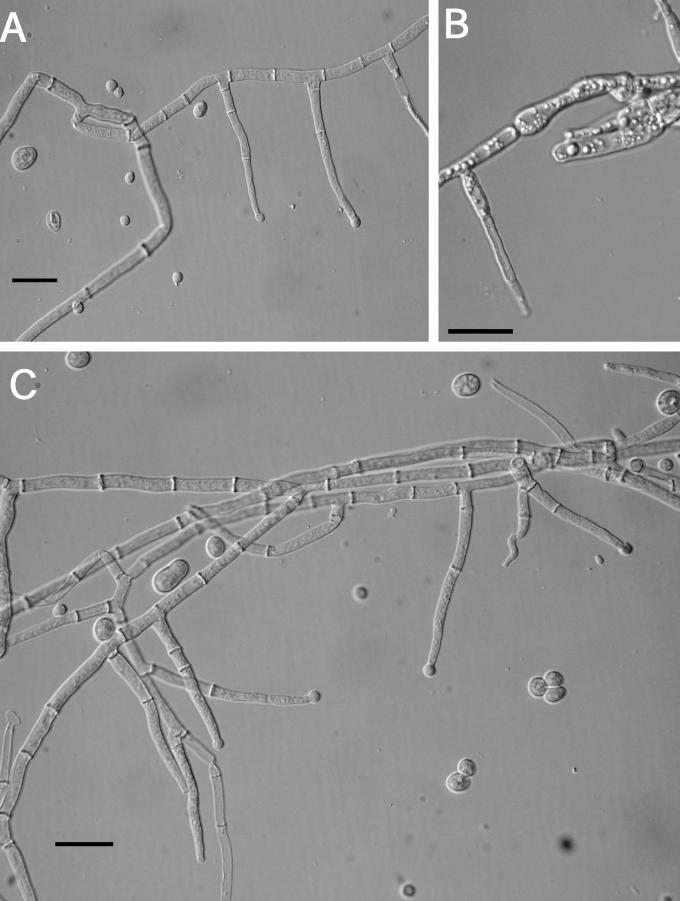
Teleomorph: Stromata effuse, extensive, largest continuous stroma, 140 × 40 mm, smallest continuous stroma, 3 × 2 mm, many stromata not larger than 25 × 10 mm, solitary to confluent, varying in colour, sometimes white to light pastel, usually yellow to greyish yellow, sometimes orange-yellow to brownish yellow (4A4–4B4; 4A6–5A7; 3A4), KOH+/–, reaction variable, usually very weak with slight browning of stroma; ostiolar canals visible at stroma surface, appearing light brown, giving rise to the greyish yellow overall appearance of the stroma. Stroma surface smooth; tissue immediately below the stroma surface and perithecia of compact to loose pseudoparenchymatous cells consisting of textura globulosa to t. angularis with some compact-vertically arranged hyphae. Perithecia completely immersed except for a slight protrusion of the ostiolar canals, ≤ 30 μm high (n = 7), generally widely spaced, compact in some regions, sometimes completely absent near the margins or regions of extensive stroma growth. Perithecia ellipsoidal, (263–)275–332(–360) μm long (including the length of the ostiolar canal, n = 22); width of perithecia near the base (measured from 3/4 total length of the perithecium) (99–)122–192(–214) μm (n = 22); length of ostiolar canal (42–)52–74(–82) μm; width of the ostiolar canal from the outer perithecial wall to the opposing internal perithecial wall (29–)34–54(–65) μm (n = 22); wall KOH+/–, reaction variable, weak, usually brownish orange. Asci cylindrical, (65–)80–102 (–114) × (3.7–)6.2–7.2(–9.6) μm (n = 149); tip slightly thickened. Part-ascospores hyaline, thick-walled, minutely spinulose, dimorphic; distal part subglobose, sometimes subellipsoidal, (3.2–)4.1–5.2(–6.3) × (2.9–) 3.7–4.8(–5.7) μm, L/W ratio, (0.7–)0.99-1.2(–1.5) (n = 166); proximal part, ellipsoidal, sometimes subglobose to ovate, (3.3–)4.4–6(–7.1) × (2.5–)3.3–4.9(–6.3) μm, L/W ratio, (0.6–)1–1.6(–2.1) (n = 166).
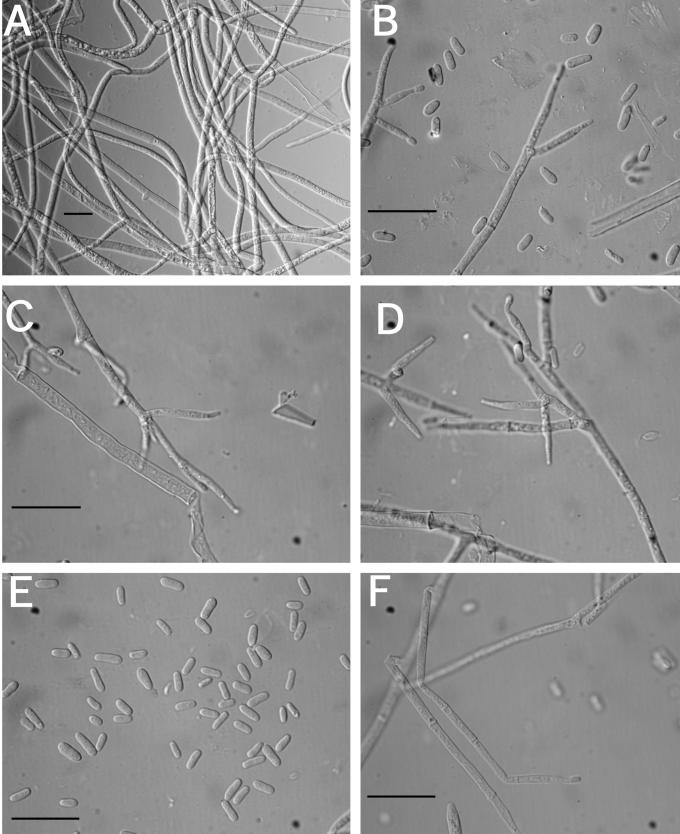
Anamorph: Colony covering the surface of a 100 mm diam Petri plate with PDA in 10 d, not producing concentric rings or radial rays of mycelium; a layer of cottony aerial mycelium along the entire Petri plate; no distinctive odour or pigmentation. Conidiophores irregularly branched on long hyphal elements, usually verticillium-like; phialides in whorls of 3–4(–5), sometimes solitary; phialides subulate, (8–)14–19 (–26) × (2.3–)2.9–3.7(–4.3) μm (n = 61); conidia variable in size, subglobose to subellipsoidal, (3–)3.3–5.8 (–10.8) × (3–)3.5–4.3(–5.1) μm (n = 61), some conidia asymmetric with a truncate base; chlamydospores cylindrical to subglobose when intercalary and bulbous when terminal, (5–)14-20(–22) × (9–)10–15(–18) μm (n = 16). After 10 d, conidia begin to swell and are more variable in size. Conidiophores not visible at 10 d on SNA or CMD. Conidiophores developed on CMD and SNA at 15–20 d, primarily near the point of inoculation. Optimal temperature for growth is 25 °C on all three media.
Habitat: On leaf litter and rich soils, especially soil near decaying stumps, less commonly on decorticated wood, and moss-covered bark.
Known distribution: Europe, Japan, and North America.
Neotype (designated here): Belgium, Hestreux near Eupen: on leaf litter including pine needles, Oct. 1985, W. Gams 4031 (CBS 894.85).
Other specimens examined: Austria, Steiermark, Lassnitzhöhe: vic. of Graz, elev. 480 m, on leaf litter and pine needles, 1995, H. Voglmayr (BPI 737697; culture G.J.S. 95-96); Niederösterreich, near Krems at Waldhof, beside Lake Egelsee, on leaf litter of Fagus/Picea, 28 Aug. 2000, W. Klofac, comm. W. Jaklitsch 1617 (BPI 748251; culture G.J.S. 00-161). England, Devon, Budleigh, on ground near base of Picea sitchensis stump, moss present, 22 Oct. 1989, J. Webster (BPI 1107145; culture G.J.S. 89-145). Germany, Pelmer Wald near Gerolstein, Oct. 1970, W. Gams (CBS 853.70). Netherlands, Baarn (Zandheuvelweg), Aug. 1973, W. Gams (CBS 708.73). Sweden, in mixed forest between Heina and Saara, on birch bark, 14 Aug. 1979, Lars Fagerström (BPI 744475); Småland, Femsjö, on wood, date not known, E. M. Fries (UPS, as Hypocrea lactea); Femsjö, on dicot leaf, date not known, E. M. Fries (UPS, as Sphaeria lactea), designated here as lectotype for H. lactea; Uppland, Arentuna parish, Storvreta, Eastern outskirts, on stump of Salix cinerea, 12 Sep. 1987, N. Lundqvist 17028 (UPS). USA, Georgia, Rabun Co., Chattahoochee Natl. Forest, along Little Creek Rd., near Double Bridge Creek, 34°59' N, 83°10' W, 17 Oct. 1990, A. Y. Rossman & G. J. Samuels, G.J.S. 90-137 (BPI 1107189); Kentucky, Laurel Co., Daniel Boone National Forest, elev. 350 m, Laurel River Lake Recreation Area, Cane Creek Wildlife Refuge, on decorticated wood, 27 Sep. 1995, G. J. Samuels (BPI 737759; culture G.J.S. 95-183); New York, Oswego County, Vandercamp Trail, on the moss Dreplanocladus uncinatus, 2 Oct. 1999, B.E. Overton, B.E.O. 99-29 (BPI); Warren County, Pack forest North of Warrensburg, on litter under white pine, 9 Sep. 1963, R.L. Gilbertson 4195 (UPS); North Carolina, Giles Co., off Bullpen Rd., 35°01' N, 83° 08' W, elev. 3000 ft., 14 Oct. 1990, Y. Doi, A.Y. Rossman & G.J. Samuels, G.J.S. 90-104 (BPI 1107172). Ohio: on Salix sp., 3 Oct. 1896, A. P. Morgan No. 46 (BPI 631486).
Comments: Gams 4031 (herb. CBS, ex-neotype culture CBS 894.85) is designated here as the neotype specimen of H. citrina. This European specimen is well suited to serve as the neotype because it is yellow, effuse, and occurs on leaf litter just as Persoon described. Hypocrea citrina is similar in overall appearance to H. sulphurea, but H. sulphurea is not as effuse near the stroma margin, is intensely yellow throughout, and occurs on Exidia sp. on twigs, branches, and trunks.
A lectotype is designated here for Hypocrea lactea [Sweden, Småland, Femsjö (as Sphaeria lactea, UPS herb. E.M. Fries)], assuming that this material was collected before 1822 (otherwise it would be neotype). Hypocrea lactea as lectotypified here is therefore a synonym of H. citrina. Doi (1972) illustrated stroma characters and part-ascospore measurements in his composite description of H. lactea that are not present in, or indicative of the lectotype specimen designated here for H. lactea s.s. Doi's (1979) annotation of Fries's historical specimen of H. lactea (cited in his specimens examined) listed part-ascospore measurements as follows: distal part-spores 3.5–4.5 × 3.4–3.9 μm, proximal part-spores 4.5–5.5 × 3.2–3.5 μm. These measurements differ substantially from the measurements given by Doi (1972) in his composite description of H. lactea; distal part-ascospores 4.5–5.2 × 4.5–4.8 μm, proximal part-spores, 5.5–7.1 × 4.2–4.4 μm. The Japanese specimens cited by Doi under the name H. lactea likely represent an undescribed species from Japan, especially given that Doi illustrated hyphal protrusions near the stroma surface, which are not indicative of H. lactea.
-
Hypocrea pulvinata Fuckel, Jahrb. Nassau. Ver. Naturk. 23–24: 185. 1870 [“1869”]. Figs 9, 10, 11.
- = Hypocrea citrina * fungicola P. Karst., Mycol. Fenn. 2: 204. 1873.
- ≡ Hypocrea karsteniana Niessl in Rehm. Ascomyceten, Hedwigia 22: 53 [Apr.] 1883.
- ≡ Hypocrea fungicola (P. Karst.) Sacc., Syll. Fung. 2: 528 [13 June] 1883.
- ≡ Protocrea fungicola (P. Karst) Lar.N. Vassiljeva, Nizshie Rasteniya, Griby i Mokhoobraznye Dal'nego Vostoka Rossii, Griby. Tom 4. Pirenomitsety i Lokuloaskomitsety (Sankt-Peterburg): 162. 1998.
- = Hypocrea citrina (Fr.) Fr. var. citrina sensu Cunham, Mycologia 61: 318. 1969.
Anamorph: Trichoderma sp. [sect. Hypocreanum].
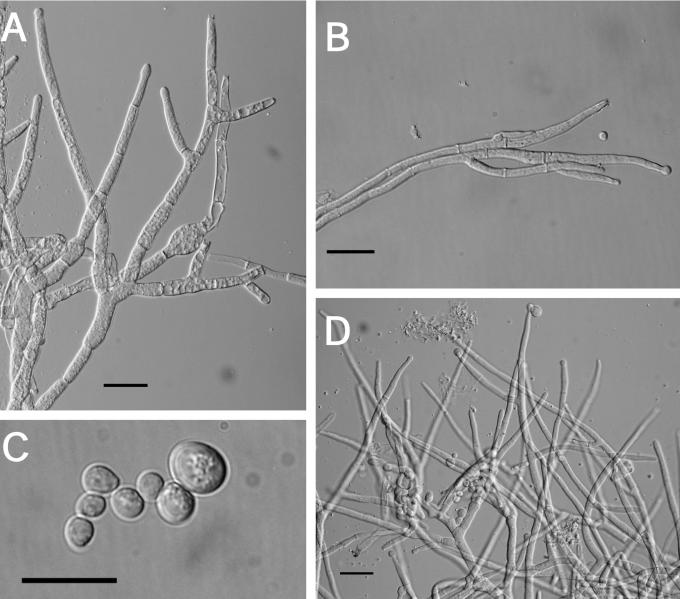
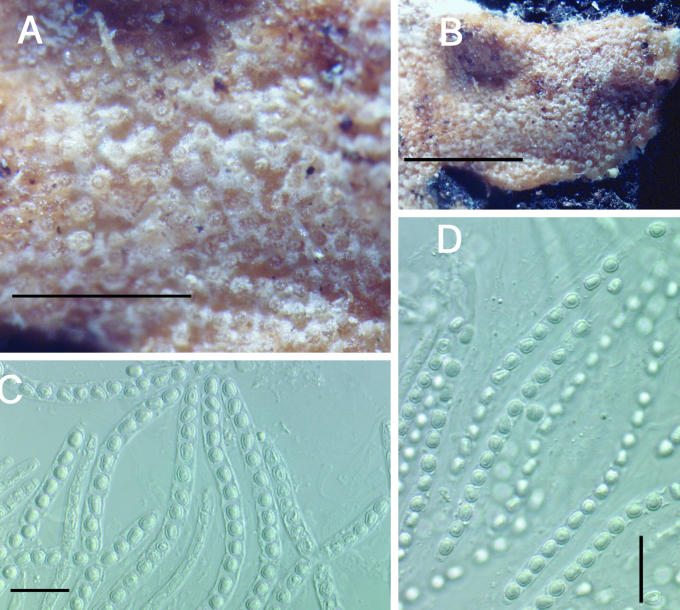
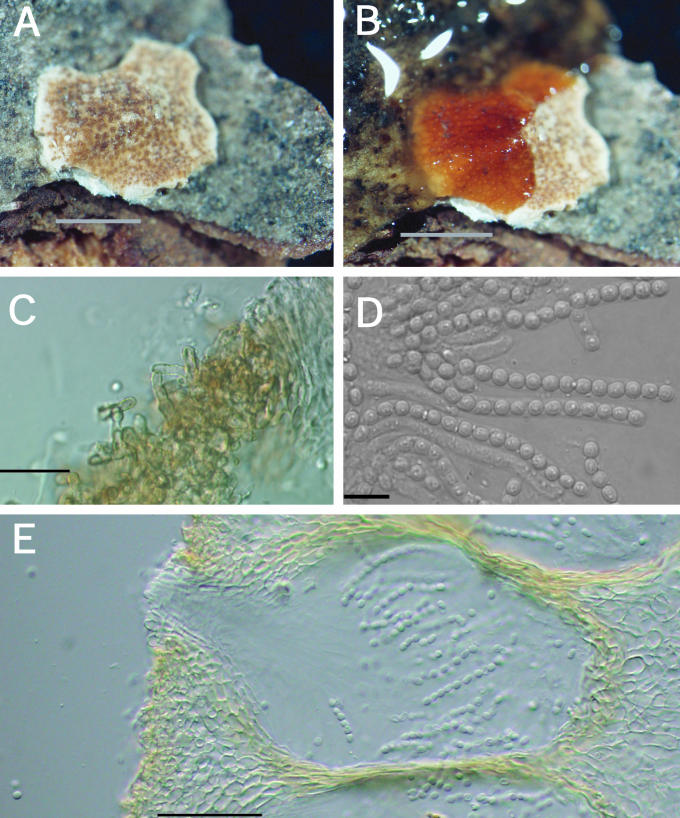
Teleomorph: Stromata effuse to subpulvinate, extensive, solitary to confluent, largest continuous stroma 60 × 20 mm, smallest continuous stroma 3 × 2 mm, varying in colour, orangish yellow to greyish orange, sometimes brownish yellow to golden-yellow, or brown-yellow (4B7–4B6; 5C7–5B7; 5C5); KOH+, reaction variable, sometimes weak, generally with stromata becoming orange or red; ostiolar openings visible from stroma surface. Warted hairs at surface of stromata, tissue immediately below the stroma surface formed of compact to loose pseudoparenchymatous cells, textura globulosa to t. angularis. Perithecia numerous and completely immersed in the stromata, generally widely spaced but compact near the centre, ellipsoidal, (189–) 206–246(–275) μm long (including length of ostiolar canal, n = 28); width of perithecia near the base (3/4 total length of perithecium) (96–)105–161(–186) μm (n = 28); length of ostiolar canal (36–)48–72(–82) μm; width of ostiolar canal (29–)43–63(–72) μm (n = 28); perithecial wall KOH+, reaction variable, usually brownish orange. Asci cylindrical, (44–)66–90(–115) × (2.8–)3.6–5.2(–7.9) μm (n = 321), tip slightly thickened. Part-ascospores hyaline, thin-walled, smooth to minutely spinulose, generally monomorphic and longer than broad; distal part ellipsoidal, sometimes subglobose, (2.8–)3.7–4.7(–6.0) × (2.3–)2.9–3.7(–4.6) μm, L/W ratio (0.8–)1.1–1.5(–2.2) (n = 335); proximal part, ellipsoidal, sometimes subglobose, (2.8–)3.8–5 (–6.7) × (2.3–)2.8-3.8(–4.7) μm, L/W ratio, (0.8–)1.1–1.6(–2.2) (n = 335).
Anamorph: Colonies covering a 100 mm diam Petri plate with PDA in 10 d; producing concentric growth pattern with radial rays; small white cottony aggregates near the edge of the Petri dish composed of mycelium and acremonium-like conidiophores, no aerial conidiophores; stromata and perithecia produced near the edge of the plate; reddish brown pigment (9E8) produced near the centre of the plate and brown pigment (7E8) near the edge of the plate; no odour. Colonies covering a 100 mm diam Petri plate with SNA also in 10 d; forming a thin layer with small white cottony aggregates of conidiophores (at random places on the plate); no aerial mycelium and only a few scattered conidiophores with conidia; no pigment production, no odour. Conidiophores developed and started producing conidia on SNA at 10 d. Colonies covering a 100 mm diam Petri plate with CMD in 10 d; forming a thin layer with small white cottony aggregates near the edge of the Petri plate; trace of brown pigment (7E8) near the inoculum; no odour. Conidiophores developing on CMD, only a few producing conidia. Optimal temperature for growth on all 3 media is 25 °C.
Measurements of micro-morphological characters were the same on all three media. A description based on measurements from all three media at 10 d follows: conidiophores variable in branching pattern and phialide number; phialides generally solitary, subulate, variable in length, (7–)24–44(–50) × (2.7–)3.6–4.6 (–5.3) μm (n = 32); below the phialides chlamydospore-like hyphal thickenings sometimes observed; conidia variable in size, many asymmetric, having a truncate base, subglobose to subellipsoidal, some bulb-shaped, (2.7–)4.1–7.1(–11) × (2.1–)3.3–5.6(–9) μm (n = 92). After 10 d conidia began to swell and were more variable in size.
Habitat: Found on a variety of polypores, including Laetiporus sulphureus, Fomitopsis pinicola, Piptoporus betulinus, and Ganoderma spp.
Known distribution: Europe, Japan, and North America.
Lectotype (designated here): Germany, Geis, Hattenheimer Wald, on Polyporus sulphureus (= Laetiporus sulphureus), L. Fuckel, autumn, No. 876 (FH).
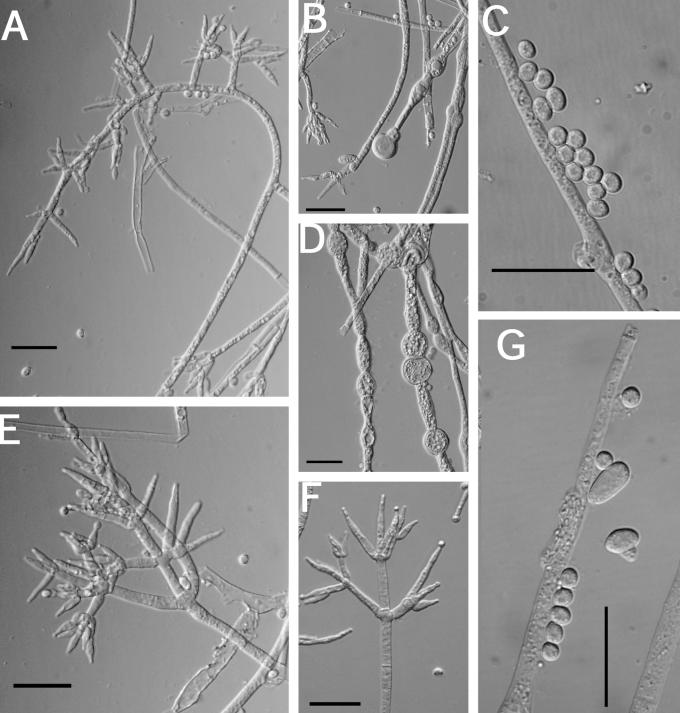
Other specimens examined: Austria, Wachau District, Krems an der Donau, Mosingraben, North of Spitz in mixed forest, elev. 300 m, on undetermined polypore, 2 June 1996, I. Krisai-Greilhuber (BPI 744499; culture G.J.S. 96-142); Waldviertel, Virgin Forest of Dobra, on Fomitopsis pinicola, 10 Oct. 1995, H. Voglmayr (BPI 737803; culture G.J.S. 95-220). Finland, Locality unknown, Fungi Fennici Exs. 264, identified as H. citrina f. fungicola (K); Wasa (= Vassa), on polypore, May 1869, P.A. Karsten s.n. (H, herb. Karsten 4479, as H. citrina f. fungicola); Tammela, Mustiala, on polypore, 25 Sep. 1890, P. A. Karsten s.n. (H, herb. Karsten 4475); Satakunta Tyrvis (= Vammala, Tyrvää), on polypore, date unknown, P.A. Karsten s. n. (H, herb. Karsten 4478; syntype of H. citrina f. fungicola); Karelia Keretina, on Polyporus (Piptoporus) betulinus, 9 Aug. 1861, P.A. Karsten s.n. (H, herb. Karsten 4477, as H. citrina f. fungicola; syntype). Germany, Geis, on “Polyporus resinosus”, substrate determined as Laetiporus sulphureus by E. Parmasto (Oct. 1999), Fuckel, Fungi Rhenani 2467 (K 61843); Saarland, Naturpark Saar-Hundsrück, vic. Ritzenberg, 90°40' N, 7°0' W, elev. 600–650 m, on Ganoderma sp., 12 Oct. 1998, G. J. Samuels & H. J. Schroers, G.J.S. 98-104 (BPI 746114); BPI 746115; culture G.J.S. 98-108. Great Britain, Yorks., Wrathe Woods, Pately Bridge, on Piptoporus betulinus, 25 Sep. 1955, C. Booth, IMI 61207 (BPI 631478). Luxembourg, Nassebesh, Hachy, on Piptoporus betulinus, 19 Sep. 1996, Vanhalle S. Mull 40055 (BPI 744722; culture G.J.S. 96-283). Poland, Silesia, Karlsbrunn, on Polyporus pinicola (= Fomitopsis pinicola, conf. E. Parmasto), Aug. 1882, von Niessl, Rehm Ascomyceten 678 (K, 61844). Sweden, on polypore, E.M. Fries, Scleromyceti Sueciae 31 (K). U.S.A., New Hampshire, Chocorua, on Fomitopsis pinicola, 16 July 1906, W.G. Farlow No. 34, identified as H. fungicola (K); New York, Franklin County, Little Green Pond, vic. Paul Smith College, on Ganoderma sp., 17 Aug. 1991, R. Lowen R.L. 886 (BPI 112828; culture G.J.S. 91-55A); Oneida County, on Fomitopsis pinicola, Barrie Overton, B.E.O. 99-34 (BPI); Utah, Beaver County, Tushar Mountains, Fishlake National Forest, on Fomitopsis pinicola, 31 Aug. 1994, C.T. Rogerson (BPI 737831; culture G.J.S. 94-20); Washington, Olympia National Park, Twin Creek, on Fomitopsis pinicola, 18 Oct. 1992, H. Burdsall, H.B. 14889 (BPI 802876; culture G.J.S. 92-127); Olympia National Park, Twin Creek, on Fomitopsis pinicola, 18 Oct. 1992, H. Burdsall, H.B. 14910 (BPI 802877; culture G.J.S. 92-128).
-
Hypocrea americana (Canham) Overton, stat. nov. MycoBank MB501053. Figs 12, 13, 14.
- ≡ Hypocrea citrina (Fr.) Fr. var. americana Canham, Mycologia 61: 320. 1969.
Anamorph: Trichoderma sp. [sect. Hypocreanum].
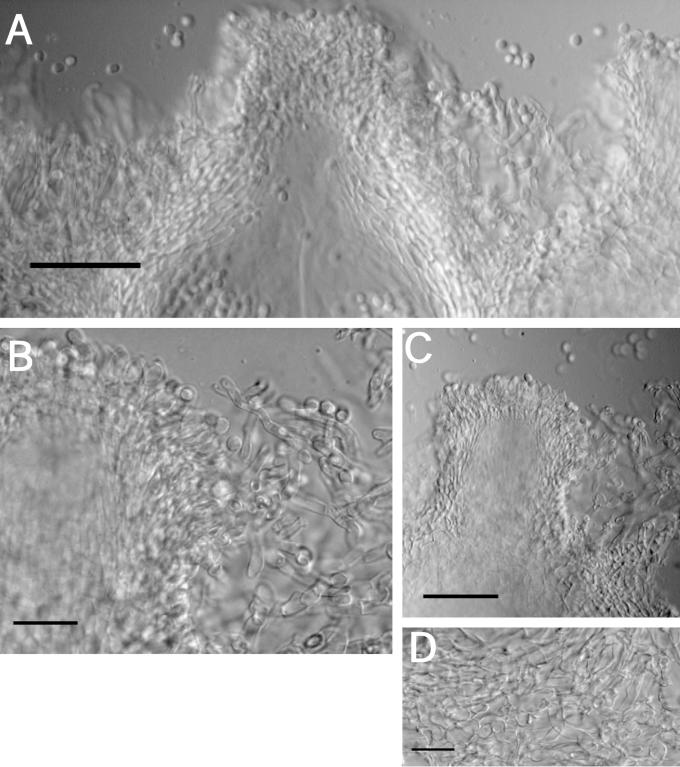
Teleomorph: Stromata effuse to subpulvinate, solitary to confluent, extensive, largest continuous stroma 50 × 20 mm, smallest continuous stroma 3 × 2 mm, varying in colour, usually orange to brownish yellow, or orangish yellow to greyish yellow (5B8–5C8; 4B7–4B6); KOH+, reaction variable, generally with stromata becoming dark orange or red; perithecial necks or openings barely visible from stroma surface. Warted hairs at stroma surface; tissue immediately below the stroma surface formed of compact to loose pseudoparenchymatous cells composed of textura globulosa to t. angularis. Perithecia numerous and completely immersed in the stromata, generally widely spaced but compact near the centre of the stroma, ellipsoidal, (149–)167–199 (–213) μm long (including the length of the ostiolar canal, n = 15); width of perithecium near the base, measured from 3/4 the total length of the perithecium, (73–)80–106 (–119) μm (n = 15); length of ostiolar canal (44–)54–74 (–84) μm; width of ostiolar canal (41–)45–56(–62); wall KOH+, reaction variable, usually brownish orange or red. Asci cylindrical, (49–)59–79(–106) × (3.3–)4–5(–6.2) μm (n = 167), tip slightly thickened. Part-ascospores hyaline, thin-walled, smooth, monomorphic, usually subglobose to rhomboidal by mutual pressure, broader than long; distal part subglobose to rhomboidal, (1.7–) 2.7–3.7(–6.2) × (2.4–)3.2–4.2(–5.3) μm, L/W ratio (0.5–) 0.84–0.89(–1.5) (n = 212); proximal part subglobose to rhomboidal, (1.8–)2.6–3.8(–5.7) × (2.5–)3.2–4.2(–5.4), L/W ratio (0.5–)0.82–0.86(–1.4) (n = 212).
Anamorph: Colonies covering a 100 mm diam Petri plate with PDA in 10 d; mycelium growing tightly pressed to the surface, appearing wet, no aerial mycelium; conidiophores produced in cottony tufts concentrated near the edge of the plate, tufts thicker and rising further from the agar surface than on CMD; stromata and perithecia emerging from several regions where conidiophores are found in the greatest number; brownish orange (7C8) to reddish brown (8E8) pigment diffusing in the medium, pigments visible within the hyphae. Colonies covering a 100 mm diam Petri plate with SNA in 10 d; mycelium thin, watery in appearance; conidiophores scattered, conidial production poor or absent. Colonies covering a 100 mm diam Petri plate with CMD in 10 d; mycelium tightly pressed to the surface, appearance watery, no aerial mycelium; conidiophores produced in cottony aggregates and tufts concentrated near the edge of the plate; brownish orange (7C8) to light brown pigment (7D8) diffusing into the medium. Optimal temperature for growth on all 3 media is 25°C.
Measurements of micro-morphological characters were the same on all three media. A description based on measurements from all three media at 10 d follows: conidiophores variable in branching pattern and phialide number; phialides generally solitary, subulate, variable in length, (12–)20–40(–56) × (1.8–)3.3–5.1(–6.4) μm (n = 41), with chlamydospore-like thickenings just below the phialides on CMD and PDA; conidia variable in size and shape, typically subglobose to subellipsoidal, sometimes bulb-shaped, many asymmetric, having a truncate base, (3.2–)3.6–6.8(–10.2) × (2.6–)3.2–5.9 (–8.8) μm (n = 43). After 10 d conidia begin to swell, becoming larger and more variable in size.
Habitat: On Fomitopsis pinicola and Piptoporus betulinus.
Known distribution: North America.
Holotype: USA, New York, Madison Co., Nelson Swamp, on F. pinicola, 9 Oct. 1949, C.T. Rogerson 3284 & Huttleston (NY ex CUP 38045, ex-type culture ATCC 18574).
Other specimens examined: U.S.A., Arizona, Apache Co., White Mountains, 19.1 miles from forest boundary, on F. pinicola, 21 Aug. 1994, C.T. Rogerson (BPI 737849; culture G.J.S. 94-79); Graham Co., Pin leno Mt., Coronado National Forest, Rt. 366, 4.1 miles south of Riggs Lake, on F. pinicola, 15 Aug. 1996, C.T. Rogerson (BPI 744432; G.J.S. 96-191); New Hampshire, East Monroe, on Piptoporus betulinus, 22 Aug. 1937, C.L. Shear (BPI 631477); New Mexico, Upper Fernando de Taos, forest service map, T25n R15e S15, on Fomes sp., 15 Aug. 1992, R. Lowen 1003 (BPI 802838; culture G.J.S. 92-93); New York, Oswego Co., Vandercamp Trail, on F. pinicola, 2 Oct. 1999, B.E. Overton, B.E.O. 99-30 (BPI); also B.E.O. 99-32 and B.E.O. 99-33 (all BPI); Ulster County, Clinton dale Swamp, on P. betulinus, 11 Oct. 1963, S. J. Smith, R. Detroit, & C. T. Rogerson (NY; paratype).
Comments: Hypocrea americana is easily distinguished from H. pulvinata by size and shape of the ascospores and degree of visibility of the ostiolar openings. The ascospores of H. americana are globose to rhomboidal with width greater than the length. In H. pulvinata, the ascospores are ellipsoidal, the length exceeding the width. The ostiolar openings in H. pulvinata are visible and the stroma surface has less of an overall rugulose appearance than in H. americana. In the specimen BPI 802838, both H. pulvinata and H. americana occur on the same host.
-
Hypocrea protopulvinata Yoshim. Doi, Bull. Natl. Sci. Mus. 15: 695. 1972. Figs 15, 16.
Anamorph: Trichoderma sp. [sect. Hypocreanum].
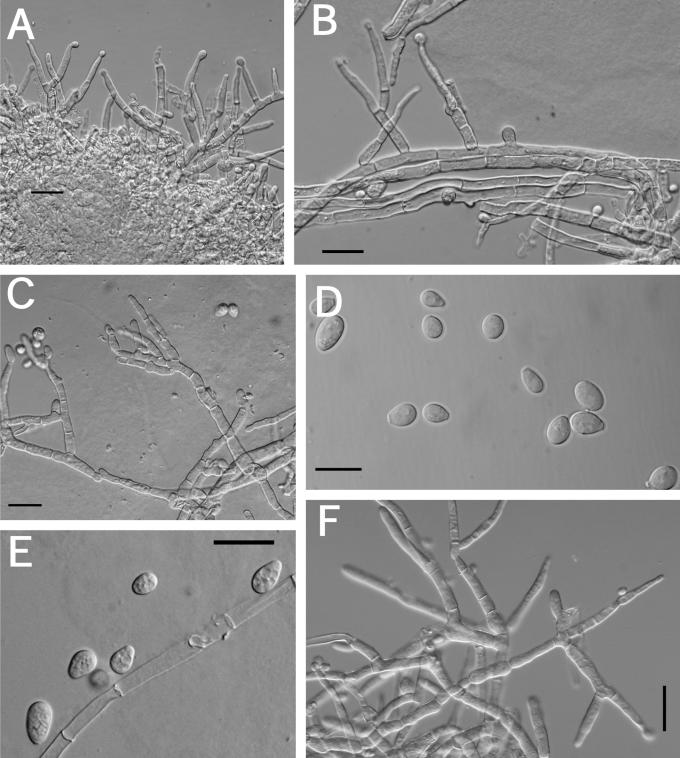
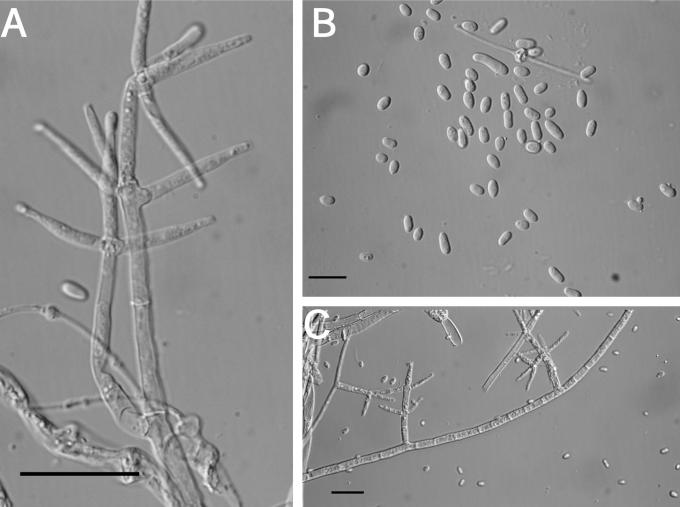
Teleomorph: Stromata effuse, sometimes subpulvinate, solitary to confluent, extensive, varying in colour, usually orangish yellow to light brown, young stromata white, becoming pale yellow where perithecia are developing (4B6–6D7; 4A3); KOH+/–, reaction variable, weak, generally with stromata becoming darker orange; largest continuous stroma 22 × 15 mm, smallest continuous stroma 5 × 3 mm; ostiolar openings visible from stroma surface. Hyphal protrusions at stroma surface subglobose to pyriform, slightly roughened, not appearing warted like in H. pulvinata. Tissue immediately below the stroma surface variable; cells adjacent to ostiolar canals composed of rather thick-walled t. angularis; cells between perithecia of t. globulosa with some t. intricata intermixed; cells near the base of the perithecia composed of tightly packed irregular pseudoparenchymatous tissue in between t. globulosa, t. intricata, and t. angularis. Perithecia numerous near the stroma centre, scarce near the margin, generally widely spaced, subglobose to ellipsoidal, (151–)180–286(–310) μm long (including the length of the ostiolar canal, n = 11); width of perithecia near the base (99–)106–153(–176) μm, n = 11; length of ostiolar canal (33–)33–57(–68) μm; width of ostiolar canal (27–)30–46(–53) μm, n = 11; wall KOH+/–, reaction variable, weak, usually brownish orange. Asci cylindrical, (45–)56–72(–76) × (3.7–)4–5(–5.4) μm (n = 52), tip slightly thickened. Part-ascospores hyaline, thin-walled, smooth, sometimes minutely spinulose, dimorphic; distal part subglobose, (3–)3.3–3.9(–4.3) × (2.6–)3–3.5(–4.2) μm, L/W ratio, (0.8–)0.99–1.2 (–1.5) (n = 52); proximal part ellipsoidal, sometimes subglobose, (3.2–)3.6–4.2(–4.8) × (2.4–)2.7–3.3(–3.8), L/W ratio (0.9–)1.1–1.5(–1.8) (n = 52).
Anamorph: Colonies growing across the surface of a 100 mm diam Petri plate with PDA in 10 d; radial growth with radial rays, mycelium tightly pressed to the surface, sometimes with web-like sectors of rapidly growing mycelium, no aerial mycelium; conidiophores produced near the edge of the colonies in tufts; several large aggregates appear to be stroma initials; other large aggregates consist of clustered conidiophores; agar with a yellowish tint from the reverse, brown pigment (5D7) being produced near the edge of the plate and the agar plugs; sometimes odour (fruity of sour apples) produced when plates are tightly sealed; stromata produced 25–30 d following inoculation in sealed plates of the ex-type strain. Colonies growing across the surface of a 100 mm diam Petri plate with SNA in 10 d; mycelium web-like, random tufts of conidiophores arising from the agar surface, very few mature conidiophores; no pigment production. Colonies growing across the surface of a 100 mm diam Petri plate with CMD in 10 d; mycelium tightly pressed to the surface, appearing wet, no aerial mycelium; conidiophores produced near the edge of the plate, very rarely in tufts arising from the surface, very few mature conidiophores. Optimal temperature for growth on all 3 media is 25–30 °C with cultures growing ≤ 3 mm at 35 °C.
Measurements of micro-morphological characters were the same on all three media. A description based on measurements from all three media at 10 d follows: conidiophores irregular, acremonium-like, variable in branching pattern and phialide number; phialides generally solitary, subulate, variable in length, (8–)20–38(–49) × (3–)3.7–4.7(–6) μm (n = 54); chlamydospore-like swellings below the phialides not as prominent on PDA and CMD; conidia variable in size and shape, generally subellipsoidal, some subglobose to subellipsoidal, most conidia have a well-developed truncate base, (4–)5.9–9.9(–16) × (2.8–)4–7(–9.5) μm (n = 76); chlamydospores not observed after 10 d. After 10 d conidia begin to swell and are more variable in size.
Habitat: On Fomitopsis pinicola and unidentified polypores.
Known distribution: Japan and North America.
Isotype: Japan, Chiba Pref., Fudagou Mt., on unidentified polypore, 24 Oct. 1967, Y. Doi (NY ex TNS.D-365 = TNS-F-223421; ex-type culture CBS 739.83).
Other specimens examined: U.S.A., Maryland, on unidentified polypore, autumn 2000, Kadri Põldmaa, K.P. 00-56 (BPI); New York, Cranberry Lake, Suckers Trail, on unidentified polypore, 15 Sep. 2001, B.E. Overton & D. Geiser, B.E.O. 00-01 (BPI); Ohio, on unidentified polypore, 11 Nov. 1883, A.P. Morgan No. 29 (BPI 631312; specimen degraded).
Comments: Hypocrea protopulvinata is easily distinguished from H. pulvinata and H. americana because of its dimorphic ascospores and more effuse stromata with roughened, not warted, hyphal protuberances. The subglobose distal part-spores of H. protopulvinata are similar in size to the monomorphic subglobose part-spores found in H. americana: (3–)3.3–3.9(–4.3) × (2.6–)3–3.5(–4.2) μm in H. protopulvinata versus (1.7–)2.7–3.7(–6.2) × (2.4–)3.2–4.2(–5.3) μm in H. americana; In addition, the ellipsoidal proximal part-spores of H. protopulvinata are similar in size to the monomorphic ellipsoidal part-spores found in H. pulvinata: (3.2–)3.6–4.2(–4.8) × (2.4–)2.7–3.3(–3.8) in H. protopulvinata versus (2.8–)3.8–5(–6.7) × (2.3–)2.8–3.8(–4.7) in H. pulvinata. All three of these species have thin-walled part-ascospores that are smooth to minutely spinulose, characters not common in Hypocrea.
-
Hypocrea platypulvinata Yoshim. Doi, Bull. Natl. Sci. Mus. 15: 698. 1972.
Anamorph: Trichoderma sp. [sect. Hypocreanum].
Teleomorph: Stromata in aggregates or scattered, disciform, attached to decayed logs via a narrow base, deep yellow-ochre to ochre-brown, 2–6 mm diam, 0.8–1.6 mm thick, cells at surface of stromata roughened; perithecia ellipsoid, 200–240 μm in length; part-ascospores hyaline, warted, generally monomorphic, globose, 2.1–2.3 μm diam or sometimes obovate ellipsoid, 2.0–3.0 μm long (Doi 1972).
Anamorph: Conidiophores acremonium-like; phialides subulate toward the apices, 15–35 × 1.5–2.0 μm; conidia hyaline, smooth, cylindrical to obovate-ellipsoid, with a truncate base, 4.0–14 × 2.3–5.1 μm; chlamydospore-like, thick-walled cells often produced, 2–12 × 6–8 μm in culture (Doi 1972).
Habitat: On decayed logs of broad-leaved trees.
Known distribution: Japan.
Specimens examined: Type material not available for examination.
-
Hypocrea megalocitrina Yoshim. Doi, Bull. Natl. Sci. Mus. 15: 669. 1972. Figs 17, 18.
Anamorph: Trichoderma sp. [sect. Hypocreanum].
Teleomorph: Stromata effuse, solitary to confluent, extensive, largest continuous stroma, 40 × 30 mm, smallest continuous stroma 3 × 2 mm, varying in colour, usually light brown (6D7); KOH–; surface of stromata appearing roughened due to ostiolar canals projecting from the stroma surface, which are covered by a thin layer of t. intricata. Tissue immediately below the stroma surface formed of compact to loose t. intricata and sporadic pseudoparenchymatous cells varying from textura globulosa to t. angularis. Perithecia numerous, generally widely spaced but compact near the centre of the stroma, ellipsoidal-elongate, (207–)240–320 (–345) μm high (including the length of the ostiolar canal, n = 24); width of perithecia near the base, measured from 3/4 the total length of each perithecium, (113–) 127–181(–208) μm (n = 24); length of ostiolar canal (54–)62–94(–130) μm (n = 24); width of ostiolar canal (54–)57–75(–84) (n = 24); wall KOH–. Asci cylindrical, (66–)79–97(–118) × (4.7–)5.3–6.6(–7.4) μm (n = 93), tip slightly thickened. Part-ascospores hyaline, thick-walled, nodulose, dimorphic; distal part subellipsoidal to conical, (3.2–)4.5–5.8(–6.6) × (3.0–)3.8–5(–6) μm, L/W ratio (0.9–)1–1.3(–1.9) (n = 121); proximal part ellipsoidal, rarely subellipsoidal, sometimes appearing thimble-shaped or conical, (3.7–)4.6–5.9(–7) × (3.1–) 3.7–4.8(–5.8), L/W ratio (0.8–)1.0–1.4(–1.8) (n = 121).
Anamorph: Colonies on n PDA covering the surface of a 100 mm diam Petri plate in 10 d, producing an extensive layer of aerial mycelium; conidiophores concentrated near the agar plug, some areas with thicker layers of aerial mycelium than others; conidiophores in dense clusters near the agar plug, but also on thin layers of aerial mycelium near the edge of the plate; no diffusible pigment observed in the agar; conidiophores typically verticillately branched; phialides slender, subulate, typically in whorls of 3–5, but also solitary; phialides with conidia appearing light yellow when compacted in the aerial mycelium, (11–)15–24(–30) × (2–)2.3–3.4(–4) μm; conidia hyaline, smooth, subglobose to ellipsoidal, variable in size and shape, sometimes with a truncate base, (3.8–)4–6.5(–9) × (3.3–)3.4–4(–4.3) μm; chlamydospores not observed at 10 d. Cultures on SNA or CMD did not produce conidiophores within 10 d.
Habitat: On decayed trunks of broadleaf trees including Acer sp.
Known distribution: Japan and North America.
Isotype: Japan, Mie, Hirkura Forestry Experimental Station, 7 Sep. 1965, Doi (NY ex TNS-F-223220).
Other specimens examined: U.S.A., Maryland, Howard Co. and Caroll Co. border, Patapsco Valley State Park, N. of Ellicot city, on wood, 14 Oct. 1999, probably Acer sp., B.E. Overton, B.E.O. 99-42 (BPI); North Carolina, Macon Co., Glen Falls lower trail, Nantahala National Forest, 12 July 2000, L. Grand & C. Vernia (BPI, culture B.E.O. 00-09).
-
Hypocrea aurantiistroma Overton, sp. nov. MycoBank MB501054. Figs 19, 20.
Anamorph: Unknown.
Stromata effusa, solitaria vel confluentia, ad 8 × 5 mm, griseo-aurantia, ostiolis protrudentibus verrucosa. Ascosporae hyalinae, crassitunicatae, exigue nodulosae, partes quasi monomorphicae; pars distalis quasi conica, (3.6–)4.5–5(–5.9) × (2.5–)3.3–4(–4.6) μm, pars proxima plus minusve ellipsoidea, (3.6–)4.2–5.4(–6.4) × (3.1–)3.3-4(–4.6) μm. Anamorphosis ignota.
Teleomorph: Stromata effuse, solitary to confluent, extensive, largest continuous stroma, 8 × 5 mm, smallest continuous stroma, 2 × 2 mm, greyish orange (6B4); KOH–; surface of stromata appearing roughened because of ostiolar canals projecting from stroma surface; projecting ostiolar canals covered by a thin layer of t. intricata. Tissue immediately below the stroma surface formed of compact to loose t. intricata and sporadic pseudoparenchymatous cells, typically textura globulosa. Perithecia numerous and completely immersed in stromata, generally widely spaced but compact near the centre of the stroma, ellipsoidal-elongate, (207–)240–258(–283) μm high (including the length of the ostiolar canal, n = 10); width of the perithecium near the base, measured from 3/4 the total length of each perithecium, (113–)135–149(–193) μm, n = 10; length of ostiolar canal (54–)64–90(–99) μm, n = 10; width of ostiolar canal (67–)69–79(–84) (n = 10); wall KOH–. Asci cylindrical, (66–)80–98(–104) × (4.7–) 5–5.8(–6.4) μm (n = 30), tip slightly thickened; part-ascospores uniseriate. Part-ascospores, hyaline, thick-walled, minutely nodulose, slightly dimorphic, almost appearing monomorphic; distal part subellipsoidal, slightly conical, (3.6–)4.5–5(–5.9) × (2.5–)3.3–4(–4.6) μm, L/W ratio, (0.9–)1–1.4(–1.9) (n = 30); proximal part ellipsoidal, rarely subellipsoidal, slightly conical, (3.6–)4.2–5.4(–6.4) × (3.1–)3.3-4(–4.6) μm, L/W ratio, (0.8–)1.1–1.5(–1.7) (n = 30).
Habitat: On bark of Pinus strobus.
Known distribution: North America, Japan (?).
Holotype: U.S.A., North Carolina, Macon, Clear Creek road, 35°00' N, 83°15' W, on Pinus strobus, 16 Oct. 1990, Y. Doi, A. Y. Rossman, G.J. Samuels, (BPI 1107193; Doi-68 in TNS, isotype).
Comments: Hypocrea aurantiistroma can be distinguished from H. megalocitrina by its greyish orange stroma and minutely nodulose ascospores. This species is similar in overall appearance to H. fulva sensu Yoshim. Doi, which differs substantially from H. fulva s.str. Japanese specimens included under the name H. fulva were not available for study. It is likely that specimens from Japan included under the name H. fulva by Doi represent an undescribed sister species close to H. aurantiistroma or possibly, the same species.
-
Hypocrea fulva Penz. & Sacc., Malpighia 11: 520. 1897. Fig. 21.
Anamorph: unknown.
Teleomorph: Stromata subpulvinate to pulvinate, discrete but sometimes confluent, largest stroma composed of confluent fructifications, 1.5 × 1.2 mm, smallest continuous stroma 0.5 × 0.5 mm, stromata generally discrete, 0.55–0.9 mm diam (n = 26), brownish yellow in lighter regions to yellowish brown in darker regions (5C8–5E8); tissue immediately below the stroma surface formed of a thin layer (3–4 cells thick) of loose to compact pseudoparenchymatous cells, typically textura globulosa to t. angularis, stromata otherwise composed of vertically arranged t. intricata. Perithecia completely immersed in the stromata, generally widely spaced, ellipsoidal, (283–)290–340(–345) μm high (including the length of the ostiolar canal (n = 4); width of perithecia near the base, measured from 3/4 the total length of each perithecium, (200–)210–250(–258) μm (n = 3); length of ostiolar canal (63–)64–84(–88) μm (n = 4). Asci cylindrical, (110–)125–140(–162) × (5.6–)6.3–8.0(–9.6) μm (n = 26), tip slightly thickened. Part-ascospores hyaline, thick-walled, nodulose, slightly dimorphic; distal part subellipsoidal, slightly conical, (3.9–)4.5–5.5(–6.2) × (2.8–)3.2–3.8(–4.3) μm, L/W ratio, (1.1–)1.2–1.6(–2.0) (n = 30); proximal part ellipsoidal, rarely conical, (4.3–)4.6–5.6(–6.5) × (2.8–) 3.0-3.6(–4.1), L/W ratio, (1.2–)1.3–1.7(–1.9) (n = 30).
Syntypes examined: Indonesia, Tjibodas, on herbaceous stem, 104 and 410 (PAD).
Comments: Hypocrea fulva is very similar to H. aurantiistroma and H. megalocitrina as it has ostiolar canals projecting from the stroma surface, and nodulose part-ascospores. Hypocrea fulva is distinguished by its yellow-brown pulvinate stroma, which has a thin layer of pseudoparenchymatous tissue, and its slightly conical part-ascospores. The part-ascospores of H. megalocitrina are much more conical and the nodules more developed, while the part-ascospores of H. aurantiistroma are even less conical with nodules that are present but not as distinct. The syntypes of H. fulva differ morphologically from the illustrations provided by Doi (1972) under the name H. fulva both in colour and the presence of a well-defined layer of pseudoparenchymatous tissue at the stroma surface. Hypocrea fulva sensu Doi is likely an undescribed species closely related to H. aurantiistroma. Nodulose ascospores are unusual in Subsection Citrinae as all other species Doi included in Citrinae have smooth to minutely spinulose part-ascospores.
-
Hypocrea mikurajimensis Yoshim. Doi, Mem. Natl. Sci. Mus., Tokyo 37: 113. 2001.
Anamorph: Trichoderma sp. [sect. Hypocreanum].
Teleomorph: Stromata pulvinate, gregarious or dispersed, sometimes fused together to form larger and irregular stroma masses, wax-yellow or mustard-yellow, 3–8 mm broad, 0.3–0.8 mm thick; surface of the stromata smooth; perithecia completely immersed in the stroma, obovate-subglobose, generally crowded and elongate, 150–200 μm high; asci 48–56 × 2.6–3.0 μm; part-spores colourless, minutely pustulate-tuberculate; generally globose to subglobose, often with a depressed surface between paired part-spores, or the proximal part-spores often obovate, sometimes ellipsoid, 2.2–3.2 × 2.0–2.8 μm (Doi 2001).
Anamorph: Conidiophores verticillate, phialides in whorls of 3–5, slender, attenuated toward the tip, 14–32 × 1.7–2.8 μm; conidia colourless, ovate, obovate, ellipsoid, subcylindrical with a minutely truncate base, smooth, 2.6–6.2 × 1.4–2.3 μm; chlamydospores not formed (Doi 2001).
Habitat: Dead branches and twigs of Castanopsis cuspidata.
Known distribution: Japan.
Specimens examined: Type material was not available for examination.
Comments: This species can be identified from the others discussed here by its unique colour and pulvinate stroma. Pustulate-turberculate or nodulose ascospores are not common among species of Hypocrea with extensively effuse stromata and anamorphs assignable to Trichoderma sect. Hypocreanum. The minutely pustulate-tuberculate ascospores, and elongate perithecia of H. mikurajimensis suggest a close relationship to H. aurantiistroma, H. fulva, and H. megalocitrina.
-
Hypocrea pseudostraminea Yoshim. Doi, Bull. Natl. Sci. Mus. 15: 676. 1972. Figs 22, 23.
Anamorph: Trichoderma sp. [sect. Hypocreanum].
Teleomorph: Stromata effuse, varying from solitary and discrete to extensive confluent; largest continuous stroma 8 × 5 mm, smallest continuous stroma 3 × 2 mm, with a white byssoid margin, varying in colour, usually brownish orange to light brown (5C7–5E6); KOH+/–, reaction variable, weak when hyphal protrusions not abundant; KOH+, reaction strong, when hyphal protrusions abundant, stroma typically turning reddish brown; ostiolar canals visible at stroma surface, appearing brown. Stroma surface appearing granular due to minutely roughened hyphal protrusions at the stroma surface; tissue immediately below the stroma surface formed of loose pseudoparenchymatous cells and t. intricata, anastomosing to form a more compact layer of t. angularis; stroma tissue more angular near the centre of the stroma, tissue near the margin becoming more hyphal. Pseudoparenchymatous tissue below the perithecia formed of a loose t. globulosa and t. intricata. Perithecia completely immersed, tightly compacted near the stroma centre, fewer near the margin. Perithecia subglobose to ellipsoidal, (133–)145–190(–207) μm long (including the length of the ostiolar canal (n = 29); width of the perithecia near the base (measured from 3/4 total length of the perithecium), (74–)89–127 (–147) μm (n = 29); length of ostiolar canal (32–)40–58 (–67) μm; width of ostiolar canal (25–)29–41(–43) μm (n = 29); wall KOH+/–, reaction variable, weak, usually orangish brown. Asci cylindrical, (48–)58–79(–92) × (3.3–)4.1–5.7(–7) μm (n = 158), tip slightly thickened. Part-ascospores hyaline, spinulose, dimorphic; distal part subglobose, sometimes subellipsoidal, (2.3–)3–4 (–5.5) × (2.3–)2.8–3.6(–4.2) μm, L/W ratio (0.8–)0.97–1.2(–1.5) (n = 192); proximal part ellipsoidal, sometimes subglobose to ovate, (2.2–)3.5–4.5(–6.2) × (2–)2.6–3.3(–4) μm, L/W ratio (0.9–)1.2–1.6(–2) (n = 192).
Anamorph: Colonies growing across the surface of a 100 mm diam Petri plate with PDA in 10 d, with a dense white layer of mycelium close to the agar surface and a uniform layer of cottony aerial mycelium above; conidiophores visible in the aerial mycelium, borne on long elements of aerial hyphae, irregularly branched; not all isolates producing conidiophores on PDA; no odour; light brown pigment (7D7) near the edge of the plate; stroma initials observed on PDA at 10 d in the same isolates that produce conidiophores; chlamydospores not observed at 10 d. Colonies growing across the surface of a 100 mm diam Petri plate with SNA in 10 d, producing a thin layer of effuse mycelium covering the agar surface; no odour; no pigment; no conidiophore production. Colonies growing across the surface of a 100 mm diam Petri plate with CMD in 10 d, producing a thin layer of mycelium close to the agar surface; conidiophores visible in the aerial mycelium, especially near the edge of the plate; conidiophores verticillium-like, irregularly branched, borne on long hyphal elements; no odour; no pigment production; stroma initials (aggregates of mycelium leading to the production of stromata) present; some isolates do not form conidiophores in culture; chlamydospore-like swellings observed on CMD. Optimal temperature for growth is 25–30 °C.
Measurements of micro-morphological characters were the same on all three media. A description based on measurements from all three media at 10 d follows: conidiophores irregularly verticillate, phialides solitary and alternating, or in whorls of 3–5, subulate, (9–)14–21(–36) × (1.5–)2.3–3.2(–3.8) μm (n = 67); conidia variable, subellipsoidal to elongate ellipsoidal, (3–)4.7–6.4(–10) × (2–)2.4–3.1(–3.4) μm (n = 67), sometimes with asymmetric, truncate base.
Habitat: On bark of Quercus, Rhododendron, possibly lichenicolous, possibly fungicolous as found growing on Armillaria rhizomorphs.
Known distribution: Japan, North America, and France.
Paratypes examined: Japan, Hirakura, the Forestry Experiment Station, Mie Pef., on bark, 7 Sep. 1965, Yoshim. Doi (NY ex TNS.D-56 = TNSF 19051); Tsushima Island, Nagasaki Pref., on bark, 27 Sep. 1968, Yoshim. Doi (NY ex TNS.D-487 = TNSF 223484).
Other specimens examined: France, Osserain, on Phyllostachys sp., 22 Oct. 1989, F. Candoussau No. 4805-16 (BPI 1107143). U.S.A., Indiana, Brown Co., vic. of Pike's Peak, Happy Hollow Camp, 39°09' N, 86°06' W, elev. 250 m, on root, 29 Sep. 1995, G. J. Samuels (BPI 737763; culture G.J.S. 95-189); Kentucky, Daniel Boone National Forest, Cave Run Lake, Sheltowee Trail from Stone Cave, on decayed lichen on bark, 26 Sep. 1995, G. J. Samuels (BPI 737729, culture G.J.S. 95-169); Maryland, Howard Co. and Carroll Co. border, Paptapsco State Park, outside of Sykesville, on rotten wood and Armillaria sp. rhizomorphs, 12 Oct. 1999, B.E. Overton, B.E.O. 99-36 (BPI; culture B.E.O. 99-36); Prince Georges Co., E. of Largo in old growth forest at Church Rd., on decorticated wood, 11 Oct. 1991, G. J. Samuels, S. Rehner, A.Y. Rossman, & F.A. Uecker (BPI 1112904); New York, Dutchess Co., E. side of Pawling Nature Reserve, on bark, 6 Oct. 1990, G.J. Samuels & C.T. Rogerson (BPI 1107187; culture G.J.S. 90-74).
Comments: Specimen B.E.O. 99-36 has roughened hyphal protrusions near the stroma surface that are more prevalent and developed than in the other specimens examined and it was the only collection from those examined on Armillaria sp. rhizomorphs. In addition, molecular data suggest that this collection represents a unique phylogenetic species. However, the ascospore measurements and anamorph characteristics of B.E.O. 99-36 are identical to the type of H. pseudostraminea. At this time, it is unclear whether this collection represents a unique species as more isolates have to be sequenced. The orange-brown stromata, granular appearance of the stromata, and part-ascospore measurements allow H. pseudostraminea to be distinguished from H. microcitrina. Hypocrea lactea sensu Doi has hyphal protrusions near the stroma surface similar to those observed in H. pseudostraminea.
-
Hypocrea microcitrina Yoshim. Doi, Bull. Natl. Sci. Mus. 15: 667. 1972. Figs 24, 25.
Anamorph: Trichoderma sp. [sect. Hypocreanum].
Teleomorph: Stromata effuse, extensive and confluent, largest continuous stroma 12 × 11 mm, smallest continuous stroma 2 × 2 mm, with a white byssoid margin, varying in colour, greyish yellow to orangish brown, sometimes light yellow (4B5–5E4, 3A4), ostiolar canals visible at stroma surface, appearing orange (5A4). KOH+, reaction variable, stromata becoming darker orange. Stroma surface appearing granular due to minutely roughened hyphal protrusions, tissue at stroma surface formed of loose to compact t. intricata. Pseudoparenchymatous tissue below the perithecia of t. intricata or t. globulosa, variable among collections, sometimes compact or sometimes in loose aggregates. Perithecia completely immersed, tightly compacted near the stroma centre, fewer near the margin. Perithecia subglobose to ellipsoidal, (176–)180–205(–210) μm high (including the length of the ostiolar canal (n = 16); width of perithecia near the base (measured from 3/4 total length of the perithecium); (80–)88–126(–140) μm (n = 16); length of ostiolar canal (33–)38–53(–58) μm; width of ostiolar canal (35–)38–50(–53) μm (n = 16); wall KOH+/–, reaction variable, usually becoming dark orange. Asci cylindrical, (48–)53–67(–77) × (3.3–) 3.9–4.9(–5.8) μm (n = 105), tip slightly thickened. Part-ascospores hyaline, thick-walled, spinulose, dimorphic; distal part subglobose, sometimes subellipsoidal, (2.3–) 2.7–3.5(–4) × (2.3–)2.7–3.3(–3.8) μm, L/W ratio (0.75–)0.9–1.2(–1.4) (n = 140); proximal part, ellipsoidal, sometimes subglobose to ovoidal, (2.6–)3.1–3.9(–4.7) × (1.9–)2.4–2.9(–3.6) μm, L/W ratio (0.9–)1.1–1.5(–1.8) (n = 192).
Anamorph: Colonies growing across the surface of a 100 mm diam Petri plate with PDA in 10 d; not producing concentric rings or radial rays of mycelium; a dense layer of cottony aerial mycelium covering the entire Petri plate; sometimes aerial mycelium producing yellowish brown exudates; conidiophores not visible in the aerial mycelium at 10 d; only some conidiophore aggregates near the inoculation plug and the edge of the plate; conidiophores irregularly verticillate; phialides subulate, typically in whorls of 3–5, but also solitary or in groups of two, (8–)13–22(–33) × (1.3–)2.3–3.2(–4.3) μm (n = 50); conidia hyaline, smooth, ellipsoidal to elongate ellipsoidal, variable in size, (3.7–)4.8–6(–12) × (1.9–)2.3–2.9(–3.6) μm (n = 69), without a well-defined flat edge, rarely with a septum; chlamydospores not observed at 10 d on PDA; culture producing yellowish grey diffusible pigment (4B6), near the agar plug appearing darker orange (5B8) to brown (5D7). After 10 d conidia begin to swell, becoming larger and more variable in size. Colonies growing across the surface of a 100 mm diam Petri plate with SNA in 10 d; a thin layer of effuse mycelium covering the whole surface; no odour, no pigment production; no conidiophore production. Colonies growing across the surface of a 100 mm diam Petri plate with CMD in 10 d, producing a thin layer of effuse mycelium covering the agar; no conidiophore production, no odour, and no pigment production. Optimal temperature for growth is 25 °C on all three media.
Habitat: On bark, possibly fungicolous.
Known distribution: Japan and North America.
Type: The type of H. microcitrina was not available for study.
Specimens examined: U.S.A., Georgia, Rayburn Co., Chatachoochie National Forest, vic. Clayton, Warwomen Dell., elev. 750 m, E. Lieckfeldt & G. J. Samuels (BPI 744660; culture G.J.S. 97-248); New Jersey, Newfield, on leaf, Oct. 1894, Ellis & Everhart 3217 (BPI 631561); Virginia, Giles Co., Mt. Lake Biological Station, vic. Little Spruce Bog, 37°22' N, 80°31' W, elev. 1170 m, growing on black perithecial fungus on bark, 17 Sep. 1991, G. J. Samuels, C. Rogerson, S. Huhndorf, S. Rehner & M. Williams (BPI 1112833; culture G.J.S. 91-61); Giles Co., Mt. Lake Biological Station, Horse Barn Trail, 37°22' N, 80°3' W, on decorticated wood, 19 Sep. 1991, G. J. Samuels, C. Rogerson, S. Huhndorf, S. Rehner & M. Williams (BPI 1112839).
Comments: Hypocrea microcitrina and H. pseudostraminea are similar species and difficult to distinguish (Doi 1972). The stroma surface of H. microcitrina is composed of t. intricata with hyphae that do not anastomose to form a thick-walled t. globulosa or t. angularis as in H. pseudostraminea. It is difficult to interpret whether the hyphal elements near the stroma surface of H. microcitrina are hyphal protuberances as in H. pseudostraminea or just a loose layer of t. intricata. Regardless of homology, the hyphal elements in H. microcitrina macroscopically make the stroma surface appear granular as in H. pseudostraminea. Hypocrea microcitrina has slightly smaller ascospores than H. pseudostraminea. Hypocrea microcitrina was previously only known from Japan and has not previously been reported from the United States, where it also appears to be common on woody substrata.
-
Hypocrea albocitrina Yoshim. Doi, Bull. Natl. Sci. Mus. 15: 661. 1972. Fig. 26.
Anamorph: Trichoderma sp. [sect. Hypocreanum].
Teleomorph: Stromata effuse, solitary to confluent, largest continuous stromata 5 × 3 mm, smallest continuous stromata 1 × 1 mm, stromata pastel-yellow (3A4), KOH–, tissue below the stroma surface of t. globulosa to t. intricata, loosely arranged; ostiolar canals visible from the stroma surface, appearing yellow; perithecia globose, widely spaced, not abundant. Asci cylindrical, (63–)66–78(–83) × (4.8–)4.9–5.7(–6) μm (n = 17), tip slightly thickened. Asci with (12–14–)16 part-ascospores; ascospores often segregating into 4 large or 4 small spores; segregation pattern of part-spores 4 large to 4 small and 8 large to 8 small common, with some 4 to 8 to 4. Asci with fewer than 16 part-spores usually showing segregation patterns of 2 to 8 to 2, or 2 to 8 to 4; the smaller part-ascospores then appearing to be aborted or missing; regardless of segregation pattern, the smaller part-ascospores are not as mature or well-formed as the larger part-ascospores. Part-ascospores hyaline, thick-walled, spinulose; smaller part-spores subglobose to subellipsoidal, (2.3–)2.8–3.6(–3.8) × (2.4–)3–3.7(–4.0) μm, L/W ratio (0.7–)0.84–1.08(–1.4) (n = 38); larger part-spores subglobose to ellipsoidal, (3.9–)4.3–5.1(–5.5) × (3.3–)3.7–4.5(–4.8) μm, L/W ratio (0.9–)1–1.3(–1.5) (n = 38).
Anamorph: verticillium-like (fide Doi 1972). Conidiophores irregularly verticillate, forming minute colonies; phialides slender, 13–45 × 2–4 μm; conidia hyaline, smooth, cylindrical to ellipsoidal, with a truncate base, 2.8–19 × 2–4 μm (Doi 1972).
Habitat: On decayed branches of Abies firma.
Known distribution: Japan.
Isotype: Japan, Tsushima Island, Nagasaki Pref., Mt. Mitake, on bark, 28 Sep. 1968, Y. Doi (NY ex TNS-F-190517).
Comments: The isotype material is in good condition. The segregation of ascospores based on size is a unique character that distinguishes this species from others in subsect. Citrinae.
-
Hypocrea protocitrina Yoshim. Doi, Bull. Natl. Sci. Mus. 15: 660. 1972.
Anamorph: Unknown.
Teleomorph: Stromata effuse, thin, yellow, up to 4 × 12 mm, 200–240 μm thick; internal tissue of the stromata of t. intricata; perithecia entirely embedded in the stroma, obovate-subglobose, 110–130 μm high; perithecial necks, 20–30 μm long; part-ascospores hyaline, very minutely warted; distal part subglobose to obovate-ellipsoid, 1.9–2.5 × 1.7–2.1 μm; proximal part obovate-ellipsoid, 2.5–3.5 × 1.6–2.0 μm (Doi 1972).
Habitat: On decayed bamboo.
Known distribution: Japan.
Isotype: Japan, Tsushima Island, Nagasaki Pref., Toshibachi Izuhara-Cho, on bamboo, 26 Sep. 1968, Y. Doi (NY ex TNS D-482).
Comments: The isotype material is immature and limited in quantity not allowing for destructive sampling. The minute ascospores of H. protocitrina distinguish this species from all others that Doi (1972) included in subsect. Citrinae.
Fig. 8.
A–G. Anamorph of Hypocrea citrina. A. Aerial mycelium with irregular verticillium-like conidiophores, CBS 894.85 on PDA; bar = 20 μm. B, D. Bulbous terminal and globose intercalary chlamydospores, B.E.O. 99-29 on PDA; bar = 20 μm. C, G. Variation in conidial size and shape, B.E.O. 99-29 on PDA and CBS 894.85 on PDA, respectively; bar = 20 μm. E–F. Variation in phialide arrangement with typically 3–5 phialides per whorl, CBS 894.85 on PDA and B.E.O. 99-29, respectively; bar = 20 μm.
Fig. 9.
A–C. Hypocrea pulvinata, BPI 744722. A. Subpulvinate stromata; bar = 2 mm. B. Orange brown subpulvinate stromata and positive KOH reaction; bar = 2 mm. C. Ostiolar openings visible at stroma surface; bar = 1 mm.
Fig. 10.
A–F. Hypocrea pulvinata, BPI 744722. A. Section through stroma; bar = 40 μm. B. Ostiolar canal with pointed papillae; bar = 20 μm. C. Warted hyphal protuberances at stroma surface; bar = 20 μm. D. Vertically elongate t. angularis to tightly packed t. intricata below perithecia; bar = 20 μm. E–F. Asci with ascospores; bar = 20 μm.
Fig. 11.
A–C. Anamorph of Hypocrea pulvinata, G.J.S. 98-104. A. Solitary phialides on long hyphal branches, on PDA; bar = 20 μm. B. Chlamydospore-like swellings below phialides, on PDA; bar = 20 μm. C. Multiple phialides on branched hyphal elements, on PDA; bar = 20 μm.
Fig. 12.
A–C. Hypocrea americana, CUP 38045. A. Subpulvinate stroma; bar = 2 mm. B. Stromata with rugulose appearance, ostiolar openings not readily visible; bar = 1 mm. C. Colour reaction in KOH; bar = 1 mm.
Fig. 13.
A–E. Hypocrea americana, CUP 38045. A. Section of stroma showing tissue types; bar = 40 μm. B, E. Asci with ascospores; bar = 20 μm. C. Warted hyphal protuberances near stroma surface; bar = 20 μm. D. Tissue below perithecia; bar = 20 μm.
Fig. 14.
A–D. Anamorph of H. americana. A. Chlamydospore-like swellings below phialides, on PDA. B. Phialides on long hyphal elements, on SNA, both G.J.S. 96-191. C. Variability in conidial size and shape, on CMD, G.J.S. 92-93. D. Conidiophores with multiple branches, on CMD, G.J.S. 96-191; bars = 20 μm.
Fig. 15.
A–G. Hypocrea protopulvinata. A. Effuse to subpulvinate stromata, K.P. 00-56; bar = 2 mm. B. Reaction of stromata in KOH, B.E.O. 01-01. C. Asci with ascospores; bar = 20 μm. D. Roughened hyphal protuberances near stroma surface and t. intricata, bar = 20 μm; C. and D. TNS 223431. E. Cross section of perithecium; bar = 20 μm. F. Tissue near ostiolar canals of t. globulosa approaching t. angularis; bar = 20 μm; E. and F. K.P. 00-56. G. Tissue below base of perithecium, t. globulosa, sometimes with angular edges due to mutual compaction, TNS 223431; bar = 20 μm.
Fig. 16.
A–F. Anamorph of H. protopulvinata. A. Conidiophores arising from compact hyphal aggregates, on PDA; bar = 20 μm. B. Conidiophores arising from long hyphal elements, on CMD; bar = 20 μm. C. Branched conidiophores, on CMD; bar = 20 μm; A–C. CBS 739.83. D–E. Variation of conidial size and shape in K.P. 00-56 on PDA, and CBS 739.83 on CMD, respectively; bar = 20 μm. F. Branched conidiophores with slight swelling of hyphae below phialides, K.P. 00-56, on PDA; bar = 20 μm.
Fig. 17.
A–E. Hypocrea megalocitrina. A. Effuse stromata with projecting ostiolar canals, B.E.O. 99-42; bar = 2 mm. B. Stroma characteristics of the type, TNS 223220; bar = 2 mm. C. Variation in conidial size and shape; bar = 20 μm. D. Verticillium-like anamorph; bar = 20 μm. E. Phialides in whorls, usually of 3–5; bar = 20 μm; C–E. B.E.O. 00-09.
Fig. 18.
A–F. Hypocrea megalocitrina. A. Section of stroma showing protruding ostiolar canals and tissue types, B.E.O. 99-42; bar = 40 μm. B. Discharged nodulose ascospores, TNS 223220; bar = 20 μm. C. Tightly packed t. intricata near stroma surface, sometimes appearing angular when tightly packed, sometimes loosely woven; bar = 20 μm. D. Vertically elongate t. globulosa to t. intricata below perithecium, no space between the hyphae; bar = 20 μm; C–D. L.G.1. E, F. Asci and conical ascospores, TNS 223220 and L.G.1, respectively; bar = 20 μm.
Fig. 19.
A–D. Hypocrea aurantiistroma, BPI 1107193. A. Ostiolar canals projecting from stroma surface; bar = 1 mm. B. Stroma surface including margin; bar = 2 mm. C–D. Asci and minutely nodulose ascospores; bar = 20 μm.
Fig. 20.
A–D. Hypocrea aurantiistroma, BPI 1107193. A. Section through stroma; bar = 40 μm. B. Textura intricata near ostiolar canal; bar = 20 μm. C. Cross section of protruding ostiolar canal; bar = 20 μm. D. Loose t. globulosa to tightly packed t. intricata below perithecium; bar = 20 μm.
Fig. 21.
A–E. Hypocrea fulva, syntype 410. A. Stromata; bar = 2 mm. B. Section of stroma surface including margin; bar = 40 μm. C–D. Cross sections showing stroma anatomy; bar = 40 μm. E. Asci and minutely nodulose ascospores; bar = 20 μm.
Fig. 22.
A–E. Hypocrea pseudostraminea. A. Effuse to subpulvinate stroma with white byssoid margin; bar = 1 mm. B. Reaction in KOH; bar = 1 mm; both TNS 223484. C. KOH-positive roughened hyphal protuberances, B.E.O. 99-36; bar = 20 μm. D. Asci and ascospores, TNS 223484; bar = 20 ìm. E. Cross section showing tissue near ostiolar canal and below perithecium, BPI 1112904; bar = 40 μm.
Fig. 23.
A–C. Anamorph of H. pseudostraminea, G.J.S. 91-135. A. Verticillium-like anamorph, on CMD. B. Variation in conidial size and shape, on CMD. C. Conidiophores on long hyphal element, on CMD; bars = 20 μm.
Fig. 24.
A–C. Hypocrea microcitrina, BPI 1112833. A. Effuse stroma with white byssoid margin; bar = 2 mm. B. Section of stroma in KOH; bar = 40 μm. C. KOH reaction of stroma; bar = 1 mm. D–E. Section showing tissue types; bar = 40 μm. F. Tissue below perithecium composed of t. globulosa with angular edges due to mutual compaction; bar = 20 μm. G. Ascus with ascospores; bar = 20 μm.
Fig. 25.
A–F. Anamorph of H. microcitrina. A. Sterile aerial mycelium, G.J.S. 91-61; bar = 20 μm. B. Irregular verticillium-like anamorph with variable branching pattern, on PDA; bar = 20 μm. C–D. Phialides in varying numbers, usually not more than 3 per node, on PDA; bar = 20 μm. E. Variation in conidial size and shape, on PDA; bar = 20 μm. F. Solitary phialides on long hyphal extensions, on PDA; bar = 20 μm; B–F. G.J.S. 97-248.
Fig. 26.
A–F. Hypocrea albocitrina, TNS 190517. A. Effuse stromata; bar = 1 mm. B. Ostiolar canals visible at stroma surface; bar = 1.5 mm. C. Section of stroma showing tissue types; bar = 40 μm. D. Asci with ascospores segregating, different sizes; bar = 20 μm. E–F. Stroma tissue composed of loosely woven t. globulosa and t. intricata near the stroma surface and below the perithecium; bar = 20 μm.
KEY TO HYPOCREA CITRINA AND ALLIES
1. Stromata attached by a narrow base; part-ascospores spinulose, monomorphic, globose, 2.1–2.3 μm diam, or sometimes obovate-ellipsoid, 2.0–3.0 μm long................................... 5. H. platypulvinata
1. Stromata effuse to pulvinate............................................................................................................................... 2
2. Part-ascospores monomorphic without ornamentation; on polypores................................................................ 3
2. Part-ascospores not as above; mostly on other substrata................................................................................. 4
3. Part-ascospores ellipsoidal, (2.8–)3.8–5(–6.7) × (2.3–)2.8-3.8(–4.7) μm..................................... 2. H. pulvinata
3. Part-ascospores, subglobose to rhomboidal, (1.7–)2.6–3.8(–6.2) × (2.4–)3.2–4.2(–5.4) μm............................................................................................................ 3. H. americana
4. Part-ascospores smooth, dimorphic; distal part subglobose, (3–)3.3–3.9 (–4.3) × (2.6–)3–3.5(–4.2) μm; proximal part ellipsoidal, sometimes subglobose, (3.2–)3.0–4.2 (–4.8) × (2.4–)2.7–3.3(–3.8) μm; on polypores (Fomitopsis and others).............................. 5. H. protopulvinata [If part-ascospores slightly smaller and anamorph Gliocladium; on soft polypores (e.g. Tyromeces), see H. pallida]
4. Part-ascospores spinulose or nodulose; not on polypores................................................................................. 5
-
5. Stromata white to yellow, effuse, and with pseudoparenchymatous tissue near the surface; on leaf litter, soil, bark, or moss; part-ascospores spinulose, distal part subglobose, sometimes subellipsoidal, (3.2–)4.1–5.2(–6.3) × (2.9–)3.7–4.8(–5.7) μm; proximal part ellipsoidal, sometimes subglobose to ovate, (3.3–)4.4–6(–7.1) × (2.5–)3.3–4.9(–6.3) μm................1. H. citrina
5. Stromata variable in colour, effuse or pulvinate, hyphal to pseudoparenchymatous; growing on decorticated wood, or old fructifications of fungi on bark; part-ascospores spinulose or nodulose........................................................................................................................................ 6
6. Stroma surface with hyphal protuberances, extensive in some specimens, minimal in others, arising from a well-defined layer of pseudoparenchymatous tissue, KOH+/–, variable, stromata orange-brown to light brown; on bark, possibly fungicolous; part-ascospores spinulose; distal part subglobose, sometimes subellipsoidal, (2.3–)3–4(–5.5) × (2.3–)2.8–3.6(–4.2) μm; proximal part ellipsoidal, sometimes subglobose to ovate, (2.2–)3.5–4.5(–6.2) × (2–)2.6–3.3(–4) μm.................................. 10. H. pseudostraminea
6. Stroma surface without hyphal protuberances arising from a pseudoparenchymatous layer near the surface............................................................................................................................ 7
7. Part-ascospores spinulose................................................................................................................................. 8
7. Part-ascospores nodulose................................................................................................................................. 10
8. On bark; stroma surface composed of t. intricata, greyish yellow to orange-brown; distal part-ascospores subglobose, sometimes subellipsoidal, (2.3–)2.7–3.5(–4) × (2.3–)2.7–3.3(–3.8) μm; proximal part ellipsoidal, sometimes subglobose to ovoidal, (2.6–)3.1–3.9(–4.7) × (1.9–)2.4–2.9(–3.6) μm......................................................... 11. H. microcitrina
8. On bamboo or with eight large and eight small part-ascospores........................................................................ 9
9. On bamboo; stroma surface composed of t. intricata, yellow; part-ascospores hyaline, minutely warted; distal part subglobose to obovate-ellipsoid, 1.9–2.5 × 1.7–2.1 μm, proximal part obovate-ellipsoid, 2.5–3.5 × 1.6–2.0 μm................................... 13. H. protocitrina
9. On decayed tree branches; stroma surface of loose to compated t. globulosa with t. intricata, yellow; with eight large and eight small, hyaline part-ascospores; smaller part-spores subglobose to subellipsoidal, (2.3–)2.8–3.6(–3.8) × (2.4–)3–3.7(–4.0) μm; larger part-spores subglobose to ellipsoidal, (3.9–)4.3–5.1(–5.5) × (3.3–)3.7–4.5(–4.8) μm............................................................................................................................ 12. H. albocitrina
10. Stroma surface effuse to subpulvinate, composed mostly of t. intricata with ostiolar canals projecting from stroma surface.............................................................................................................. 11
10. Stroma surface pulvinate with pseudoparenchymatous tissue......................................................................... 12
11. Stroma surface greyish orange; part-ascospores slightly dimorphic, almost appearing monomorphic; distal part subellipsoidal, slightly conical, (3.6–)4.5–5(–5.9) × (2.5–)3.3–4(–4.6) μm; proximal part ellipsoidal, rarely subellipsoidal, slightly conical, (3.6–)4.2–5.4(–6.4) × (3.1–)3.3-4(–4.6) μm......................................................................... 7. H. aurantiistroma
11. Stroma surface light brown; part-ascospores dimorphic, distal part subellipsoidal to conical, (3.2–)4.5–5.8(–6.6) × (3–)3.8–5(–6) μm; proximal part ellipsoidal, rarely subellipsoidal, sometimes appearing thimble-shaped or conical, (3.7–)4.6–5.9(–7) × (3.1–)3.7–4.8(–5.8) μm......................................................................... 6. H. megalocitrina
12. Part-ascospores dimorphic; distal part subellipsoidal, slightly conical, (3.9–)4.5–5.5(–6.2) × (2.8–)3.2–3.8(–4.3) μm; proximal part ellipsoidal, rarely conical, (4.3–)4.6–5.6(–6.5) × (2.8–)3.0–3.6 (–4.1); stromata yellow-brown.................................................................................... 8. H. fulva
12. Part-ascospores monomorphic, globose to subglobose, 2.2–3.2 × 2.0–2.8 μm; stromata mustard-yellow................................................................................................... 9. H. mikurajimensis
Acknowledgments
We wish to acknowledge the following persons for their contributions to this manuscript: Dr Gary J. Samuels for his comments on the systematics of H. citrina, for the photographs of H. lactea, and access to specimens, Dr Ronald Petersen for his assistance in questions of nomenclature, Dr E. Parmasto for his identification of polypores in our study, Dr Elke Lieckfeldt (deceased Aug. 2002) for her support and comments with the initial planning stages of the molecular portion of this project, Dr Yoshimichi Doi for making specimens available for study during the first author's work in Japan, and Dr Walter Gams for rendering the Latin diagnoses and his editorial comments on the manuscript. Dr. James Bray identified the moss Drepanocladus uncinatus (Hedw.) Warnst. This study was supported by the United States National Science Foundation (PEET) grant 9712308 “Monographic Studies of Hypocrealean Fungi: Hypocrea and Hypomyces”.
Taxonomic novelties: A neotype is designated for Hypocrea citrina (Pers: Fr.) Fr. and a lectotype for Hypocrea lactea (Fr.: Fr.) Fr.; H. pulvinata Fuckel is lectotypified; Hypocrea aurantiistroma Overton, sp. nov., Hypocrea americana, stat. nov.
References
- Bissett J (1991a). A revision of the genus Trichoderma. II. Infrageneric classification. Canadian Journal of Botany 69: 2357-2372. [Google Scholar]
- Bissett J (1991b). A revisjon of the genus Trichoderma. III. Section Pachybasium. Canadian Journal of Botany 69: 2372-2418. [Google Scholar]
- Canham SC (1969). Taxonomy and morphology of Hypocrea citrina. Mycologia 51: 315–331. [Google Scholar]
- Chaverri P, Castlebury LA, Samuels GJ, Geiser DM (2003a). Multilocus phylogenetic structure within the Trichoderma harzianum/Hypocrea lixii complex. Molecular Phylogenetics and Evolution 27: 302–313. [DOI] [PubMed] [Google Scholar]
- Chaverri P, Castlebury LA, Overton BE, Samuels GJ (2003b). Hypocrea/Trichoderma: species with conidiophore elongations and green conidia. Mycologia 95: 1100–1140. [PubMed] [Google Scholar]
- Chaverri P, Samuels GJ, Stewart EL (2001). Hypocrea virens sp. nov., the teleomorph of Trichoderma virens. Mycologia 93: 1113–1124. [Google Scholar]
- Dingley JM (1955). Notes on Hypocrea citrina (Pers.) Fr., H. lactea Fr., H. pulvinata Fuckel and H. sulphurea (Schw.) Sacc. Kew Bulletin 9: 575–578. [Google Scholar]
- Dodd SL, Lieckfeldt E, Chaverri P, Overton BE, Samuels GJ (2002). Taxonomy and phylogenetic relationships of two species of Hypocrea with Trichoderma anamorphs. Mycological Progress 1: 409–428. [Google Scholar]
- Doi Y (1972). Revision of the Hypocreales with cultural observations IV. The genus Hypocrea and its allies in Japan (2). Enumeration of the species. Bulletin of the National Science Museum Tokyo, Japan 15: 649–751. [Google Scholar]
- Doi Y (2001). A new species of Hypocrea (Ascomycota, Hypocreales) from Mikurajima Island, Japan. Memoirs of the National Science Museum Tokyo, Japan 37: 113–118. [Google Scholar]
- Fries EM (1816). Upstallning af de i Sverige funne Vartsvampar (Scleromyci). Kungliga Svenska VetenskapsAkademiens Handlingar II, 37: 126–157. [Google Scholar]
- Fries EM (1818). Observationes Mycologicae. II. Hafniae.
- Fries EM (1823). Systema Mycologicum. 2(2): 275–620. Lund. [Google Scholar]
- Fries EM (1849). Summa vegetabilium Scandinaviae. Sectio posterior p. 259–572.
- Fuckel L (1870). Symbolae Mycologicae. Jahrbücher des Naussauischen Vereins für Naturkunde 23–24, 459 pp., 6 plates. [Google Scholar]
- Greuter W, McNeill J, Barrie FR, Burdet HM, Demoulin V, Filgueras TS, Nicholson DH, Silva PC, Skog JE, Trehane P, Turland NJ, Hawksworth DL (2000). International Code of Botanical Nomenclature (St. Louis Code). Regnum Vegetabile 138: 389 pp. http://www.bgbm.fuberlin.de/iapt/nomenclature/code/SaintLouis/0000St.Luistitle.htm.
- Karsten PA (1873). Mycologia Fennica 2: 1–250. [Google Scholar]
- Kornerup A, Wanscher JH (1981). Taschenlexikon der Farben. 3rd ed. Muster-Schmidt Verlag, Zürich, Göttingen. 242 pp.
- Kullnig-Gradinger CM, Szakács G, Kubicek CP (2002). Phylogeny and evolution of the genus Trichoderma: a multigene approach. Mycological Research 106: 757–767. [Google Scholar]
- Liu YJ, Whelen S, Hall BD (1999). Phylogenetic relationships among Ascomycetes: Evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Müller E, Aebi B, Webster J (1972). Culture studies on Hypocrea and Trichoderma V. Hypocrea psychrophila sp. nov. Transactions of the British Mycological Society 58: 1–4. [Google Scholar]
- Niessl G (1883). In Rehm H, Ascomyceten, fasc. XIV. Hedwigia 22: 52–61 (p. 53). [Google Scholar]
- Nirenberg HI (1976). Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 169: 1–117. [Google Scholar]
- O'Donnell K, Kistler HC, Cigelnik E, Ploetz RC (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Science USA. 95: 2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton BE, Samuels GJ, Stewart EL (1999). Hypocrea citrina var. americana; a new polyporicolous Hypocrea sp. or an old concept? Abstr. 16th International Botanical Congress 2923 [Poster 68].
- Põldmaa K, Larsson E, Kõljalg U (2000). Phylogenetic relationships in Hypomyces and allied genera, with emphasis on species growing on wood-decaying homobasidiomycetes. Canadian Journal of Botany 77: 1756–1768. [Google Scholar]
- Persoon CH (1796). Observationes Mycologicae. I. Lipsiae. 115 pp.
- Rifai MA, Webster J (1966). Culture studies on Hypocrea and Trichoderma. III. H. lactea (= H. citrina) and H. pulvinata. Transactions of the British Mycological Society 49: 297–310. [Google Scholar]
- Saccardo PA (1883). Sylloge Fungorum. 2: 1–815. Padova. [Google Scholar]
- Seaver FJ (1910). The Hypocreales of North America. III. Mycologia 2: 48–92. [Google Scholar]
- Stewart EL, Liu Z, Crous PW, Szabó LJ (1999). Phylogenetic relationships among some cercosporoid anamorphs of Mycosphaerella based on rDNA sequence analysis. Mycological Research 103: 1491–1499. [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC (2000). Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31: 21–32. [DOI] [PubMed] [Google Scholar]
- Weese J (1927). Über Hypocrea pulvinata Fuckel. Mitteilungen aus dem Botanischen Institut der Technischen Hochschule in Wien 4: 28–32. [Google Scholar]
- White TJ, Bruns, T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for pylogenetics. In: Innis MA, Gefland DH, Sninsky JJ, & White TJ. (eds.): PCR protocols: a guide to methods and applications. New York: Academic Press: p. 315–322.
- Zhang N, Blackwell M (2002). Molecular phylogeny of Melanospora and similar pyrenomycetous fungi. Mycological Research 106: 148–155. [Google Scholar]