Abstract
The type species of the genus Hypocrea (Hypocreaceae, Hypocreales, Ascomycota, Fungi), H. rufa, is re-defined and epitypified using a combination of phenotype (morphology of teleomorphs and anamorphs, and characteristics in culture) and phylogenetic analyses of the translation-elongation factor 1α gene. Its anamorph, T. viride, the type species of Trichoderma, is re-described and epitypified. Eidamia viridescens is combined as Trichoderma viridescens and is recognised as one of the most morphologically and phylogenetically similar relatives of T. viride. Its teleomorph is newly described as Hypocrea viridescens. Contrary to frequent citations of H. rufa and T. viride in the literature, this species is relatively rare. Although both T. viride and T. viridescens have a wide geographic distribution, their greatest genetic diversity appears to be in Europe and North America. Hypocrea vinosa is characterised and its anamorph, T. vinosum sp. nov., is described. Conidia of T. vinosum are subglobose and warted. The new species T. gamsii is proposed. It shares eidamia-like morphology of conidiophores with T. viridescens, but it has smooth, ellipsoidal conidia that have the longest L/W ratio that we have seen in Trichoderma. Trichoderma scalesiae, an endophyte of trunks of Scalesia pedunculata in the Galapagos Islands, is described as new. It only produces conidia on a low-nutrient agar to which filter paper has been added. Additional phylogenetically distinct clades are recognised and provisionally delimited from the species here described. Trichoderma neokoningii, a T. koningii-like species, is described from a collection made in Peru on a fruit of Theobroma cacao infected with Moniliophthora roreri.
Keywords: Bayesian phylogeny, biogeography, biological control, cacao, endophytes, Hypocrea, Hypocreales, Hypocreaceae, molecular identification, morphological key, nomenclature, species identification, systematics, translation elongation factor 1-alpha
INTRODUCTION
Trichoderma viride Pers. (Hypocreales, Hypocreaceae) is one of the most commonly reported species of fungi. In only the two years 2004 and 2005 T. viride appeared in nearly 200 articles that were abstracted by CAB. The species is encountered in widely diverse contexts; a few examples of activities include organochlorine degradation as a soil fungus (Smith 1995), biological control in fungus-induced plant disease (Brown & Bruce 1999; Brown et al. 1999), and as the cause of disease in button mushrooms in India (Mishra & Singh 2005). It is said to effect seed germination of flowering plants (Celar & Valic 2005), and enhance phosphorus uptake by plants (Rudresh et al. 2005). It produces enzymes (Nobe et al. 2004), degrades cellulosic agricultural waste to alcohol (Baig et al. 2004), colonises leaf litter (Osono 2005) and is a normal inhabitant of soils (Roiger et al. 1991, Hagn et al. 2003). Do all these citations refer to only one species, T. viride? Kullnig et al. (2001) detected a shockingly high level of misidentification of strains that were reported in the literature as T. harzianum. If this experience is representative of the genus, as it is likely, then not all of these reports actually refer to T. viride. One example that is representative of the degree of inaccuracy in identification is that of a biocontrol fungus reported in the literature as T. viride (Bastos 1988, 1996 a, b) that was ultimately described as the new species T. stromaticum Samuels & Pardo-Schultheiss (Samuels et al. 2000); these two species are distantly related and morphologically and biologically highly dissimilar. Obviously, it is important to clarify the identity of T. viride, otherwise the literature is meaningless.
Bisby in 1939 stated that essentially there was only one species of Trichoderma, T. viride. In spite of some discordant indications, that view held sway until 1969 when Rifai (1969) monographed the genus and characterised T. viride as the only species having globose, warted conidia. This immediately raised suspicion about all reports of activity by Trichoderma species prior to 1969. Even with the description of T. saturnisporum and T. ghanense, both having warted conidia and both being members of T. sect. Longibrachiatum Bissett (Samuels et al. 1998), T. viride stood out because its conidia were globose as compared to ellipsoidal in the other species. Scanning electron microscopy (Meyer & Plaskowitz 1989) revealed the existence of two distinct patterns of conidial ornamentation within strains identified as T. viride, viz. more and less strongly warted. Strains having the less strongly warted conidia were segregated as T. asperellum Samuels et al. (Lieckfeldt et al. 1999; Samuels et al. 1999). In a study of variation within the morphological species T. viride, in addition to recognising T. asperellum and T. viride s. str., Lieckfeldt et al. (1999) noted the existence of two additional ITS-defined groups that had warted conidia, which they referred to as Vd and Ve. The group Vd was very closely related to Vb in its ITS1 and 2 sequences and its morphology. The group Ve was more distantly related and was phenotypically diverse, some of the few included strains having smooth conidia and others having warted conidia. They (Samuels et al. 1999) determined that the group Vb was “true” T. viride by comparison with the over two-hundred-year-old type specimen of the species that is preserved in Leiden. Despite differences in ITS sequences, Samuels et al. (1999) could not see consistent phenotypic differences between Vb and Vd that would support recognition of Vd as a separate taxon.
Bissett (1991a) proposed to include H. rufa/T. viride and its relatives in Trichoderma sect. Trichoderma, including also T. koningii Oudem. and T. atroviride P. Karst. The monophyly of this group either as Trichoderma sect. Trichoderma (e.g. Kullnig-Gradinger et al. 2002) or more recently simply as “the viride clade” (Samuels 2006), has been affirmed by DNA sequence analysis. Since the work of Lieckfeldt et al. (1999) we have obtained many additional specimens and cultures referable to the viride clade and are able to propose a revised taxonomy for this clade. In the present work we re-evaluate T. viride groups Vb and Vd and recognise group Vd as a distinct species.
Since the middle of the 19th century (Tulasne & Tulasne 1865), T. viride has been recognised as the anamorph of Hypocrea rufa (Pers.: Fr.) Fr., the type species of Hypocrea Fr. Like T. viride, H. rufa is possibly the most common name used in the identification of Hypocrea specimens. Hundreds of specimens in herbaria throughout the world are labelled “Hypocrea rufa”. However, even a quick glance at specimens shows that a plethora of species has been lumped under this name. For example, species such as H. minutispora B.S. Lu et al./T. minutisporum Bissett and H. pachybasioides Yoshim. Doi/T. polysporum (Link: Fr.) Rifai have both been incorrectly identified as the only distantly related H. rufa.
Webster (1964) provided the first modern description of H. rufa. It is a species that has a stroma that starts out semieffused and whitish to tan to reddish brown and pruinose and with age becomes darker and cushion-shaped; the ascospores are hyaline. In our continuing work with the viride clade we have found that especially the young stroma of most members of the clade is distinctive of a number of often sympatric species that are best distinguished by their Trichoderma anamorphs (Samuels et al. 2006a). We have found indistinguishable teleomorphs for both T. viride groups, Vb and Vd. This calls for a redefinition and redescription of H. rufa. In the present work we refine the description of H. rufa and provide an epitype for the species, we describe as new a teleomorph for T. viride group Vd, redescribe Hypocrea vinosa with its new anamorph T. vinosum, and describe the new species T. gamsii, T. neokoningii and T scalesiae.
MATERIALS AND METHODS
Isolates including NCBI GenBank accession numbers of gene sequences investigated in this study are listed in Table 1. The locations in European countries are indicated with coordinates and map sheets (MTB = Messtischblatt).
Table 1.
Strains used in phylogenetic analysis, their origin and GenBank numbers.
| Species | Strain1 | Geography | Substratum |
GenBank accession number
|
|
|---|---|---|---|---|---|
| ITS1 and 2 | tef1 | ||||
| H./T. viridescens (An)3 | G.J.S. 04-232 | Mexico | soil under Agave tequillensis | DQ841736 | DQ841716 |
| H./T. viridescens (An) | CBS 439.95 | Northern Ireland | mushroom compost | DQ315439 | AY937413 |
| H./T. viridescens | CBS 333.72 | Netherlands | unknown | DQ315441 | DQ307523 |
| H./T. viridescens (An) | CBS 438.95 | Northern Ireland | mushroom compost | DQ315438 | DQ307522 |
| H./T. viridescens | G.J.S. 99-175 | Australia (VI) | Hypoxylon sp. on Nothofagus cunninghamii | DQ315437 | DQ307521 |
| H./T. viridescens (An) | G.J.S. 97-274 = BBA 68432 | Russia | cardboard | DQ315440 | DQ307505 |
| H./T. viridescens (An) | J.B. NZ61 | New Zealand, Northland | Poukani Forest, soil under fern | DQ845419 | |
| H./T. viridescens | G.J.S. 99-142 | Australia (VI) | bark | DQ315427 | DQ307512 |
| H./T. viridescens | G.J.S. 99-128 | Australia (VI) | bark | DQ315431 | DQ307515 |
| H./T. viridescens (An) | G.J.S. 04-81 | Italy | soil | DQ841740 | DQ841709 |
| H./T. viridescens | G.J.S. 98-182 = W.J. 1223 = CBS 120067 | Austria | Carpinus | DQ315425 | DQ307511 |
| H./T. viridescens | G.J.S. 89-142 | U.S.A. (NC) | decorticated wood | DQ109532 | AY376049 |
| H./T. viridescens | G.J.S. 94-118 = IMI 374788 | France | Carpinus bark | DQ315424 | DQ307510 |
| H./T. viridescens | G.J.S. 98-129 = CBS 101928 | France | bark | AY737773 | DQ307542 |
| H./T. viridescens | CBS 119323 | Germany | Picea abies, wood | DQ677648 | DQ672607 |
| H./T. viridescens | C.P.K. 2046 | U.K. | Fagus sylvatica, wood | DQ677649 | DQ672608 |
| H./T. viridescens (An) | ATCC 32630 | Sweden | Fagus wood | DQ315445 | DQ307526 |
| H./T. viridescens (An) | G.J.S. 99-11 = N.R. 6969 | Germany | soil | DQ841743 | DQ841717 |
| H./T. viridescens (An) | C.P.K. 2138 | Germany | Picea abies, wood | DQ677647 | DQ672606 |
| H./T. viridescens (An) | Tr 6 | U.S.A. (OR) | Pseudotsuga menziesii, root infected with Phellinus weirii | DQ315444 | AY376050 |
| H./T. viridescens (An) | Tr 5 | U.S.A. (OR) | Pseudotsuga menziesii, root infected with Phellinus weirii | DQ315443 | DQ307525 |
| H./T. viridescens (An) | G.J.S. 92-11 = ICMP 16297 | New Zealand | Pinus radiata, wood | DQ315442 | DQ307524 |
| H./T. viridescens | CBS 119321 = C.P.K. 2140 T4 of H. viridescens, Epitype of T. viridescens | Austria | Fagus sylvatica, wood | DQ677651 | DQ672610 |
| H./T. viridescens (An) | G.J.S. 99-8 = N.R. 5541 | Japan | leaf litter | DQ315433 | DQ307517 |
| H./T. viridescens (An) | CBS 274.79 | Austria | wood | DQ315428 | DQ307513 |
| H./T. viridescens | C.P.K. 947 | Austria | Picea abies, wood | AY665592 | DQ672604 |
| H./T. viridescens (An) | C.P.K. 2069 | Italy (Sardinia) | soil | DQ790657 | |
| H./T. viridescens | C.P.K. 2043 | Austria | Fagus sylvatica, wood | DQ677646 | DQ672605 |
| H./T. viridescens (An) | G.J.S. 05-464 | U.K. | Fagus sylvatica, trunk endophyte | DQ841714 | |
| H./T. viridescens (An) | G.J.S. 04-202 | Switzerland | soil | DQ841729 | DQ841710 |
| H./T. viridescens (An) | G.J.S. 97-272 = BBA 66069 | Germany | soil | DQ315429 | DQ307504 |
| H./T. viridescens (An) | G.J.S. 99-10 = N.R. 5510 | Czech Republic | soil | DQ315430 | DQ307514 |
| H./T. viridescens (An) | ATCC 20898 | U.S.A. (NY) | soil | DQ315434 | DQ307518 |
| H./T. viridescens (An) | C.P.K. 2084 | Italy (Sardinia) | soil | DQ790658 | |
| H./T. viridescens (An) | J.B. PER43 | Peru (Cuzco Dept, La Raya) | soil | DQ845420 | |
| H./T. viridescens (An) | J.B. PER52 | Peru (Lima Dept, San Luís) | soil | DQ845421 | |
| H./T. viridescens | G.J.S. 05-185 | Iran | Vitis sylvestris | DQ841732 | DQ841720 |
| H./T. viridescens (An) | G.J.S. 98-86 | Mexico | decorticated wood | DQ315423 | DQ307509 |
| H./T. viridescens (An) | CBS 119322 | Great Britain | Fagus sylvatica, wood | DQ677650 | DQ672609 |
| H./T. viridescens (An) | C.P.K. 999 | Russia (Central St. For. Biosphere Reserve) | soil | AY665699 | AY665707 |
| H./T. viridescens (An) | C.P.K. 998 | Russia (Central St. For. Biosphere Reserve) | soil | AY665698 | AY665706 |
| H./T. viridescens(An) | G.J.S. 05-482 | U.K. | Fagus sylvatica, stem endophyte | DQ841728 | |
| H./T. viridescens (An) | G.J.S. 99-18 | Japan | Pinus radiata, wood | DQ315435 | DQ307519 |
| H./T. viridescens | Tr 4 | U.S.A. (OR) | Pseudotsuga menziesii, root | DQ315436 | DQ307520 |
| H./T. viridescens (An) | CBS 433.34 T of Eidamia viridescens | apple fruit | AF456922 | AF456905 | |
| Vd 3 (An) | G.J.S. 00-67 | U.S.A. (WV) | decorticated wood | DQ315418 | DQ307502 |
| Vd 3 (An) | G.J.S. 97-243 | U.S.A. (GA) | decorticated wood | DQ315419 | DQ307503 |
| Vd 3 (An) | G.J.S. 94-11 | Taiwan | bark | DQ315422 | DQ307508 |
| Vd 3 (An) | G.J.S. 94-10 | Taiwan | decorticated wood | DQ315420 | DQ307506 |
| Vd 3 (An) | G.J.S. 94-9 | Taiwan | bark | DQ315421 | DQ307507 |
| H. vinosa/T. vinosum | G.J.S. 99-158 = ICMP 16294 = CBS 119087, T of T. vinosum, epitype of H. vinosa | New Zealand | Nothofagus menziesii | AY380904 | AY376047 |
| H. vinosa/T. vinosum | G.J.S. 02-54 = ICMP 16295 = CBS 119086 | New Zealand | Nothofagus menziesii | DQ315447 | DQ307528 |
| H. vinosa/T. vinosum | G.J.S. 99-183 | Australia | bark | DQ841744 | DQ841719 |
| H. vinosa/T. vinosum | G.J.S. 99-156 = ICMP 16293 | Australia | bark | DQ315446 | DQ307527 |
| H. vinosa/T. vinosum (An) | DAOM 176335 | unknown | unknown | ||
| Hypocrea sp. | RMF 78322 | unknown | unknown | ||
| Vd 1 | G.J.S. 03-151 = CBS 120068 | Ghana | pyrenomycete | DQ841738 | DQ841711 |
| Vd 1 | G.J.S. 02-87 | Sri Lanka | bark | DQ315461 | DQ307544 |
| Vd 2 (An) | C.P.K. 1488 | Italy (Sardinia) | soil | DQ845431 | DQ845416 |
| Vd 2 (An) | C.P.K. 2071 | Italy (Sardinia) | soil | DQ845429 | |
| Vd 2 (An) | G.J.S. 05-186 | Iran | Vitis sylvestris | DQ841731 | DQ841713 |
| Vd 2 (An) | J.B. NZ151 | New Zealand (Northland, Christchurch) | clay soil | DQ845422 | |
| Vd 2 (An) | PER232 | Peru (Puno Dept, Sillustani) | soil | DQ845423 | |
| T. gamsii (An) | C.P.K. 2070 = G.J.S. 06-07 = CBS 120073 | Italy (Sardinia) | soil | DQ790645 | |
| T. gamsii (An) | C.P.K. 2093 | Italy (Sardinia) | soil | DQ790656 | |
| T. gamsii (An) | C.P.K. 2092 | Italy (Sardinia) | soil | DQ790655 | |
| T. gamsii (An) | C.P.K. 2091 = G.J.S. 06-15 | Italy (Sardinia) | soil | DQ790654 | |
| T. gamsii (An) | C.P.K. 2090 = G.J.S. 06-14 | Italy (Sardinia) | soil | DQ790653 | |
| T. gamsii (An) | C.P.K. 2077 = G.J.S. 06-11 = CBS 120077 | Italy (Sardinia) | soil | DQ790650 | |
| T. gamsii (An) | C.P.K. 2076 | Italy (Sardinia) | soil | DQ790649 | |
| T. gamsii (An) | C.P.K. 2075 = G.J.S. 06-10 = CBS 120076 | Italy (Sardinia) | soil | DQ790648 | |
| T. gamsii (An) | C.P.K. 2073 = G.J.S. 06-09 = CBS 120075 T | Italy (Sardinia) | soil | DQ790647 | |
| T. gamsii (An) | C.P.K. 2071 = G.J.S. 06-08 = CBS 120074 | Italy (Sardinia) | soil | DQ790646 | |
| T. gamsii (An) | J.B. RSA9665 | Rep. South Africa (Kwa-Zulu Natal) | soil in Eucalyptus plantation | DQ845424r | |
| T. gamsii (An) | J.B. GS33 | Guatemala (Zacapa Dept, San Lorenzo) | soil in Pine-oak forest | DQ845425 | |
| T. gamsii (An) | J.B. R414 | Ruanda (Kigali) | soil | DQ845426 | |
| T. gamsii (An) | J.B. RSA9642A | Rep. South Africa (Cape Province, Capetown) | soil under Leucodendron | DQ845427 | |
| T. gamsii (An) | J.B. RO42B | Romania (Brasov) | flower garden soil | DQ845428 | |
| T. gamsii (An) | J.B. RSA75 | Rep. South Africa (Cape Province) | soil under Protea | DQ845430 | |
| T. gamsii (An) | C.P.K.2079 = G.J.S. 06-13 | Italy (Sardinia) | soil | DQ790652 | |
| T. gamsii (An) | G.J.S. 04-09 | Texas | soil | DQ315459 | DQ307541 |
| T. gamsii (An) | G.J.S. 92-60 | Australia | Eucalyptus nitens, stem endophyte | DQ315448 | DQ307529 |
| T. gamsii (An) | G.J.S. 05-111 = CBS 120072 | Italy (Pisa) | Ricinus communis, stem | DQ841730 | DQ841722 |
| T. gamsii (An) | C.P.K. 1010 | Russia (Central St.For. Biosphere Reserve) | soil | DQ845432 | DQ845417 |
| T. gamsii (An) | C.P.K. 1011 | Russia (Central St.For. Biosphere Reserve) | soil | DQ845433 | DQ845418 |
| T. gamsii (An) | C.P.K. 2078 = G.J.S. 06-12 | Italy (Sardinia) | soil | DQ790651 | |
| T. neokoningii (An) | G.J.S. 04-216 = CBS 120070 T | Peru | Moniliophthora roreri on Theobroma cacao | DQ841734 | DQ841718 |
| H. rufa/T. viride | G.J.S. 05-104 = CBS 120071 | Italy | peat | DQ841741 | DQ841727 |
| H. rufa/T. viride (An) | CBS 101526 | Netherlands | cellulosic tissue | X93979 | AY376053 |
| H. rufa/T. viride (An) | CBS 119326 | Sweden | Pinus sylvestris, wood | AY665593 | DQ672612 |
| H. rufa/T. viride (An) | G.J.S. 99-13 = NRRL 6955 | Finland | soil | DQ841733 | DQ841712 |
| H. rufa/T. viride | C.P.K. 1995 = G.J.S. 04-371 | France | Quercus robur, wood, bark | DQ677653 | DQ672613 |
| H. rufa/T. viride (An) | C.P.K. 1007 | Russia (Central St.For. Biosphere Reserve) | soil | DQ838533 | DQ838539 |
| H. rufa/T. viride (An) | C.P.K. 1006 | Russia (Central St.For. Biosphere Reserve) | soil | DQ838532 | DQ838538 |
| H. rufa/T. viride (An) | G.J.S. 05-463 | U.K. | Fagus sylvatica, trunk endophyte | DQ841723 | |
| H. rufa/T. viride (An) | G.J.S. 97-271 = ITB 8212 = BBA 70239 | Denmark | water-damaged building | DQ315456 | AF348116 |
| H. rufa/T. viride (An) | ATCC 28020 | U.S.A. (VA) | soil | DQ109535 | AY937449 |
| H. rufa/T. viride | C.P.K. 965 | Czech Republic | Picea abies, wood | DQ677652 | DQ672611 |
| H. rufa/T. viride (An) | CBS 586.95 | Estonia | Phellinus igniarius | ||
| H. rufa/T. viride | C.P.K. 1998 | Czech Republic | Pinus sylvestris, wood | DQ677656 | DQ672616 |
| H. rufa/T. viride (An) | Tr 2 | U.S.A. (WA) | soil | DQ315457 | AY376052 |
| H. rufa/T. viride (An) | G.J.S. 99-14 = NR 6896 | U.K. | leaf litter | DQ841737 | DQ841715 |
| H. rufa/T. viride | G.J.S. 91-62 | U.S.A. (VA) | Acer sp., trunk | DQ846665 | DQ846670 |
| H. rufa/T. viride (An) | G.J.S. 92-14 = ICMP 16298 | New Zealand | Pinus radiata, wood | DQ313155 | DQ288988 |
| H. rufa/T. viride | C.P.K. 2001 | Austria | Picea abies, wood | DQ677659 | DQ672619 |
| H. rufa/T. viride | C.P.K. 1996 | U.K. | Acer pseudoplatanus, wood | DQ677654 | DQ672614 |
| H. rufa/T. viride | CBS 119327 = G.J.S. 04-369 = G.J.S. 04-370 | Austria | Picea abies, wood | DQ677657 | DQ672617 |
| H. rufa/T. viride | CBS 119325 = G.J.S. 04-372, epitype of H. rufa and T. viride | Czech Republic | Pinus sylvestris, wood | DQ677655 | DQ672615 |
| H. rufa/T. viride | C.P.K. 2000 | Austria | Pinus sylvestris, wood | DQ677658 | DQ672618 |
| H. rufa/T. viride (An) | C.P.K. 1009 | Russia (Central St.For. Biosphere Reserve) | soil | DQ838535 | DQ838541 |
| H. rufa/T. viride (An) | C.P.K. 1002 | Russia (Central St.For. Biosphere Reserve) | soil | DQ838531 | DQ838537 |
| H. rufa/T. viride (An) | C.P.K. 1001 | Russia (Central St.For. Biosphere Reserve) | soil | DQ838530 | DQ838536 |
| H. rufa/T. viride (An) | G.J.S. 99-16 | Japan? | Pinus sylvestris | DQ315460 | DQ307543 |
| H. rufa/T. viride (An) | G.J.S. 92-15 | Canada | peat | DQ315452 | DQ307537 |
| H. rufa/T. viride (An) | G.J.S. 04-86 | Italy? | peat | DQ841745 | DQ841725 |
| H. rufa/T. viride (An) | J.B. NSW13 | Australia (NSW, Wentworth Falls near Katoomba) | Eucalyptus-Banksia forest soil | DQ845430 | |
| T.viride Vb 3 (An) | Tr 21 | U.S.A. (VA) | soil | AY380909 | AY376054 |
| T.viride Vb 3 (An) | G.J.S. 90-95 = IMI 352470 | U.S.A. (NC) | wood | DQ315455 | DQ307535 |
| T.viride Vb 1 (An) | DIS 328g = IMI 394148 | Ecuador | Theobroma gileri, trunk endophyte | DQ841739 | DQ841724 |
| T.viride Vb 2 (An) | G.J.S. 04-40 | Brazil | Theobroma cacao, trunk endophyte | DQ315454 | DQ307534 |
| T. scalesiae (An) | G.J.S. 03-74 = CBS 120069 T | Galapagos Islands | Scalesia pedunculata, trunk endophyte | DQ841742 | DQ841726 |
| H. ochroleuca | C.P.K. 1895 | U.K. | bark | ||
| H. ochroleuca | G.J.S. 01-234 | Thailand | Hypoxylon sp. | DQ846666 | DQ846668 |
| T. viride Ve | G.J.S. 99-127 | Australia (VI) | wood | DQ315453 | DQ307533 |
| T. viride Ve | G.J.S. 99-204 | New Zealand | Metrosideros sp., bark | DQ315450 | DQ307531 |
| T. viride Ve | G.J.S. 99-83 | Australia (VI) | bark | AF456921 | AF348118 |
| T. viride Ve | G.J.S. 99-86 = ICMP 16290 | Australia (VI) | Eucalptus ? regnans, bark | DQ315432 | DQ307516 |
| T. viride Ve | G.J.S. 99-191 | Australia (VI) | wood | DQ315451 | DQ307532 |
| T. viride Ve | G.J.S. 04-353 | U.S.A. (TN) | Rhododendron maximum, bark | DQ323418 | DQ307551 |
| T. viride Ve (An) | G.J.S. 90-97 | U.S.A. (NC) | bark | DQ315449 | DQ307530 |
| T. viride Ve (An) | G.J.S. 90-20 | U.S.A. (WI) | decorticated wood | ||
| T. viride Ve (An) | DIS 217i | Ecuador | Theobroma gileri, trunk endophyte | DQ323420 | DQ307549 |
| H. neorufa | G.J.S. 96-141 = ATCC MYA-2680 | U.S.A. (NJ) | Acer, bark | DQ846667 | DQ846669 |
| H. neorufa | G.J.S. 96-132 | U.S.A. (NJ) | wood | AF487653 | AF487669 |
| H. neorufa | G.J.S. 96-135, T | U.S.A. (NJ) | bark | AF487655 | AF487670 |
| H. flaviconidia | G.J.S. 99-49, T | Costa Rica | bark | AY665696/AY665700 | AY665720 |
| T. paucisporum (An) | G.J.S. 03-69 = CBS 118978 = ATCC MYA-3642 | Ecuador | Theobroma cacao, rotting pod | DQ109527 | DQ109541 |
| T. paucisporum (An) | G.J.S. 01-13 = CBS 118645 = ATCC MYA-3641, T | Ecuador | Theobroma cacao, rotting pod | DQ109526 | DQ109540 |
| T. theobromicola (An) | DIS 85f, T | Peru | Theobroma cacao, stem endophyte | DQ109525 | DQ109539 |
| H. pezizoides | G.J.S. 01-257 | Thailand | wood | DQ000632 | AY937438 |
| T. hamatum (An) | DAOM 167057 = CBS 102160, neotype | Canada | spruce forest soil | Z48816 | AY750893 |
| T. pubescens (An) | DAOM 166162 | U.S.A. (NC) | forest soil | DQ083027 | AY937442 |
| T. asperellum (An) | CBS 433.97, T | U.S.A. (MD) | sclerotia of Sclerotinia minor | X93981 | AY376058 |
| T. asperellum (An) | G.J.S. 04-217 | Peru | Moniliophthora roreri on Theobroma cacao | DQ381957 | DQ381958 |
Abbreviations of culture collections and collectors as follows: ATCC = American Type Culture Collection, Manassas, VA, U.S.A., BBA = Biologisches Bundesanstalt, Berlin, Germany; CBS = Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; C.P.K. = Collection of C.P. Kubicek, Technische Universität, Abteilung für Mikrobielle Biochemie, Vienna; G.J.S., Tr = Culture collection of the United States Department of Agriculture, Systematic Botany and Mycology Lab, Beltsville, MD, U.S.A; DAOM = Canadian Collection of Fungal Cultures, Ottawa, Canada; DIS refers to CABI-Bioscience, Ascot, cultures held by G.J.S; ICMP = International Collection of Microorganisms from Plants, Manaaki Whenua Landcare Research, Auckland, New Zealand; IMI = CABI-Bioscience, Egham, U.K.; J.B. = John Bissett, Agriculture and Agri-Food, Eastern Cereal and Oilseed Research Centre, Ottawa, Canada; W.J. = Culture collection of Walter Jaklitsch, Technische Universität, Abteilung für Mikrobielle Biochemie, Vienna; NR = Nippon Roche Corp. Tokyo, Japan
From the culture collection of John Bissett, Agriculture and Agri-Food, Eastern Cereal and Oilseed Research Centre, Ottawa, Canada.
An = isolation made from conidia or directly from substratum, all other cultures derived from ascospores.
T = ex-type culture.
Collections and analysis of phenotype
The isolates originated from three natural sources: isolations from ascospores of Hypocrea specimens, direct isolations by a variety of means from soil or dead herbaceous tissue, and isolations as endophytes from sapwood of living stems of Theobroma and related tree species, and from Fagus sylvatica. Isolation of the stem endophytes was done as reported by Evans et al. (2003). A smaller number of isolates was obtained from the American Type Culture Collection (ATCC), Biologische Bundesanstalt (Berlin), the Centraalbureau voor Schimmelcultures (CBS), and from individual colleagues. Cultures derived from single part-ascospores that were germinated on cornmeal agar with 2 % dextrose (CMD, Difco cornmeal agar + 2 % dextrose w/v) and isolated by means of a micromanipulator; usually two or more single-spore cultures were combined in a single stock culture, and such polyspore cultures were used in all subsequent analyses. The working set of cultures is maintained on cornmeal agar slants at ca. 8 °C, in 20 % glycerine at -80 °C, or in liquid nitrogen.
Representative isolates are deposited at the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands (CBS) and the American Type Culture Collection, Manassas, VA (ATCC). Isolates listed as C.P.K. are those maintained in the collection of Christian P. Kubicek, Institute of Chemical Engineering, Research Area Gene Technology and Applied Biochemistry, Vienna University of Technology, Vienna. Kornerup & Wanscher (1978) was used as the colour standard. The name of the most commonly cited collectors, G.J. Samuels and W.M. Jaklitsch, are abbreviated as G.J.S. and W.J.
Cultures used for study of anamorph micromorphology were grown on CMD, PDA or SNA (Nirenberg 1976), at 20 or 25 °C for 5–11 d under alternating 12 h cool white fluorescent light and 12 h darkness; in the descriptions that follow, these alternating light conditions are referred to when the word “light” is used.
Morphological analyses of microscopic characters were undertaken from material that was first hydrated in the case of herbarium material, or wetted in the case of living cultures, in 3 % KOH. Autolytic activity, which is here defined as usually circular excretions at the tips of hyphae, was assessed under direct microscopic observation using a 10 × objective. Coilings, defined as circularly oriented and coiled intercalary or terminal parts of hyphae, were detected in the same way as autolytic excretions.
Measurements were made from KOH or water mounts and we did not observe any differences when the respective reagents were used. Where possible, at least 30 units of each parameter were measured for each collection. Ninety-five percent confidence intervals of the means (CI) are provided; this figure represents the interval within which 95 % of the individuals of the parameter was found in the analysed isolates. The parameters used for analysis are listed in Table 2. Chlamydospores were measured by inverting a 7–15 d old CMD culture on the stage of a compound microscope and observing with a 40 × objective. Data were gathered using a Nikon DXM1200 or a Nikon Coolpix 4500 digital camera and Nikon ACT 1 software and measured either directly on the microscope or by using Scion Image (release Beta 4.0.2; Scioncorp, Frederick, MD).
Table 2.
Continuous characters, geographic distribution and colony phenotype of the Trichoderma species discussed
|
Taxa/clades
|
||||||||||||||||||||
|
Character
|
||||||||||||||||||||
| T. viride / H. rufa | T.viridescens /H.viridescens | H. vinosa /T. vinosum | T. gamsii | Vd 1 | Vd 2 | Vb 1 | Vb 2 | T. scalesiae | T. neokoningii | |||||||||||
| dominant distribution | Europe, North America | Europe, North America, Australia and New Zealand | New Zealand, Australia | Europe, North America, Australia | Sri Lanka, Ghana | Cosmopolitan temperate | Ecuador | Brazil | Galapagos Islands | Peru | ||||||||||
|
Conidia
|
||||||||||||||||||||
| ornamentation | grossly warted | warted | warted | smooth | warted | smooth | grossly warted | grossly warted | smooth | smooth | ||||||||||
| shape | subglobose | subglobose to ellipsoidal | subglobose | ellipsoidal to oblong | subglobose to broadly ellipsoidal | ellipsoidal to oblong | subglobose | subglobose | subglobose | ellipsoidal to oblong | ||||||||||
| length (μm) | (3.0-)3.5-4.5(-5.5) | (2.7-)3.5-4.5(-8.5) | (3.2-)3.5-4.5(-4.7) | (3.2-)4.0-5.0(-5.8) | (2.7-)3.0-3.7(-4.2) | (3.5-)3.7-4.5(-5.5) | (3.0-)3.2-4.0(-4.5) | (3.0-)3.5-4.0(-4.2) | (2.5-)3.0-3.7(-4.0) | (3.2-)3.5-4.0(-4.5) | ||||||||||
| 95 % CI | 3.99-4.05 | 3.97-4.02 | 3.9-4.0 | 4.3-4.5 | 3.4-3.5 | 4.0-4.1 | 3.6-3.8 | 3.5-3.8 | 3.1-3.4 | 3.7-4.0 | ||||||||||
| N | 720 | 1233 | 120 | 90 | 60 | 30 | 30 | 30 | 30 | 30 | ||||||||||
| width (μm) | (2.8-)3.4-4.0(-5.0) | (2.2-)3.0-3.7(-4.7) | (2.7-)3.0-4.0(-4.2) | (2.2-)2.5-3.0(-3.2) | (2.2-)2.5-3.2(-3.5) | (2.5-)2.7-3.5(-3.7) | (2.7-)3.0-3.5(-4.0) | (3.0-)3.2-3.7(-4.2) | 2.2-)2.7-3.2(-3.5) | 2.5-3.0 | ||||||||||
| 95 % CI | 3.75-3.80 | 3.31-3.35 | 3.5-3.6 | 2.7-2.8 | 2.7-2.9 | 3.0-3.2 | 3.2-3.4 | 3.4-3.6 | 2.8-3.1 | 2.6-2.7 | ||||||||||
| N | 720 | 1233 | 120 | 90 | 60 | 30 | 30 | 30 | 30 | 30 | ||||||||||
| L/W | (0.8-)1.0-1.2(-1.5) | (0.9-)1.1-1.4(-2.0) | 1.0-1.2(-1.3) | (1.1-)1.4-1.8(-2.1) | (1.0-)1.1-1.4(-1.6) | (1.0-)1.2-1.5(-1.7) | (0.9-)1.0-1.2(-1.4) | (0.7-)0.9-1.1(-1.3) | (0.9-)1.0-1.1(-1.3) | (1.2-)1.4-1.6(-1.7) | ||||||||||
| 95 % CI | 1.06-1.08 | 1.20-1.22 | 1.10-1.13 | 1.55-1.63 | 1.0-1.1 | 1.06-1.14 | ||||||||||||||
| N | 720 | 1233 | 120 | 90 | 60 | 30 | 30 | 30 | 30 | 30 | ||||||||||
|
Ascospores
|
||||||||||||||||||||
| distal length (μm) | (3.7-)4.5-5.7(-7.7) | (3.2-)4.0-5.2(-7.5) | (3.7-)5.0-6.5(-8.0) | (2.7-)3.2-3.7(-4.2) | ||||||||||||||||
| 95 % CI | 4.91-5.14 | 4.62-4.72 | 5.5-5.9 | 3.4-3.6 | ||||||||||||||||
| N | 120 | 411 | 90 | 30 | ||||||||||||||||
| distal width (μm) | (3.2-)4.0-4.7(-6.5) | (3.2-)3.7-4.5(-5.5) | (3.7-)4.7-6.2(-7.7) | (2.5-)2.7-3.5(-3.7) | ||||||||||||||||
| 95 % CI | 4.25-4.42 | 4.15-4.23 | 5.3-5.7 | 3.0-3.2 | ||||||||||||||||
| N | 120 | 411 | 90 | 30 | ||||||||||||||||
| proximal length (μm) | (3.7-)4.7-6.5(-8.0) | (3.5-)4.5-5.7(-8.0) | (4.5-)5.0-7.0(-9.0) | (3.5-)4.0-5.0(-6.0) | ||||||||||||||||
| 95 % CI | 5.4-5.7 | 5.07-5.21 | 5.8-6.2 | 4.3-4.7 | ||||||||||||||||
| N | 120 | 411 | 90 | 30 | ||||||||||||||||
| proximal width (μm) | (3.0-)3.5-4.2(-5.2) | (2.7-)3.2-4.0(-5.2) | (3.7-)4.0-5.0(-8.0) | (2.2-)2.5-3.2(-3.5) | ||||||||||||||||
| 95 % CI | 3.77-3.93 | 3.62-3.70 | 4.8-5.2 | 2.8-3.0 | ||||||||||||||||
| N | 120 | 411 | 90 | 30 | ||||||||||||||||
|
Colony radius on PDA 72 h (mm)
|
||||||||||||||||||||
| 20 °C | 26-34 | 25-38 | 25-29 | 30-36 | 34-40 | 33 | 38 | 39 | 3 | 30 | ||||||||||
| N cultures | 18 | 29 | 3 | 4 | 2 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| 25 °C | 31-41 | 32-45 | 25-33 | 43-51 | 53-57 | 45 | 54 | 52 | 6 | 42 | ||||||||||
| N cultures | 18 | 29 | 3 | 4 | 2 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| 30 °C | 9-27 | 9-25 | 0-10 | 34-54 | 25-51 | 42 | 12 | 45 | 18 | 36 | ||||||||||
| N cultures | 17 | 29 | 3 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | ||||||||||
|
Colony radius on SNA 72 h (mm)
|
||||||||||||||||||||
| 20 °C | 15-27 | 20-30 | 14-23 | 16-24 | 18-20 | 27 | 33 | 40 | 3 | 17 | ||||||||||
| N cultures | 18 | 29 | 3 | 4 | 2 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| 25 °C | 22-34 | 24-36 | 16-27 | 29-34 | 29-31 | 42 | 42 | 50 | 10 | 30 | ||||||||||
| N cultures | 18 | 29 | 3 | 4 | 2 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| 30 °C | 7-23 | 8-22 | 0-5 | 25-39 | 17-33 | 38 | 12 | 63 | 10 | 36 | ||||||||||
| N cultures | 18 | 29 | 3 | 4 | 2 | 1 | 1 | 1 | 1 | 1 | ||||||||||
Five types of light microscopy were used, viz. stereo microscopy (stereo), bright field (BF), phase contrast (PC), Nomarski differential interference contrast (DIC), and epifluorescence (FL). The fluorescent brightener calcofluor (Sigma Fluorescent Brightener 28 C.I. 40622 Calcofluor white M2R in 2 molar phosphate buffer at pH 8.0) was used for FL.
Scanning electron microscopy (SEM) was done by one of two methods. Material for SEM studies was obtained from cultures that were grown on PDA for up to 2 weeks at 20–25 °C. Agar blocks with abundant conidia were prepared for SEM. For Figs 8 a–h all SEM procedures followed the protocols of Meyer & Plaskowitz (1989), and for Fig. 10h those of Carta et al. (2003) and Erbe et al. (2003).
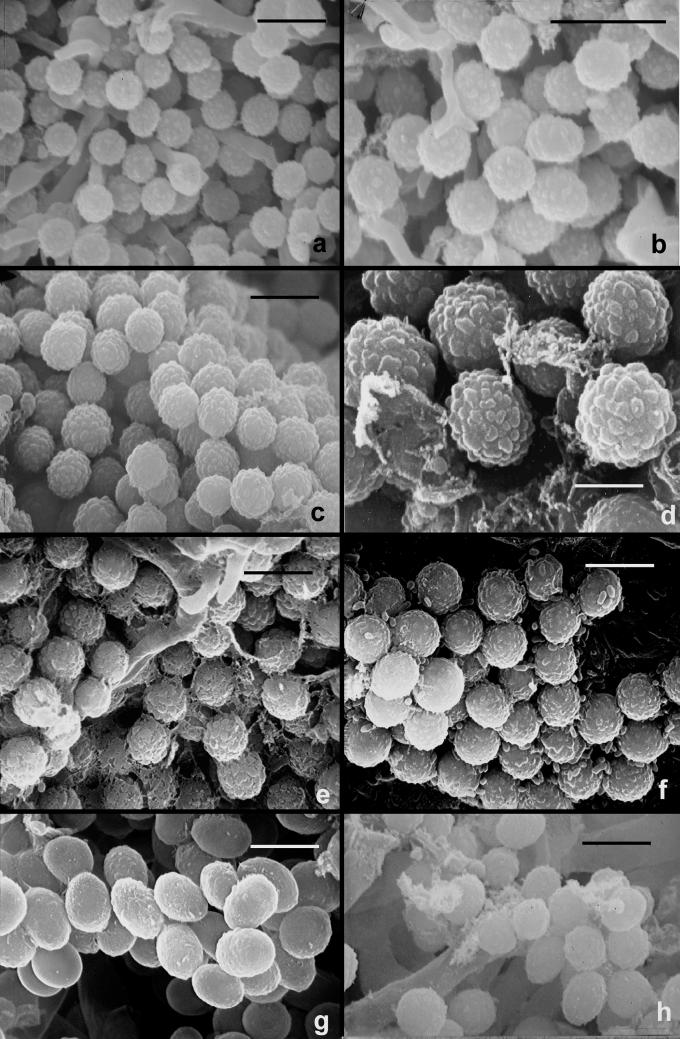
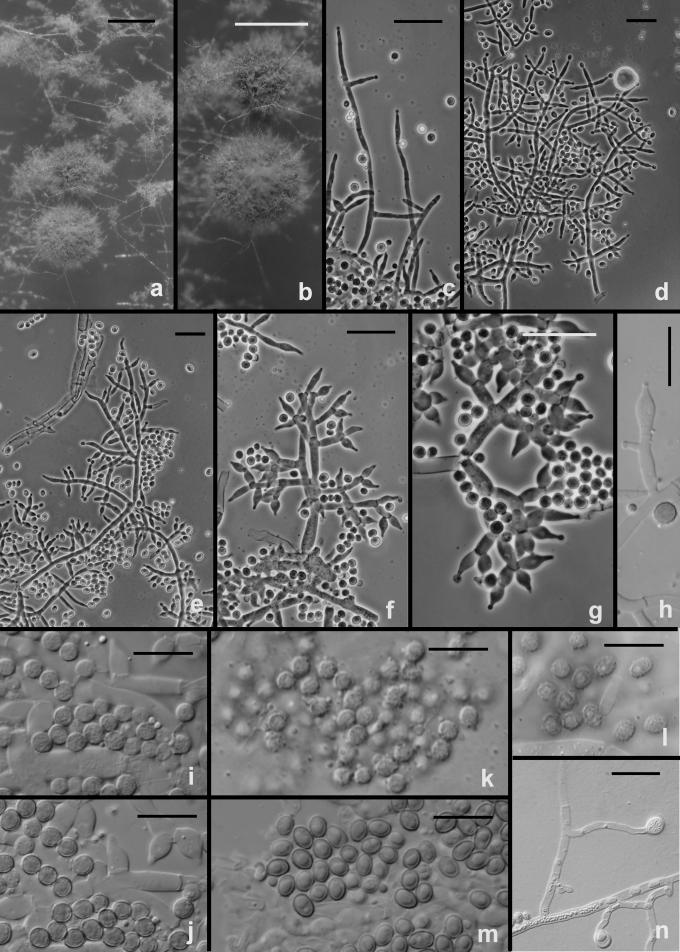
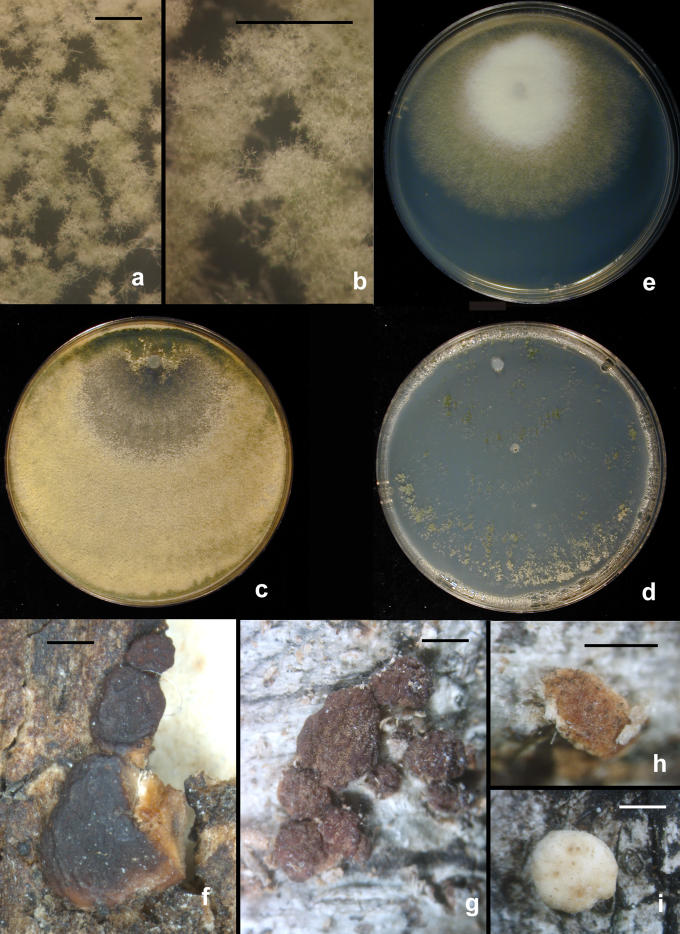
Fig. 8.
Scanning electron micrographs of conidia of T. viride and T. viridescens. a–e. T. viride. f–h. T. viridescens. a–b. from type specimen (L); c–d: G.J.S. 92-15; e: G.J.S. 90-95; f: G.J.S. 92-11; g: G.J.S. 94-11; h: G.J.S. 89-142. Scale bars = 5 μm except b = 10 μm
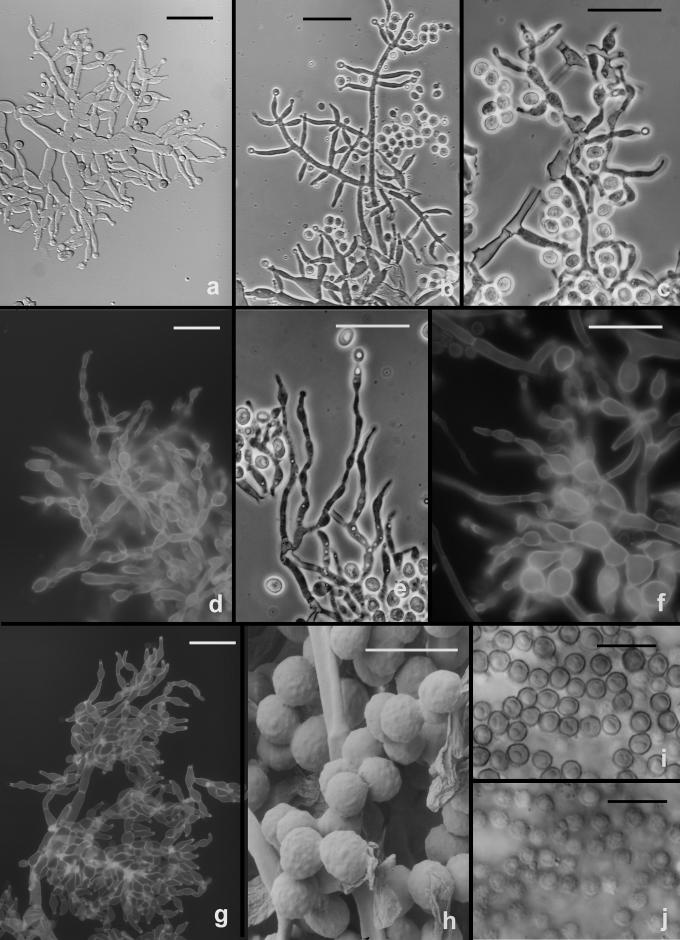
Fig. 10.
Hypocrea vinosa/T. vinosum anamorph on CMD. a–g. Conidiophores; note vesiculose development suggestive of Eidamia viridescens in a, d, f–g; phialides proliferating percurrently to form submoniliform chains in d–f. More or less typical Trichoderma conidiophores in b. h–j. Conidia. h. SEM, i. in optical section, j. in surface view. a–b, h from G.J.S. 99-158; c, e, i–j from G.J.S. 99-183; d, f from G.J.S. 99-156; g from G.J.S. 02-54. Microscopy: a, i–j. DIC; b–c, e phase contrast; d, f–g fluorescence. Scale bars: a–g = 20 μm; h = 6 μm; i–j = 10 μm.
Sections of Hypocrea stromata were prepared by rehydrating small blocks of substratum supporting stromata in 3 % KOH. The blocks were supported by Tissue Tek O.C.T. embedding medium 4583 (Miles, Inc., Elkhart, IN) and sectioned at 12–15 μm on a Microtome-Cryostat (International Equipment Co., Needham Heights, MA, or Leitz Kryostat 1720, Leica Microsystems, Vienna).
Growth rate trials were performed in darkness on potato-dextrose agar (PDA, Difco, Biolab, or Merck) and SNA following the procedure described by Samuels et al. (2002) with the addition that cultures were also grown at 25 °C under 12 h darkness/12 h cool white fluorescent light for 5–7 d. Each growth-rate trial was repeated three times and the results of the three were averaged. The slope of the growth curve was determined using the mean of the colony radius (see Samuels et al., 2006a).
DNA extraction and sequencing methods
The extraction of genomic DNA was performed as reported previously (Dodd et al. 2002). A portion of translation elongation factor 1 alpha (tef1) was amplified using the primers EF1-728F (Carbone & Kohn 1999) and TEF1 rev (Samuels et al. 2002) or TEF1LLErev (Jaklitsch et al. 2005). The PCR product of approximately 600 bp covers the large 4th and the short 5th introns of the gene. A fragment covering the internal transcribed spacers 1 and 2 (ITS1 and 2) of the rRNA gene cluster was amplified using ITS1 and ITS4 as the forward and reverse primers, respectively (White et al. 1990). DNA sequences were obtained using the BigDye Terminator cycle sequencing kit (Applied Biosystems, Foster City, California). Products were analysed directly on a 3100 DNA sequencer (Applied Biosystems). Both strands were sequenced for each locus.
Molecular phylogenetic analysis
Sequences were edited and assembled using Sequencher 4.1 (Gene Codes, Wisconsin). Clustal X 1.81 (Thompson et al. 1997) was used to align the sequences; the alignment of each locus was manually edited using MacClade or GeneDoc 2.6 (Nicholas & Nicholas 1997). The sequences were deposited in GenBank (Table 1). The MSA file for the tef1 locus is also available at http://www.isth.info/phylogeny/rufa.php.
The interleaved NEXUS file was formatted using PAUP* v. 4.0b10 (Sinauer Associates, Sunderland, MA) and manually formatted for the MrBayes v3.0B4 program. The Bayesian approach to phylogenetic reconstructions (Rannala & Yang 2005) was implemented using MrBayes 3.0B4 (Huelsenbeck & Ronquist 2001). The MODELTEST3-06 package (http://bioag.byu.edu/zoology/crandall_lab/modeltest.htm) was used to compare the likelihood of different nested models of DNA substitution and select the best-fit model for the investigated data set. Both hierarchical LRT and AIC output strategies were considered, although the preference was given to the latter. The unconstrained GTR + I + G substitution model was selected for the tef1 locus.
Metropolis-coupled Markov chain Monte Carlo (MCMCMC) sampling was performed with four incrementally heated chains (with the default heating coefficient λ = 0.2, heats for cold chains 1 and heated chains 2, 3 and 4 are 1, 0.83, 0.71 and 0.63, respectively) that were simultaneously run for 5 million generations for the tef1 alignment, which comprised 238 sequences. To check for potentially poor mixing of MCMCMC, the analysis was repeated at least three times. The convergence of MCMCMC was monitored by examining the value of the marginal likelihood through generations. Convergence of substitution rate and rate heterogeneity model parameters were also checked. Bayesian posterior probabilities (PP) were obtained from the 50 % majority rule consensus of trees sampled every 100 generations after removing the 2000 first trees using the “burn” command. According to the protocol of Leache & Reeder (2002), PP values lower than 0.95 were not considered significant while values below 0.9 are not shown on the resulting phylogram. Model parameter summaries after MCMC run and burning first samples were collected. For tef1 mean substitution values were estimated as G↔T = 1, C↔T = 3.55, C↔G = 1.28, A↔T = 1, A↔G = 4.68, A↔C = 1.5; nucleotide frequencies were estimated as 0.19 (A), 0.27 (C), 0.2 (G), 0.34 (T); alpha parameter of gamma-distribution shape was 0.29. Genetic distance was computed in PAUP* v. 4.0b10 under the GTR + I model.
RESULTS
Phylogeny
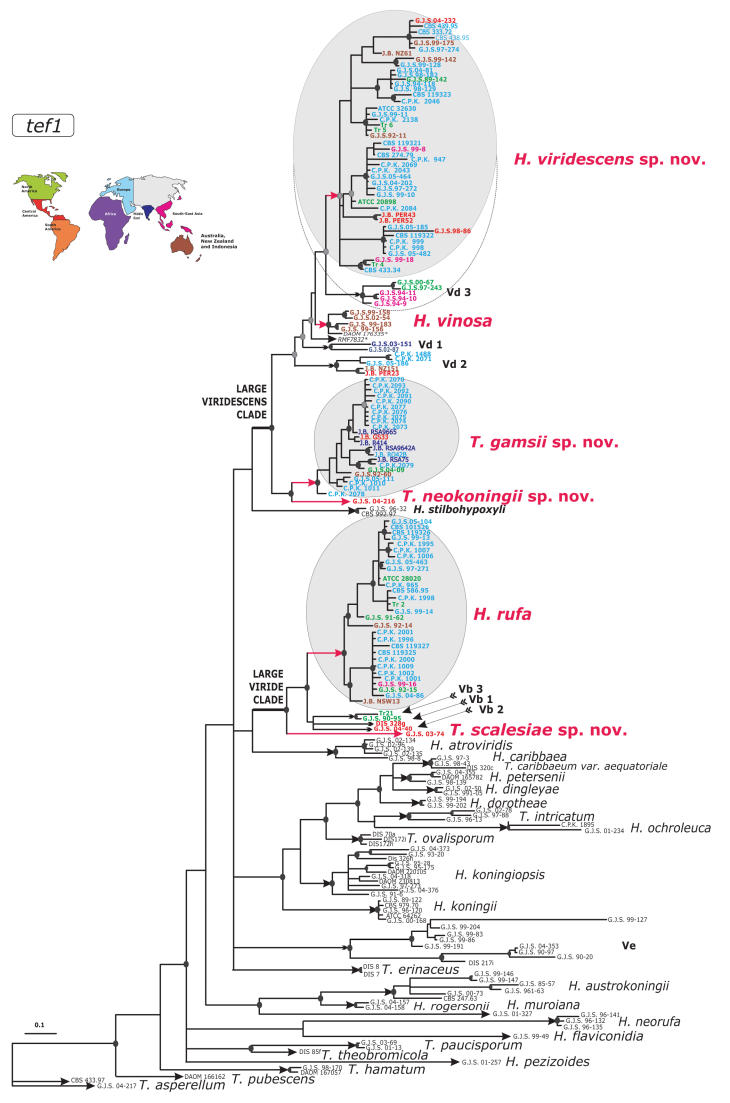
The majority of members of Trichoderma section Trichoderma share the same or very similar alleles of internal transcribed spacers 1 and 2 (ITS1 and 2), rendering this locus inappropriate for recognition of some species within the section. Therefore, to infer genetic diversity of the H. rufa/T. viride group we used intron sequences of the translation elongation factor 1-alpha (tef1), the most powerful phylogenetic marker as yet established in the genus. The resulting Bayesian phylogram (Fig. 1), which was obtained from 238 sequences, corresponds well to the previous analysis of related species with T. koningii-like morphology (Samuels et al. 2006a). Considering the analysis of phenotypes, it is obvious that there are two diverged groups named “Large Viride” and “Large Viridescens” clades, both of them with significant statistical support. Isolates of H. rufa form a compact clade composed of mainly European but also North American, Asian and Pacific strains showing its cosmopolitan nature. The “Large Viride” clade includes additional unresolved lineages that apparently represent unnamed species. The description of these taxa requires further sampling and therefore will be discussed in subsequent publications. In this study we have focused on the single endophytic strain from the Galapagos Islands, T. scalesiae sp. nov., which belongs to the “Large Viride” clade but at the same time occupies the most distant position from H. rufa. The largest group on the tree, the “Large Viridescens” clade, splits into two independent evolutionary lineages. The terminal position of the larger one represents a compact and well defined subclade with significant statistical support that contains isolates of the former Vd group (Lieckfeldt et al. 1999), described as H. viridescens below. Similar to H. rufa, this species has mainly European origin, also nearly all primary European nodes include North American, Central American, Asian and Pacific isolates, suggesting the absence of recent allopatric speciation in this group of isolates. Another well-supported clade in the vicinity of H. viridescens is composed of isolates of H. vinosa. As in the “Large Viride” clade this branch contains representatives of several well-supported speciation nodes composed of strains that are closely related to H. viridescens and H. vinosa and undoubtedly represent yet undescribed species of Hypocrea/Trichoderma. This diversity will be discussed in subsequent publications following further investigations and sampling. The material summarised in this study is sufficient to prove the existence of another phylogenetic species with eidamia-like morphology that occupies the second independent lineage within the “Large Viridescens” clade. The new species T. gamsii forms a homogeneous clade mainly represented by isolates from undisturbed soils in Sardinia and Central Russia. As in the case of H. viridescens and H. rufa, T. gamsii did not evolve as a result of any geographic isolation since we also sampled isolates from North America and Australia. We describe the most distant member of the “Large Viridescens” Clade, once again a single strain, as T. neokoningii. The detailed analysis of the highly variable intron sequences of the tef1 gene has clearly shown that, despite their close relationship, H. rufa, H. viridescens, H. vinosa, and a large group of isolates that we describe here as T. gamsii represent distinct sympatric phylogenetic species.
Fig. 1.
Bayesian radial phylogram showing the structure of the Trichoderma section Trichoderma inferred from sequences of two introns of tef1. Red colour is used to indicate species described in this study. Arrows show branches leading to species recognised within the section. Dark grey filled circles at nodes indicate posterior probability coefficients higher than 0.90 as they were obtained after 5 million generations; black filled circles at nodes show support higher than 0.95. Font colours correspond to regions of sampling on the schematic map. The dotted line around Vd 3 indicates strong phenotypic similarity despite phylogenetic divergence. * - sequences from John Bissett, collection information may be obtained from Agriculture and Agri-Food Canada, Ottawa, Ontario, Canada
Most of the Trichoderma species that have warted conidia fall within one of these two large clades. Exceptions include T. saturnisporum and T. ghanense, both of which are members of the distantly related T. sect. Longibrachiatum (Samuels et al. 1998) and clade Ve (Fig. 1). Clade Ve will be discussed in a future publication. All members of the “Large Viridescens” clade are characterised by the formation of peculiar, percurrently proliferating phialides that are diagnostic of Eidamia viridescens, the ex-type of which (CBS 433.34) falls in T. viridescens.
Phenotype: anamorph and cultures
DNA sequences referred eighty-seven strains to the “Large Viridescens” clade and thirty-four to the “Large Viride” clade. All of these fungi are typical of Trichoderma in producing copious amounts of green conidia in pustules or in extensive “lawns” on CMD, PDA and SNA. There was a tendency for conidia to form more quickly on SNA than on CMD or PDA and often conidia did not form on either of the latter media while they did form on SNA. Of the three media, SNA is overall better for the study of fungi in the viride clade in terms of more reliable production of conidia. The endophytic T. scalesiae only produced conidia on SNA to which a 1 cm2 piece of sterile filter paper had been added; the conidia only formed at the interface of the paper and the agar and on the paper itself.
There was a tendency for yellow pigment to develop in conidia in colonies of the “Large Viridescens” clade grown on PDA and SNA at 25 °C for two weeks, and a yellow pigment often diffused through CMD. No pigment was noted on SNA. Diffusing yellow pigmentation was not noted in colonies belonging to the “Large Viride” clade. A more or less strong coconut odour was detected in PDA and CMD cultures of most members of the “Large Viridescens” clade.
Conidia tended to form in pulvinate to hemispherical pustules < 1–3 mm diam. Distinct pustules measuring 1–5 mm were formed in T. viride/H. rufa on CMD. While pustules formed in T. viridescens/H. viridescens reached 3 mm diam, most often they measured less than 1 mm and often no pustules were formed, the conidiophores arising in more or less continuous cottony lawns. Often conidiophores formed apart from the larger pustules in the aerial hyphae and in minute tufts. Pustules in both groups tended to be cottony, and individual fertile branches could be seen; often conidiophores protruded beyond the surface of a pustule, producing a single phialide or a few fertile branches near the tip, the rest of the conidiophore remaining sterile or nearly so. The pustules of T. viridescens were usually less compact than in T. viride, and transparent under a 10 × objective. In pustules of T. viride produced on CMD, conidia often appeared to form at the surface of the pustule. In all cases, after one week at 20–25 °C, conidia were deep green to dark green 27–28D–F6–8, although lighter green conidia were observed in younger cultures. In some cultures of T. viridescens grown at 25 °C under alternating light on CMD and SNA, conidial masses were yellow. Conidia of T. neokoningii on PDA often were yellow at first. Often conidia of members of the “Large Viridescens” clade became greenish yellow when mounted in 3 % KOH.
Most of the fungi discussed in this work produce colonies that are recognizable as typical of Trichoderma in producing green conidia in abundance on most media. The exception is T. scalesiae, which only produced conidia sparingly on SNA to which a 1 cm2 piece of sterile filter paper had been added. Conidiophores in this species were irregularly branched, similar to what was described for T. paucisporum Samuels et al. (Samuels et al. 2006b) and for synanamorphs of pustulate species of Trichoderma (Chaverri et al. 2004). Conidia were held in drops of clear liquid, which appeared yellow to pale green because of the conidia, at the tips of the phialides.
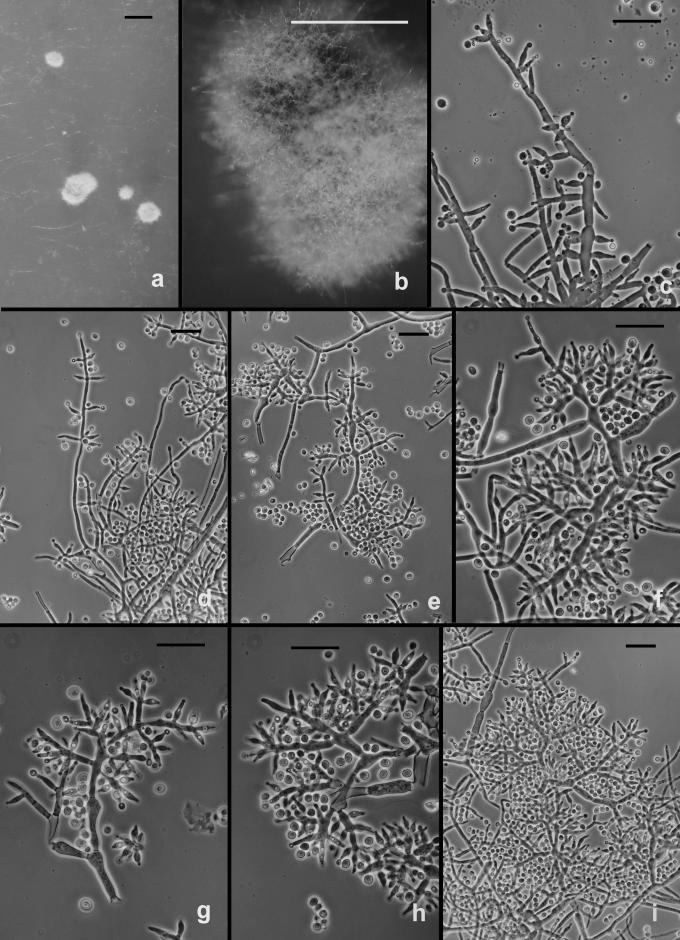
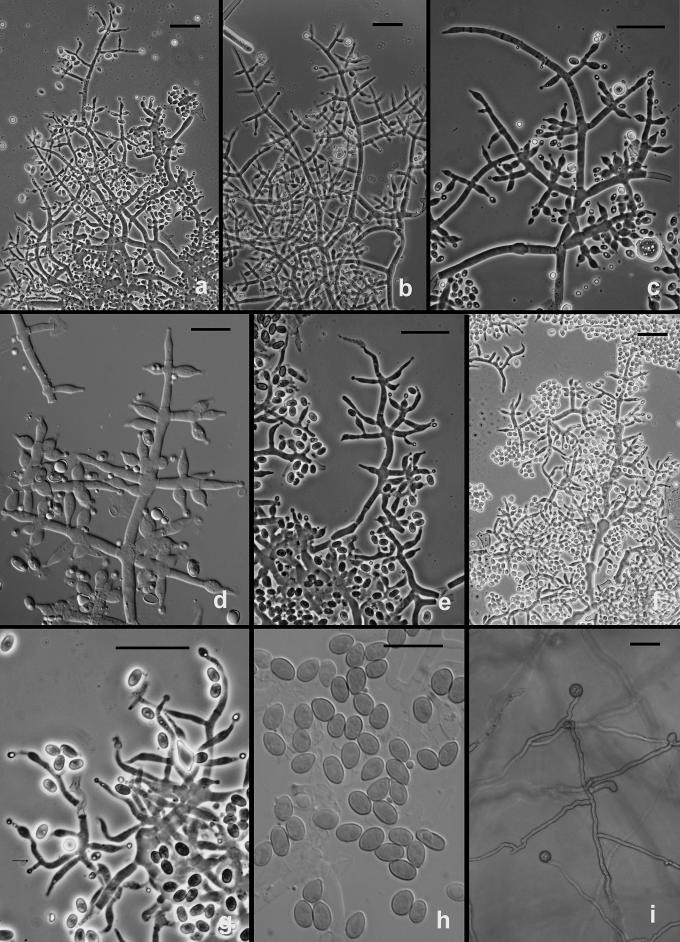
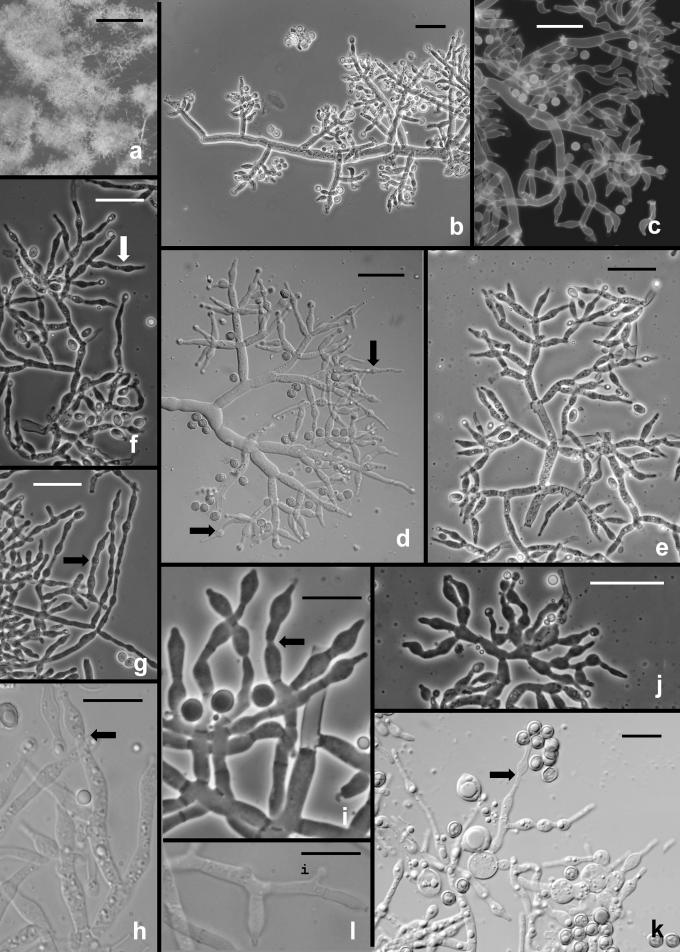
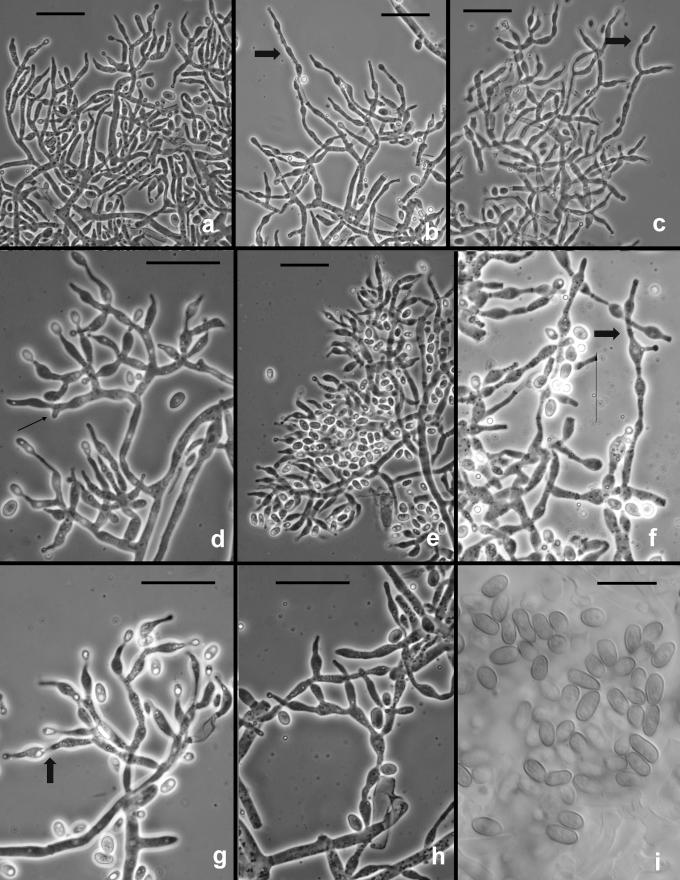
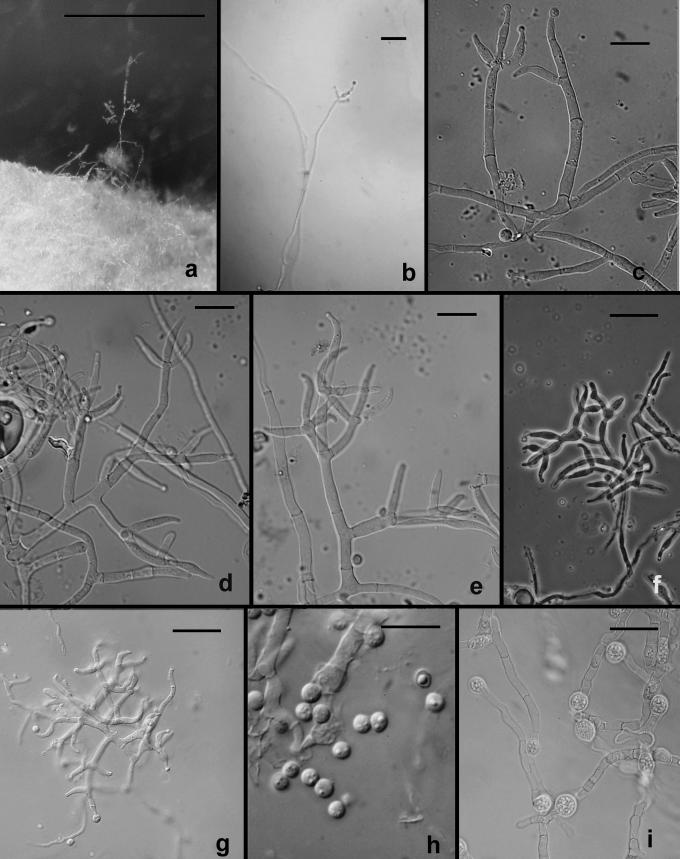
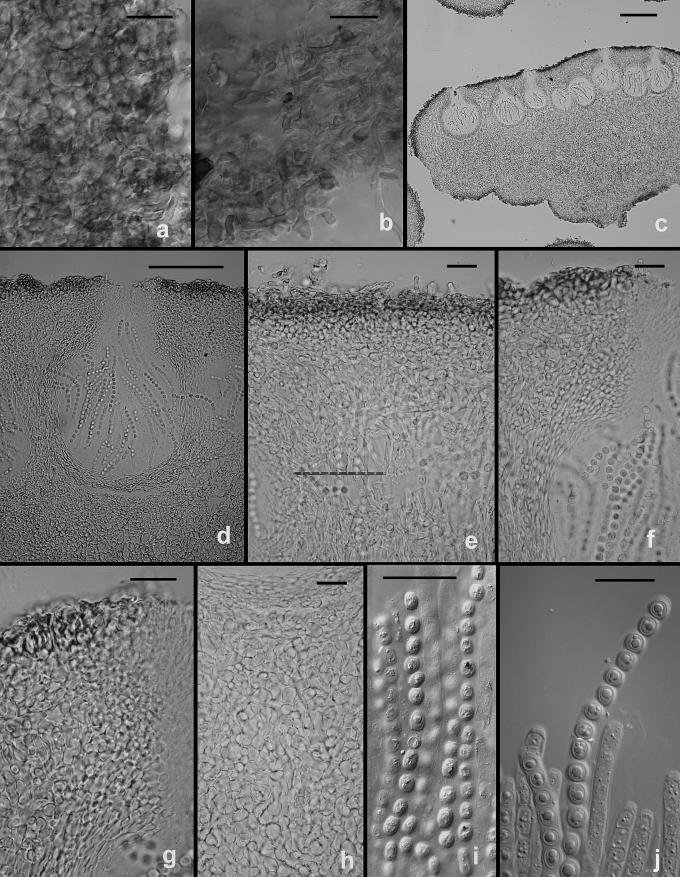
The following results pertain to the remaining species discussed in this work. It is difficult or impossible to define a conidiophore in Trichoderma. Conidiophores are mostly formed in pustules. As was noted above, pustules tend to be composed of intertwined hyphae that terminate in fertile branching systems. For the purposes of the present discussion, the conidiophore is referred to as the terminal branching system of intertwined hyphae that form the pustule. Various types of conidiophores were encountered in this study, and these were largely related to the medium and to the clade. In Type 1 (Fig. 3d, e, i), a well-developed main axis was not readily visible, or it was short and sometimes sinuous. Branching was highly irregular; branches were not paired and phialides tended to arise singly from the main axis. Phialides were often hooked or sinuous (Fig. 3d, e, j, k), cylindrical or somewhat swollen at or below the middle. This type of conidiophore was only found in the “Large Viride” clade, especially in T. viride. The Type 2 conidiophore (e.g. Figs 6, 11, 13, 14) was formed by all clades. In the Type 2 conidiophore there was a more or less readily discernable, well-developed main axis, from which lateral branches arose at or near 90°; the lateral branches were longer with distance from the tip and secondary branches were shorter with distance from the point of departure of the branch from the main axis. Branches often arose in pairs and produced secondary branches in pairs. Phialides tended to terminate branches in cruciate whorls of 3–4. The phialides were straight, cylindrical or somewhat swollen at or below the middle. In Type 3, which was common in the “Large Viridescens” clade, including T. vinosum, T. gamsii and T. neokoningii, the most distinctive characteristic is the production of percurrently proliferating phialides (Figs 5f, g, i, k; 10d–g; 12c, e, f, g; 14h–j), the branching system itself is highly variable in extent and form. At its simplest, a single phialide percurrently produces a second phialide (Fig. 14 h). What appears to be continuing percurrent proliferation of phialides results in a submoniliform chain of five or more cells (Figs 5g, 10 e, 12e, f), each cell of the chain being often abruptly swollen in the middle and separated by the cell above and below by a conspicuous septum. A main axis was discernable or not and was often reduced to a few, short, verticillately disposed branches or a reticulum of branches (e.g. Figs 5i–k; 10d, 12f–h, 14i–j). The most extreme form of the third branching type was observed in old pustules on CMD and PDA, where chains of percurrently proliferating phialides having subglobose bases and extremely long, cylindrical beaks arose from swollen, subglobose cells (Figs 5k, 10f, 12f). Percurrently proliferating phialides having this morphology were also seen occasionally on more typically branched conidiophores (Figs 5d; 12e), on conidiophores that produced typical, non-proliferating, phialides. Proliferating phialides were rarely seen on SNA. Conidiophores of DIS 328g (Vb 1) arose within well-developed pustules; they formed a reticulum with short fertile branches. The branches tended to be sinuous or curved and to be broader than is found in other clades that are studied here. The conidiophores produced often unicellular lateral branches, each of which terminated in 2–4 phialides. The phialides in DIS 328g are shorter than in any strain included in this study and have a smaller L/W ratio; they were often hooked or sinuous.
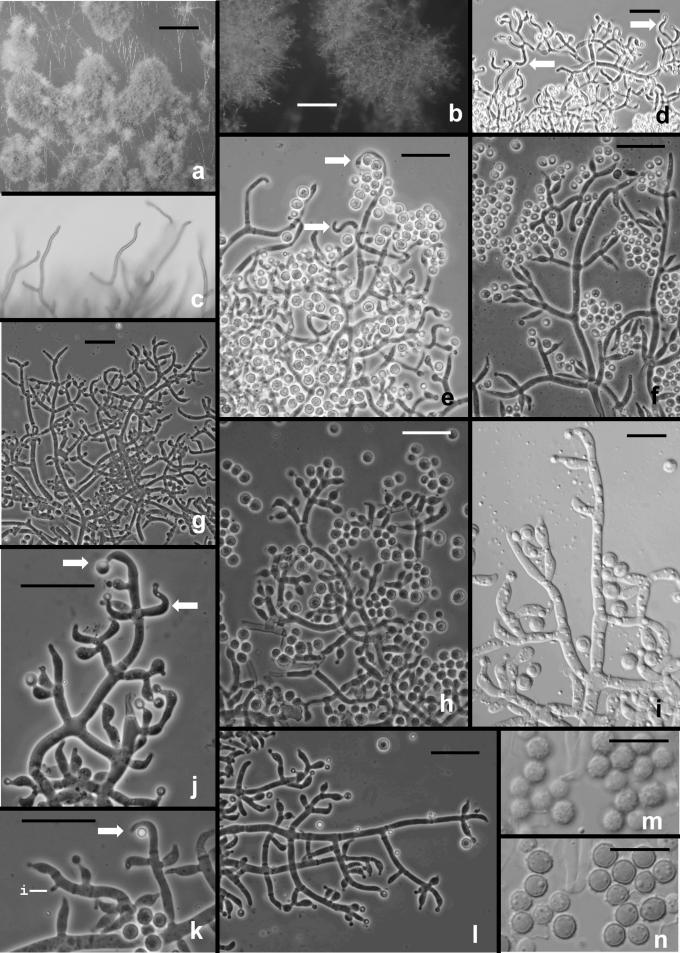
Fig. 3.
Hypocrea rufa/Trichoderma viride anamorph. a–b. Conidial pustules formed on CMD. c. Short sinuous elongations arising at the margin of pustules. d–l. Type 1 conidiophores from CMD. (d–e, h = “Type 1” conidiophores). Arrows indicate curved, hooked or sinuous phialides. Note especially solitary phialides in d and e. m–n. Conidia. Surface view of conidia shown in m, optical section shown in n. a–b from CBS 101526. Microscopy: a–b stereo; c bright field; d–h, j–l phase contrast; i, m–n DIC; c, k from CBS 119326; d–e from G.J.S. 89-127; f, m–n from Tr 8. g; l from G.J.S. 04-372; h from G.J.S. 05-463; i from G.J.S. 05-104; j from G.J.S. 99-16 9. Scale bars: a–b = 0.5 mm; d–l = 20 μm; m–n = 10 μm.
Fig. 6.
Hypocrea/Trichoderma viridescens anamorph. a–h. from SNA. a–b. Pustules. c. A long terminally fertile conidiophore extending beyond the surface of the pustule. d–g. Conidiophores typical of “Type 2,” Trichoderma branching and phialides. h. inercalary phialide. i–m. Conidia, showing variation in shape; in i surface view in optical section. i–k from SNA; l–m from CMD. n. Chlamydospores on CMD. Microscopy: a–d stereo. e–g phase contrast. h–m DIC. a–b from G.J.S. 05-115; c–d, g–i, l–m from CBS 433.34 (ex-type of Eidamia viridescens); e–f from G.J.S. 04-232; j from G.J.S. 05-115; k from G.J.S. 99-142. Scale bars: a = 1 mm; b–d = 0.5 mm; e–g, n = 20 μm; h–m = 10 μm.
Fig. 11.
Hypocrea vinosa/T. vinosum anamorph on SNA. a–b. Pustules. c–i. Conidiophores. All from G.J.S. 02-54. Scale bars: a = 1 mm, b = 0.5 mm; c–i = 20 um.
Fig. 13.
Trichoderma gamsii on SNA. a–g. “Type 2” conidiophores. Intercalary phialide shown in g (arrow). h. Conidia. I. Chlamydospores. a from C.P.K. 2073; b–d from G.J.S. 05-111; e, g from C.P.K. 2079; h from C.P.K. 2075; f, i from C.P.K. 2091. Microscopy: a–c, e–g = PC, d, h = DIC; i = Bright field. Scale bars: a–g, i = 20 μm, h = 10 μm.
Fig. 14.
Trichoderma neokoningii. a–b. Colonies on PDA (a) and SNA (b) after 1 wk at 25 °C, alternating light. c–d. Pustules from SNA. e–j. Conidiophores. e. Conidiophore protruding from the surface of a pustule with widely spaced branches and nearly cylindrical phialides. f–g. Conidiophore sparingly branched above and densely branched below. h. Percurrently proliferating phialides (arrow). i–j. Eidamia-like conidiophores with conspicuous proliferating phialides. k. Intercalary phialide. l. Conidia. m. Chlamydospore. e–h, k–m from SNA; i–j from PDA. All from G.J.S. 04-216. Microscopy: c–d = stereo; e, i, k = PC; f–h, j–l = DIC; m = bright-field. Scale bars: a–b in 9-cm-diam Petri dishes, c = 1 mm, d = 0.5 mm, e–j, m = 20 μm; k–l = 10 μm.
Fig. 5.
Hypocrea/Trichoderma viridescens anamorph from CMD. a. pustule. b–k. Conidiophores; arrows show examples of percurrently proliferated phialides. b–c. “Type 1” conidiophores more or less typical of Trichoderma. d. Conidiophore with “Type 1” branching and typical Trichoderma phialides and one branch with elongated and percurrently proliferated phialides. g. showing long submoniliform chains of proliferated phialides. i–k. showing branching typical of the original concept of Eidamia viridescens; vesicles and proliferated phialides in k very similar to the original illustration of E. viridescens. l. Intercalary phialide shown at i. a from G.J.S. 92-11; b, k from ATCC 32630; c from G.J.S. 99-3; d from G.J.S. 98-129; e–f, h from CBS 333.72; g, j from G.J.S. 05-115; i from G.J.S. 98-192; l from G.J.S. 99-142. Microscopy: a. stereo. b, e–g, i–j phase contrast. d, h, k–l DIC. c fluorescence. Scale bars: a = 0.5 mm; b–g, j, k = 20 μm; h–i, l = 10 μm.
Fig. 12.
Trichoderma gamsii on CMD. a–h. Conidiophores. More or less typical Trichoderma conidiophores in a, e; examples of percurrently proliferating phialides shown at arrows. Note submoniliform chains of percurrently proliferating phialides in b, f; thin arrow in d pointing to intercalary phialide. i. Conidia. a, d, i from C.P.K. 2077; b from C.P.K. 2078; c, e from G.J.S. 04-09; f from G.J.S. 05-111; g–h from C.P.K. 2073. Microscopy: a–h PC, i DIC. Scale bars a–h = 20 μm, i = 10 μm.
The “Large Viridescens” clade includes CBS 433.34, which is the ex-type culture of Eidamia viridescens A.S. Horne & H.S. Williamson. This species was described based on conidiophores produced on PDA; the original illustration is highly suggestive of what we have seen in the “Large Viridescens” clade, especially the extreme form described above as Type 3 and illustrated in T. viridescens (Fig. 5k) and T. vinosum (Fig. 10f). Conidiophores produced by this culture on SNA (Fig. 6f, g) were typical of Trichoderma, with a more or less uniformly branched conidiophore and typical phialides. The culture remained sterile on PDA but produced a coconut odour and a diffusing yellow pigment. On CMD mononematous conidiophores bearing green conidia in appressed phialides developed, but no pustules and no proliferating phialides were seen. These conidiophores were suggestive of the synanamorph conidiophores described by Chaverri et al. (2004) for species of Hypocrea/Trichoderma having conidiophore elongations.
Intercalary phialides were seen in some isolates but were neither common nor restricted to any particular clade (e.g. Figs 3k, 12d, 13g, 14k).
Various conidial types were observed in this study. These, like conidiophore types, were largely typical of clades. Most of the strains produced warted conidia. Conidia of T. gamsii (Fig. 12i), T. neokoningii (Fig. 14l) and T. scalesiae (Fig. 15h) are smooth. Trichoderma viride (Figs 3m, n; 8a–e), DIS 328g (Vb 1), G.J.S. 04-40 (Vb 2), and T. vinosum (Fig. 10h–j) have nearly globose conidia that have a length/width ratio 1.0–1.2. Conidia in G.J.S. 03-151/G.J.S. 02-87 (Vd 1) are ellipsoidal, length/width of 1.2–1.4. Conidia of individual collections of T. viridescens vary from subglobose to ellipsoidal (Figs 6i–m, 8f–h); although the mean L/W of all collections in this clade varies from 1.1–1.3, there is considerable overlap between this clade and DIS 328g. Conidia of T. gamsii and T. neokoningii are unusual in being ellipsoidal. Both of these species produce T. koningii-like conidiophores and conidia. Conidia of T. viride are much more coarsely warted than any of the other clades considered here. Warted conidia are also produced by members of clade Ve. Conidia in this clade are subglobose to ellipsoidal. Ornamented conidia were observed for most members of this clade. Conidial warts, while often large, are widely spaced and thus are not as conspicuous as in members of the “Large Viride” and “Large Viridescens” clades that are the focus of the present work.
Fig. 15.
Trichoderma scalesiae. a–g. Conidiophores formed in the aerial mycelium at the interface of filter paper and agar. h. Conidia. i. Chlamydospores. All from G.J.S. 03-74. Scale bars: a = 0, 5 mm, b, f–g = 20 μm, c-e, h, i = 10 μm, d = 0.5 mm; e–j, m = 20 μm; k–l = 10 μm.
Chlamydospores were inconsistently produced in most clades. Chlamydospores formed in abundance in T. gamsii (Fig. 13i) and T. neokoningii (Fig. 14m). Chamydospores were especially abundant in T. scalesiae (Fig. 15i). Chlamydospores were typical of Trichoderma in being globose to subglobose and terminal at the ends of hyphae or intercalary within hyphal cells.
Optimal temperature for growth on PDA for all clades except T. scalesiae and Vd 1 was 25 °C. The optimum for T. scalesiae was 30 °C and the two isolates in Vd 1 exhibited considerable variation at 30 °C (35–70 mm radius after 72 h). Trichoderma vinosum was unusual in having a temperature optimum of 20–25 °C and in reaching no more than 5 mm colony radius after 4 days at 30 °C.
On SNA most isolates reached a radius of no more than 40 mm, and usually less, after 72 h at 25–30 °C. On SNA only DIS 328g (Vb 1), T. viride and T. viridescens demonstrated a clear optimum at 25 °C. On SNA, the optimum for T. scalesiae was 25–30 °C, as it was for T. vinosum. The two isolates of Vd 1 were too variable to show a temperature optimum on SNA. G.J.S. 04-40 (Vb 2) was the fastest growing strain on SNA, reaching 65 mm after 72 h at 30 °C. This temperature differential was not observed on PDA. At 25 °C on PDA DIS 328g (Vb 1), G.J.S. 04-40 (Vb 2), T. gamsii, Vd 1 and Vd 2 reached or exceeded a radius of 45 mm after 72 h. Despite their phylogenetic complexity, both T. viride and T. viridescens showed very little variation in growth rate among their many isolates, both reaching a radius of 30–40 mm after 72 h at 25 °C. Significantly, growth of isolates in both of these clades, as well as in T. vinosum and DIS 328g (Vb 1), was more than 20 mm slower at 30 °C than at 20 °C. Trichoderma scalesiae was the slowest growing, reaching only 10 mm on SNA after 72 h at 25–30 °C and 18 mm on PDA at 30 °C. The fastest growing isolate at 30 °C was G.J.S. 04-40 (Vb 2) reaching 45 mm, although G.J.S. 03-151 (Vd 1) reached a radius of 70 mm after 72 h at 30 °C.
Clade Vd 3, which is a sister to T. viridescens, comprises two distinct groups of isolates. The North American isolates (G.J.S. 00-67, G.J.S. 97-243) cannot be distinguished from T. viridescens in any of their morphs and aspects. The Taiwanese isolates (G.J.S. 94-9 – G.J.S. 94-11) grow significantly more slowly than T. viridescens.
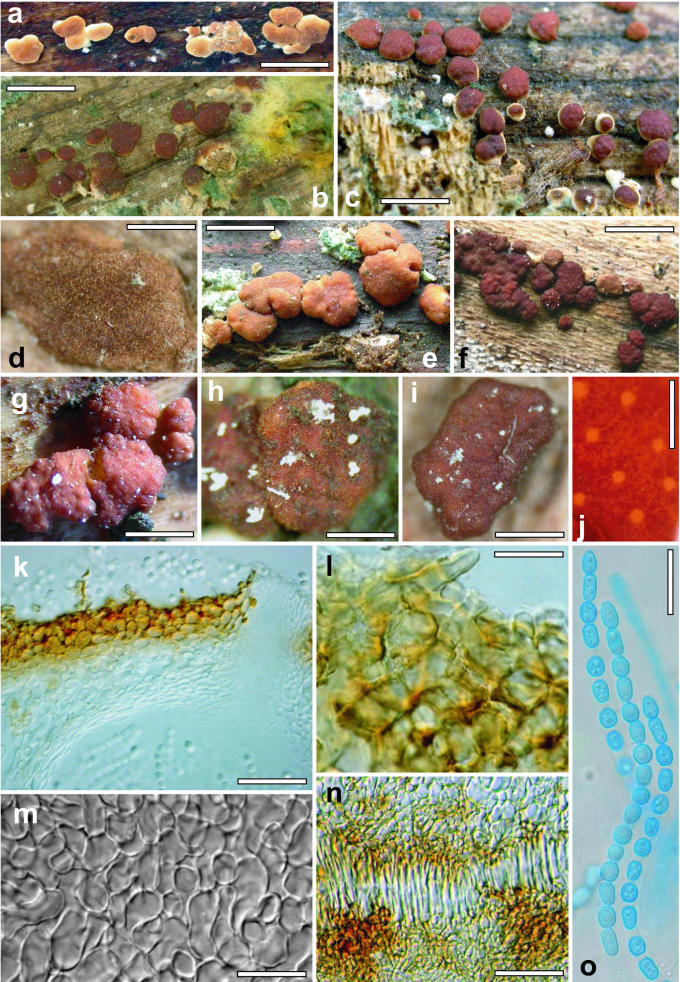
Phenotype: teleomorph
The stromata of the species included in this study are morphologically and anatomically so similar that they often cannot be distinguished. The youngest stage, when it could be observed, was semieffused, velutinous to conspicuously hairy and light tan in colour (Figs 4a, d; 2a, c). As perithecial development continued, the stroma became pulvinate to tuberculate or turbinate, and assumed a brown to rufous colour. Occasionally “albino” stromata, off-white to pale yellow, were observed in H. rufa (Fig. 2f) and in H. vinosa (Fig. 16i), in the latter only in an immature state. Often a velvety scurf was also present on mature stromata, the result of short hyphal hairs protruding from the stroma surface (Figs 2k, l; 4 l; 9b, e). Ostiolar openings were usually not visible macroscopically, or were barely visible as lighter areas on the stroma surface, sometimes with darker margins. The stroma surface, when observed in the compound microscope, was composed of small pseudoparenchymatous cells. Typically brown pigment was unevenly deposited in the walls of these cells giving a mottled appearance to the rehydrated stroma (Figs 4j, 9a). The stromata typically have a pigmented cortical layer underlain by a region of loosely arranged hyphae. Asci were cylindrical and had a thin ring in the apex; they typically contained 8 uniseriate ascospores. Ascospores were hyaline, spinulose and disarticulated early to form two halves, or part-ascospores. The part-ascospores were dimorphic, the distal part was subglobose to broadly conical and the proximal part was ellipsoidal or oblong to narrowly wedge-shaped. Ascospore sizes were clade-specific. G.J.S. 02-87 (Vd 1), a teleomorphic member of the “Large Viridescens” clade from Sri Lanka, had the smallest ascospores. Ascospores of H. vinosa were longer in the distal part than in all other species and the width of its proximal and distal parts was greater than in all others. Ascospores of H. rufa and H. viridescens are nearly identical in size. Vb 3 includes two Hypocrea collections from, respectively, Virginia and North Carolina. While these two collections are sympatric with, but phylogenetically distinct from H. rufa, we did not observe any aspect of their teleomorph, anamorph or cultural phenotypes that would serve to distinguish them from that species.
Fig. 4.
Hypocrea/Trichoderma viridescens teleomorph. a–g. Fresh stromata (a, d–e: immature, b–c, f–g: mature). h–i. Dry mature stromata. j. Surface of stroma reconstituted with water, showing ostioles and unevenly distributed pigment. k. Surface of stroma and ostiolar opening in section. l. Surface of stroma in face view, including unicellular hair. m. Subperithecial tissue in section. n. Palisade of cells above point of attachment of stroma in section. o. Asci with ascospores in cotton blue/lactic acid. Sources: a, l, o: WU 24025. b–c: WU 24027. d, f: holotype WU 24029. e: WU 24024. g, j, m–n: WU 24019. h: WU 24018. i: WU 24028. k: G.J.S. 98-182. Scale bars: a = 2.7 mm, b–c, e = 2 mm, d, g = 1 mm, f = 2.5 mm, h = 0.4 mm, i = 0.5 mm, j = 0.2 mm, k = 30 μm, l = 10 μm, m, o = 15 μm, n = 35 μm.
Fig. 2.
Hypocrea rufa/Trichoderma viride teleomorph. a–c, e–g. Dry stromata (a, c: immature, downy, b, e–g: mature, f: “albino” stroma). d, h. Fresh stromata. i. Stroma reconstituted with water. j. Ostiolar opening in section. k. Section of stroma with perithecia. l. Hairs on surface of mature stroma. m. Surface of stroma in face view. n. Subperithecial tissue in section. o–p. Ascospores, o: in cotton blue/lactic acid. q. Asci with ascospores. Sources: a–b, p: neotype Scleromyceti Sueciae 303, c: WU 24016; d, g, i–o, q: epitype WU 24013, e: BPI 872089, f: WU 24015, h: WU 24011. Scale bars: a, e, g, i = 0.5 mm, b = 0.8 mm, c–d, f = 1 mm, h = 1.5 mm, j, l, q = 20 μm, k = 0.2 mm, m = 25 μm, n = 30 μm, o = 15 μm, p = 10 μm.
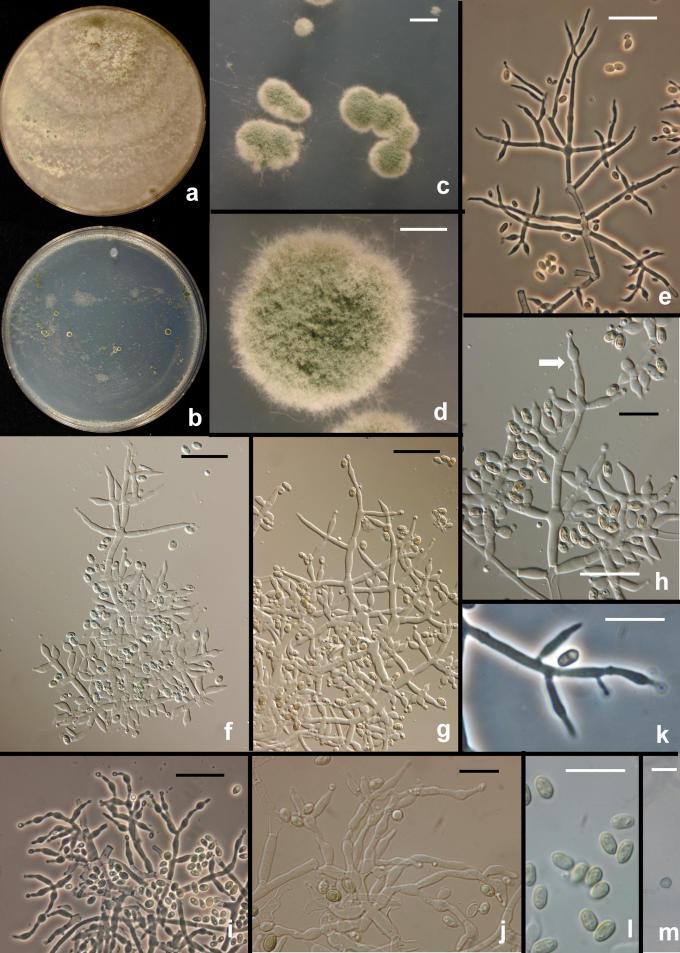
Fig. 16.
Hypocrea/Trichoderma species. a–d. Trichoderma gamsii. a–b. conidial pustules on SNA (G.J.S. 05-111). c–d. Colonies grown 1 wk at 25 °C alternating light (G.J.S. 05-111). c. PDA. d. SNA. e. T. scalesiae (G.J.S. 03-74), 1 wk at 25 °C alternating light. f–i. H. vinosa stromata. f. From the type collection. g. G.J.S. 99-158; h. G.J.S. 02-54, immature with hairs at stroma surface. i. G.J.S. 99-156, immature. Scale bars: a–b = 0.5 mm; f = 1 mm, g–i = 0.5 mm.
Fig. 9.
Hypocrea vinosa/T. vinosum teleomorph. a. Cells at surface of stroma in face view. b. Short hairs arising from the surface of the stroma. c. Longitudinal section through a stroma. d. Median, longitudinal section through a perithecium. e. Section through the upper part of a stroma showing short hairs arising from the surface, a pigmented cortical area and intertwined hyphae below. f–g. Ostiolar region of a perithecium. h. cells of the interior of a stroma below a perithecium. i–j. Asci and ascospores; a thin ring can b seen in j. All from G.J.S. 02-54. Scale bars: a–b, e–i = 20 μm; c = 200 μm, d = 100 μm, j = 10 μm.
Biogeography
Most of the clades that included more than one strain did not show strong biogeographic bias. Hypocrea vinosa was originally described from New Zealand and, in this work, it is restricted to New Zealand and Australia. The “Large Viride” and “Viridescens” clades are widely distributed but are more common in North America and Europe. These are not tropical fungi. Trichoderma viridescens has been found in Peru at high elevation. We have seen only one isolate of T. viride from a tropical region, i.e. G.J.S. 92-15, from Brazil. However, two members of the “Large Viride” clade, DIS 328g (Ecuador) and G.J.S. 04-40 (Brazil), originated in South America. These two endophytic isolates apparently represent two distinct species. Trichoderma neokoningii was isolated in a tropical region in Peru. On the basis of our collecting, T. viridescens is far more common and possibly more widespread than T. viride. Trichoderma viride and T. viridescens are common in Europe as anamorphs, but uncommon as teleomorphs if compared to common species like H. minutispora. There is a tendency for isolates originating in a geographic area (e.g. Taiwan or Europe) to cluster together but there was an equally strong tendency for clades to comprise strains of mixed origin (e.g. Japan, United Kingdom and U.S.A.). Trichoderma gamsii includes strains from widely separated locations, viz. the Tyrrhenian island of Sardinia (Italy), U.S.A. (Texas), Russia and Australia. The clade Vd 3 comprises two biogeographically distinct sister clades. Isolates G.J.S. 00-67 and G.J.S. 97-243 are from eastern U.S.A. Isolates G.J.S. 94-9 – G.J.S. 94-11 were collected in Taiwan.
The isolates G.J.S. 04-40 and DIS 328g were isolated as endophytes from trunks of Theobroma cacao and Th. gileri, respectively, and T. scalesiae was isolated as an endophyte from woody, above-ground tissue of Scalesia pedunculata.
Definition of species
Fig. 1, with T. asperellum as outgroup, demonstrates the considerable known and yet to be described taxonomic diversity in a large part of T. sect. Trichoderma. Despite the existence of several clades that no doubt merit taxonomic recognizion, in the current work we emphasize the “Large Viride” and “Large Viridescens” clades.
Each of these large clades includes several well-supported internal clades, making it difficult to delimit species. In most cases, more or less distinct phenotypic apomorphies lead to our decision as to where to draw species boundaries.
The greatest phylogenetic diversity is found in the “Large Viridescens” clade. At the most distant point of this clade, T. gamsii and T. neokoningii can be distinguished because they both have smooth, ellipsoidal conidia. Trichoderma gamsii is a common species in Europe and North America. Trichoderma neokoningii is only known from a single culture that was collected in Peru as a hyperparasite on a destructive pathogen of Theobroma cacao.
Clade Vd 2 includes European and middle-eastern (Iran) isolates that also have smooth, ellipsoidal conidia. Clade Vd 1 includes isolates from Sri Lanka and Ghana that have ellipsoidal, warted conidia. One of these, G.J.S. 02-87 (Sri Lanka), produces a H. rufa-like stroma but it has smaller ascospores than either H. rufa or H. viridescens. We did not observe an eidamia-like morphology in Vd 1 or Vd 2.
Hypocrea vinosa is distinguished from H. viridescens primarily on the basis of its faster rate of growth and on its larger ascospores. It has a conspicuous eidamia-morphology when grown on CMD.
Clade Vd 3 is phenotypically and biogeographically diverse. We had originally included all of these isolates within T. viridescens. As was noted above, the North American isolates (G.J.S. 00-67, G.J.S. 97-243) cannot be distinguished from H. viridescens, whereas the remaining isolates, all from Taiwan, have a noticeably slower rate of growth than T. viridescens. Their relationship to T. viridescens is indicated by the dotted line in Fig. 1.
Hypocrea/Trichoderma viridescens is a widely distributed species that is common in Europe. It is phenotypically, phylogenetically and geographically diverse, but the phenotypic diversity overlapped to such an extent that we were not able to subdivide the species. Hypocrea/T. viridescens is characterised by north- and south-temperate distribution, relatively slow growth, conidiophores that tend to produce paired branches on SNA, subglobose to nearly ellipsoidal, warted conidia, a coconut odour on PDA and CMD, and the conspicuous eidamia-morphology found on PDA and CMD.
The most distant point of the “Large Viride” clade is T. scalesiae. This unusual species was isolated as an endophyte from the trunk of an endemic daisy tree in the Galapagos Islands. It only produced few conidia on conidiophores that are atypical in Trichoderma. Even in the absence of conidial development, it is recognizable as a Trichoderma by its strong odour of coconut and also by the production of abundant chlamydospores that are typical of Trichoderma.
A single clade that is sister to H. rufa/T. viride includes Vb 1, Vb 2 and Vb 3. The two isolates of Vb3 were isolated in the mid Atlantic states of the U.S.A. and they cannot be distinguished from T. viride (with which they are sympatric) morphologically. Apart from the phylogenetic difference indicated by sequences of tef1, we cannot observe any way to taxonomically separate them from H. rufa/T. viride. The single strains that comprise Vb 1 and Vb 2 were isolated as endophytes from trunks of, respectively, Theobroma gileri and Th. cacao in Ecuador and Brazil. Both of them have a faster growth rate than H. rufa/T. viride, a difference that is especially marked on SNA, and Vb 2 grows faster than any of the clades included in the present study. Both of these, but especially Vb 2, have somewhat smaller conidia than T. viride. Conidiophores of Vb 2 are Type 1 described on page 144 and typical of T. viride. The unusual conidiophores of DIS 328g (Vb 1) and the short broad phialides distinguish this clade from its closest relatives, Vb 2, Vb 3 or T. viride. The data suggest that these two endophytic strains represent distinct species; their taxonomy will be discussed in a future publication.
As was the case with H./T. viridescens, H. rufa/T. viride is phylogenetically and phenotypically diverse but we did not find any hiatus in the characters that would enable us to recognise more than a single species. The hallmark of T. viride is its remarkably consistent, rather slow rate of growth, strongly warted, globose to subglobose conidia and this is consistent with the type specimen of T. viride (Fig. 8a, b and Samuels et al. 1999). Moreover, the conidiophores found in T. viride, with often solitary, hooked phialides, are consistent with what Tulasne & Tulasne (1865) illustrated for their concept of H. rufa and T. viride.
What we have called T. viridescens could have perhaps been selected as being typical of T. viride, given the overlap in phenotype characters of the anamorph, but conidia in this group are not so strongly warted and the tendency is for ellipsoidal conidia rather than globose. The ex-type culture of Eidamia viridescens (CBS 433.34) was included in our analysis. Thus we name this clade T. viridescens, with Hypocrea viridescens sp. nov. as its teleomorph.
KEY TO TAXONOMIC AND PHYLOGENETIC SPECIES OF TRICHODERMA SECT. TRICHODERMA DISCUSSED IN THIS PAPER
1. Conidia conspicuously warted, warts usually densely disposed and conspicuous............................................ 2
-
1. Conidia smooth or warted, warts scattered or inconspicuous............................................................................ 7
2. Colony radius on PDA after 72 h at 25 °C 50–60 mm........................................................................................ 3
2. Colony radius on PDA after 72h at 25 °C < 50 mm............................................................................................ 4
3. Conidia (2.7–)3.0–3.7(–4.2) × (2.2–)2.5–3.2(–3.5) μm; Sri Lanka, Ghana................................................... Vd 1
3. Conidia (3.0–)3.5–4.0(–4.2) × (3.0–)3.2–3.7(–4.2) μm; endophytic in Theobroma cacao, Brazil.............................................................................................................................. Vb 2
4. Conidia (3.0–)3.2–4.0(–4.5) × (2.7–)3.0–3.5(–4.0) μm; conidiophores typically sinuous and frequently branched; phialides short and broad, proliferating phialides and/or submoniliform hyphae not formed; endophytic in trunk of Theobroma gileri, Ecuador................................. Vb 1
4. Conidia larger, (3.0–)3.5–4.5(–8.5) × (2.2–)3.0–4.0(–4.7) μm; not endophytic.................................................. 5
5. Conidia (3.0–)3.5–4.5(–5.5) × (2.8–)3.4–4.0(–5.0) μm, typically globose, grossly warted, L/W (0.8–)1.0–1.2(–1.5); terminal conidiophores often curved, phialides often widely spaced and solitary, often hooked or sinuous; proliferating phialides usually not formed in pustules................................................................................................................. T. viride
5. Conidia (2.7–)3.5–4.5(–8.5) × (2.2–)3.0–4.0(–4.7) μm, globose to ellipsoidal, L/W (0.9–)1.0–1.4(–2.0), verruculose; phialides typically forming in whorls, straight; proliferating phialides and/or submoniliform conidiophores often formed............................................ 6
6. Conidia subglobose, (3.2–)3.5–4.5(–4.7) × (2.7–)3.0–4.0(–4.2) μm, L/W 1.0–1.2(–1.3); mean of distal part-ascospores 5.0–6.5 × 4.7–6.2 μm; mean of proximal part-ascospores 5–7 × 4–5 μm; colony radius on PDA after 72 h at 25 °C typically 25–33 mm; Australia and New Zealand.............................................................................................................................. T. vinosum
6. Conidia subglobose to ellipsoidal, (2.8–)3.5–4.5(–8.5) × (2.3–)3.0–3.7(–4.7) μm, L/W (0.9–)1.1–1.4(–2.0); mean of distal part-ascospores 4.2–5.5 × 4.2–4.7 μm; mean of proximal part-ascospores 4.5–5.5 × 3.2–4.0 μm; colony radius on PDA after 72 h at 25 °C typically 35–45 mm; cosmopolitan, more common north-temperate...................... T. viridescens
7. Conidia globose to ovoidal, smooth, finely warted or with larger scattered warts.............................................. 8
7. Conidia smooth, subglobose, ellipsoidal to ellipsoidal, smooth.......................................................................... 9
8. Conidia subglobose or ovoidal, finely spinulose (often appearing smooth in light microscope), (2.8–)3.4–3.6(–7.0) × (2.4–)3–4(–6) μm, L/W 1.0–1.7....... T. asperellum Samuels et al. (Samuels et al. 1999)
8. Conidia subglobose to ellipsoidal, smooth or with large scattered warts; (3.0–)3.2–4.5 (–5.7) × (2.2–)3.0–3.5(–4.0) μm, L/W = 0.9–1.7 (mean = 1.2)......................................................................... Ve
9. Conidia globose to subglobose or broadly ovoidal........................................................................................... 10
9. Conidia ellipsoidal to oblong............................................................................................................................. 13
10. Conidiophores and conidia typical of Trichoderma, green and typically forming in abundance on SNA, PDA and CMD; colonies fast-growing........................................................................ 11
10. Conidiophores and conidia verticillium-like, forming in wet heads, sparsely formed and very inconspicuous, reliably forming only on SNA.................................................................................... 12
11. Colonies with a strong coconut-like odour; conidia subglobose to ovoidal, smooth, (2.7–)3.0–3.8(–5.0) × (2.3–)2.8–3.5(–4.0) μm, L/W = (0.8–)1.0–1.3(–1.6); often with a strong coconut-like odor.................................................................... T. atroviride P. Karst. (Dodd et al. 2002)
11. Colonies lacking a coconut-like odor; conidia subglobose to ovoidal, finely spinulose (often appearing smooth with light microscope), (2.8–)3.4–3.6(–7.0) × (2.4–)3–4(–6) μm, L/W 1.0–1.7; rarely with a coconut-like odour.............. T. asperellum (Samuels et al. 1999)
12. Conidia (3.0–)3.5–4.5(–5.0) × 2.5–3.5 μm; colony radius 24–26 mm after 96 h at 25 °C on PDA; growing on Moniliophthora roreri on pods of Theobroma cacao, Ecuador........................................................................... T. paucisporum Samuels et al. (Samuels et al. 2006)
12. Conidia (2.5–)3.0–3.7(–4.0) × 2.2–)2.7–3.2(–3.5) μm; colony radius < 15 mm after 96 h at 25 °C on PDA; endophyte in stems of Scalesia pedunculata, Galapagos Islands.............................................................................................................................................. T. scalesiae
13. Conidia (3.2–)3.5–4.0(–4.5) × 2.5–3.0 μm; Peru.......................................................................... T. neokoningii
13. Conidia larger, (3.2–)3.5–4.5(–4.7) × (2.2–)2.5–3.5(–3.7) μm; Iran or cosmopolitan temperate...................... 14
14. Conidia (3.5–)3.7–4.5(–5.5) × (2.5–)2.7–3.5(–3.7) μm; L/W (1.0–)1.2–1.5(–1.7); colony radius on SNA after 72 h at 25 °C typically < 35 mm, north- and south-temperate................... T. gamsii
14. Conidia larger, (3.2–)4.0–5.0(–5.8) × (2.2–)2.5–3.0(–3.2) μm; L/W (1.1–)1.4–1.8(–2.1); colony radius on SNA after 72 h at 25 °C typically ca. 40–45 mm; Iran........................................................ Vd 2
DESCRIPTIONS OF THE SPECIES
Continuous characters not provided in the descriptions are given in Table 2.
-
Hypocrea rufa (Pers.: Fr.) Fr., Summa Veg. Scand., Sectio Post. 383. 1849. Figs 2, 3, 7a–f, 8 a–e.
- ≡ Sphaeria rufa Pers., Obs. Mycol. 1: 20 (1796): Fr., Syst. Mycol. 2: 335. 1822.
Anamorph: Trichoderma viride Pers., Neues Mag. Bot. [Roemer's] 1: 92. 1794: Fries, Syst. Mycol. 3: 215. 1832.
- = Trichoderma lignorum (Tode) Harz, Bull. Soc. Imp. Natur. Moscou 44: 116. 1871.
- = Trichoderma glaucum E.V. Abbott, Iowa State Coll. J. Sci. 1: 27. 1927.
Stromata when fresh (Fig. 2d, h) 1–4(–6) mm long, 0.5–1.5 mm high, solitary to gregarious, or aggregated in small numbers or crowded in lines along wood fibres, at first semi-effused, flat, velutinous, with white mycelial margin; becoming pulvinate, more rarely turbinate or discoid, circular to irregular in outline, surface smooth to slightly uneven to granular, broadly attached, margin often becoming free and concolorous with stroma surface; at first white, remaining white with yellowish ostiolar openings (“albino” form), or more commonly becoming variably coloured from the centre: first yellowish, then pale ochraceous, light brownish or yellow- to orange- to rust-brown (5A4–7, 5B4, 5C6–7, 6CD5–8), later light to dark reddish brown (7–8CD6–8, 8E7–8), sometimes with whitish to rust-coloured scurf; ostioles invisible or appearing as watery, hyaline, or indistinct darker dots, sometimes projecting, convex, often irregularly distributed.
Stromata when dry (Fig. 2a–c, e–g) (0.5–)0.6–3 (–5.7) mm long, (0.4–)0.6–2(–3.4) mm broad, (0.2–) 0.3–0.6(–0.9) mm thick (n = 31), KOH–, darker and surface more uneven than in fresh stromata, granular to finely tuberculate, sometimes extremely uneven with perithecial contours visible; ostioles not visible or partly convex or semiglobose, appearing as hyaline or brown dots, generally hyaline after addition of water (Fig. 2i); young stromata velvety to conspicuously hairy (Fig. 2a, c), with diffuse yellowish orange, yellowish brown or (orange-)brown colours, 4B4–5, 5AB5–6, 5CD7–8, 6B5, 6CD5–8, later light-, 7CD5–8, 7E7–8 to deep reddish brown, 8EF5–8 to 7F6–8, 8CD5–6, to dark brown, 7EF5–6; albino form white or becoming pale yellowish, 4A3–4, with numerous, conspicuous light brownish dots (Fig. 2f).
Stroma anatomy: Cells of stroma surface in face view (Fig. 2m) pseudoparenchymatous, (3.5–)5–9(–14.5) μm (n = 30) diam, walls to 1 μm thick, reddish brown in water, orange-brown in lactic acid, pigment unevenly deposited in cell walls, giving a mottled appearance to the stroma surface. Ostiolar area in dry stromata (32–) 40–84(–126) μm (n = 33) diam. Hairs (Fig. 2l) arising from the stroma surface, yellowish to pale brown, comprising 2–5 cells, apically rounded, rarely branched, sometimes consisting of only one inflated cell, (7.5–)10–29(–62) μm (n = 79) long, (2–)3.5–5(–6.5) μm wide (n = 49), walls 0.5–1 μm thick. Cortical region (12–)17–30(–35) μm (n = 20) thick, cells forming a textura angularis, slightly compressed, reddish brown, in lactic acid orange-brown, (2–)3.5–9(–13.5) μm (n = 60) diam in vertical section, walls up to 1 μm thick. Cells immediately below the cortex comprising a mixture of intertwined hyphae, (2.5–)3–6(–6.5) μm (n = 10) wide, vertical and parallel between perithecia, and few subglobose to angular cells similar to those of the cortex. Perithecia (Fig. 2k) (161–)182–245(–307) × (107–)140–210 (–251) μm (n = 31), flask-shaped, ellipsoidal to globose. Ostiolar canal (70–)75–107(–120) μm (n = 21) long, (32–)33–55(–69) μm (n = 15) wide at the opening (Fig. 2j), cells surrounding the ostiolar opening a palisade of hyaline, narrowly cylindrical, apically slightly expanded cells, plane with the surface or projecting to 14–43 (–69) μm (n = 15). Peridium colourless, consisting of laterally strongly compressed thin hyphae, basally and apically pseudoparenchymatous, indistinct, scarcely differentiated from and merging with the surrounding tissue, apical part flanking the ostioles conspicuously thickened. Tissue below the perithecia (Fig. 2n) of homogeneous, dense textura epidermoidea, of globose to elongate, thin-walled, hyaline cells, (4–)5–19(–26) × (3–)4.5–10(–12.5) μm (n = 30), not layered but cells gradually smaller and interspersed with few narrow hyphae towards the base of the stroma. Asci (Fig. 2q) cylindrical, (70–)87–112(–132) (n = 72) μm long, including a stipe of (5–)9–17(–22) μm (n = 30), (4–)5.5–7(–8.5) μm (n = 72) wide, stipe short, with a knob-like base, apical pore minute. Ascospores (Fig. 2o, p) hyaline, verrucose, verrucae ca. 0.5 μm long and diam; dimorphic, distal part subglobose to oval, sometimes slightly tapered towards the upper end, proximal part oblong to wedge-shaped, the lower end broadly rounded.
Colony characteristics: Optimal temperature for growth on PDA and SNA 25 °C, colony radius on PDA 30–35 mm after 72 h in darkness, on SNA 22–31 mm; not growing at 35 °C. Colonies grown on PDA 1 wk at 25 °C with alternating light (Fig. 7a–d) developing conidia in several alternating green (28DE5–7 to 27DE3–6 to 27F7–8) and dull yellow (3A3–4) concentric rings. Diffusing pigment not noted. Slight coconut odour rarely noted.
Colonies grown on CMD (Fig. 7e; 25 °C under alternating light) >7 cm, filling a Petri plate within 5–6 d, thin, hyaline, margin indistinct, diffuse, hyphae loosely arranged, no zonation, no pigment formed. Autolytic activity absent, coilings and aerial hyphae inconspicuous. A weak coconut-like odour formed in some but not all strains. Conidiation from 2 d, in pustules more or less regularly distributed on the plate or forming in a broad band around the margin, less frequently in concentric rings, usually starting close to the point of inoculation, formed exclusively or preceded or accompanied by various amounts of simple conspicuously curved conidiophores or minute tufts. Pustules (Fig. 3a–b) at first white, becoming green from the 4th day or later, depending on the isolate, 28D3–5 or 26E4–6 to 27E4–6, finally 26F5–8 to 27F6–8 after 1 wk, compact to cottony, pulvinate to hemispherical, 0.5–2.5(–5.0) mm diam, 0.5–1.6 mm high.
The structure of typical conidiophores was determined after 5–7(–11) d at conditions described above: pustules and minute tufts arising on a 8–12 μm thick stipe, often with constricted septa, bearing several thick primary branches arising at various angles, both partly verrucose, further branching dense and complex, final long branches thin, bearing short terminal branches at various angles, with 1 or 2(–3) terminal phialides. Conidiophores (Fig. 3d–l) ill-defined, no main axes discernible or at best weakly developed, conspicuously and extremely variably curved to sinuous, often seen as short elongations on the periphery of pustules; branches and phialides generally unpaired. Simple conidiophores and microtufts sometimes tending to be more regularly paired, with tree-like branching; branches sometimes originating on thickened nodes, 7–11 μm wide with up to 5 branches, often tending to be less curved. Phialides (4.0–)6.5–11.2(–18.2) μm long, (1.0–)2.5–3.2(–4.0) μm at the widest point, l/w (1.2–)2.0–4.5(–13.0), basal width (1.0–)1.7–2.5(–3.0) μm (n = 600), originating singly or in groups of 2–3, on rarely inflated, 2–3 μm thick cells, usually not paired, very variable among isolates, lageniform to long cylindrical, typically strongly curved to sinuous, less commonly straight, usually with long necks (up to 10 μm), not or slightly thickened in various positions, tending to be longer and narrower in minute tufts and shorter and more swollen when crowded. Conidia (Fig. 3m, n) globose to subglobose, infrequently nearly ovoidal, (olive-)green, basal scar sometimes visible, coarsely tuberculate (Figs 3 m, n; 8 a–e), tubercles up to ca. 0.7 μm long and 1 μm diam, containing few guttules, in aged cultures often in chains. Chlamydospores rare, typically subglobose at the tips of hyphal branches, less frequently intercalary in hyphal cells, (5–)8–12(–14) μm diam (n = 81), hyaline to pale yellowish.
Colonies grown on SNA (Fig. 7f; 25 °C, alternating light) similar to CMD, thin, hyaline, no zonation, no pigment, autolytic activity absent, aerial hyphae inconspicuous, coilings somewhat more pronounced than on CMD. Conidiation similar to CMD, asymmetrical, starting in the centre in loosely arranged compact pustules of ca. 1–2 mm diam, aggregated to ca. 4 mm diam, and on some effuse single conidiophores, green (26EF5–7 to 27F6–8) from 3–4 d, dry. Chlamydospores (after 15 d at 25 °C) inconspicuous, more frequent than on CMD, terminal and intercalary, mainly globose, (5.0–) 6.5–10.5(–12.5) μm (n = 21) diam, smooth, hyaline to pale yellowish.
Habitat: Anamorph isolated directly from soil, peat, and wood, found also on leaf litter; isolated as an endophyte from sapwood of Fagus sylvatica in the U.K., isolated from water-damaged building in Denmark, not uncommon. Teleomorph uncommon, inconspicuous, found on wood, less commonly on bark of cut branches, tree tops or 2–120 cm thick logs. In Europe found in open coniferous or mixed deciduous forests, grassland with single trees or at shady road sides, often in piles (to 3 m above the ground) of logs at the edge of forests, stored or lying on bare moist soil, in leaf litter, in grass, the wood in little to medium degree of decomposition and often hard. In Central and Northern Europe mainly on coniferous trees (Pinus sylvestris, Picea abies), in Western Europe more frequent on deciduous trees (e.g. found on Quercus robur, Acer pseudoplatanus). Associated with Trichaptum abietinum, Stereum sanguinolentum, Sarea resinae, Ophiostoma sp., Nectria fuckeliana, Valsa pini, Pezicula eucrita, Schizophyllum commune, various Corticiaceae; Amphiporthe leiphaemia, Diatrypella sp., Bulgaria inquinans.
Distribution: Teleomorph collected in Europe (confirmed in Austria, Czech Republic, U.K., France, Sweden) and United States (confirmed in North Carolina, Virginia). Anamorph north and south-temperate, including Europe (Italy, Sweden, Finland, Switzerland, Denmark), Canada, United States, New Zealand, Japan.
Neotype: Scleromyceti Sueciae No. 303. Epitype, designated here: Czech Republic, South Bohemia, Frymburk, 3.4 km north from Lipno, MTB 7351/3, 48°38'04” N, 14°11'19” E, elev. 745 m, on partly decorticated logs of Pinus sylvestris, 12–30 cm thick, on the ground or elevated in a pile of stored logs at roadside and edge of coniferous (Picea/Pinus) forest, soc. Ophiostoma sp., Nectria fuckeliana, Valsa pini, Pezicula eucrita, Schizophyllum commune, unidentified Corticiaceae, 3 Oct. 2004, W. Jaklitsch, W.J. 2753 (WU 24013; culture CBS 119325 = C.P.K. 1997 = G.J.S. 04-372).
Lectotype of Trichoderma viride: “Prope Parisiis Trichoderma viride, Hb. Pers.” Herb. Lugd. Bat. 910 263-877 (L 0018559 = “Rijksherbarium No 148-1” cited by Bisby 1939, see Webster 1964; lectotype designated by Bisby 1939). Epitype of Trichoderma viride designated here: culture C.P.K. 1997, a dry culture deposited with the epitype of H. rufa (WU 24013a; living culture CBS 119325 = C.P.K. 1997 = G.J.S. 04-372).
Other teleomorphic specimens examined: Austria, Niederösterreich, Zwettl, Traunstein, roadside, 1 km after the western end of the village, MTB 7556/4, 48°26'10” N, 15°05'57” E, elev. 830 m, on partly decorticated cut logs of Picea abies, up to 45 cm thick, in a pile stored at the edge of a Picea/Fagus forest, soc. Ophiostoma sp., 5 Oct. 2004, W. Jaklitsch, W.J. 2766 (WU 24015; culture CBS 119327 = C.P.K. 1999); Steiermark, Liezen, Kleinsölk, close to the northeast corner of the Schwarzensee, MTB 8749/1, 47°17'38” N, 13°52'36” E, elev. 1170 m, on partly decorticated cut logs of Pinus sylvestris, 20–25 cm thick, stored in a pile at roadside and edge of a spruce forest, soc. Ophiostoma sp., 7 Oct. 2004, W. Jaklitsch, W.J. 2773 (WU 24016; culture C.P.K. 2000); Liezen, Weng im Gesäuse, Ennstal, Gstatterboden, 0.9 km after the village heading east, MTB 8453/2, 47°35' 34” N, 14° 39' 09” E, elev. 570 m, on cut log of Picea abies, 120 cm thick, 2.5 m above ground, in a pile stored at roadside, soc. Trichaptum abietinum, 8 Oct. 2004, W. Jaklitsch, W.J. 2774 (WU 24017; isolate C.P.K. 2001). Czech Republic, South Bohemia, at road side 5.7 km north from Frymburk, MTB 7250/4, 48°42'36” N, 14°08'06” E, elev. 750 m, on partly decorticated cut log of Picea abies, 22 cm thick, on the ground, protected by grass, herbs, soc. Nectria fuckeliana, Stereum sanguinolentum, Sarea resinae, immature, culture from conidia, 22 Sep. 2003, W. Jaklitsch, W.J. 2408 (WU 24010; culture C.P.K. 965); 2.7 km before Frymburk approaching from Lipno, MTB 7351/3, 48°38'22” N, 14°10'52” E, elev. 740 m, on partly decorticated logs of Pinus sylvestris, 10–43 cm thick, stored in a pile between roadside and edge of coniferous (Picea/Pinus) forest, mostly immature, 3 Oct. 2004, W. Jaklitsch, W.J. 2758 (WU 24014; culture C.P.K. 1998). France, La Moselle, Parc Lorraine, Héming, between Étang du Stock and Maizières de Vic, 48°43'35” N, 06°54'07” E, elev. 180 m, on cut and mostly corticated branches and logs of Quercus robur, 2–40 cm thick, on bare ground, some branches squeezed into moist soil, soc. Amphiporthe leiphaemia, Diatrypella sp., Bulgaria inquinans, part with white mould, 5 Sep. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2677 (WU 24011; culture C.P.K. 1995). Sweden, Uppsala Län, Fredrikslund, pine forest near nature reserve Kungshamn-Morga, 1.5 km NE of Fredrikslund, 59°47'00” N, 17°39'00” E, elev. 50 m, on cut and mostly corticated tree tops and branches of Pinus sylvestris, 5–9 cm thick, on the ground, 8 Oct. 2003, W. Jaklitsch & S. Ryman, W.J. 2450 (BPI 872089; cultures CBS 119326 = C.P.K. 984, G.J.S. 04-21 from white stroma). United Kingdom, Derbyshire, Baslow, Longshaw Country Park, Peak District National Park, 53°18'26” N, 01°36'08” W, elev. 350 m, on corticated branches and logs of Acer pseudoplatanus, 2–10 cm thick, on the ground in open grassland, immature, culture from conidia, 10 Sep. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2695 (WU 24012; culture C.P.K. 1996). U.S.A., North Carolina, Macon County, northwest of Highlands, Cullasaja Gorge, Dry Falls, on bark of recently dead tree, 20 Sep. 1989, G.J.S. et al. (BPI 744477; culture G.J.S. 89-127 = CBS 114374); Macon County, Blue Valley off Clear Creek Rd., along Overflow Creek, 35°00' N, 83°15' E, on decorticated wood, 16 Oct. 1990, G.J.S. et al. (BPI 1109386, culture G.J.S. 90-95 = IMI 352470 = CBS 120066); Virginia, Giles County, Mt. Lake Biological Station, Little Spruce Bog, 37°22' N, 80°31' E, elev. 1170 m, on branch of Acer sp., 17 Sep. 1991, G.J.S. et al. (BPI 1112834; culture G.J.S. 91-62).
Anamorphic cultures studied: see Table 1.
Typification of H. rufa: This species was first described by Persoon in 1796 as Sphaeria rufa. The description, comprising just seven words, was enigmatic: “periphaerica, irregularis, applanata, mollis, rufa intus albida”, and no collecting location or specimen was cited. He (Persoon 1801) expanded the description, noting that the species was rare in dense forest on branches of Fagus and that the rufous colour and “scarcely prominent sphaerules [ostiola]” distinguished this species from all others. Fries (1822) included S. rufa in his Systema mycologicum and he later (Fries 1849) placed the species in the new genus Hypocrea. The first mention of a specimen was Fries' (1849) citation of Scleromyceti Sueciae 304. Fries created confusion in this exsiccate when he misnumbered specimens 303 and 304. The specimen list gave number 303 as H. gelatinosa and number 304 as H. rufa but the numbers on the actual specimens were reversed, 303 for H. rufa and 304 for H. gelatinosa (Holm & Nannfeldt 1962; Hennig Knudsen, in litt. 7 Oct. 2004). Tulasne & Tulasne (1865) were the first to establish a link between H. rufa and T. viride and their elegant work established the taxonomic identity of this species. Scleromyceti Sueciae 303 (UPS!) was designated as neotype for S. rufa by Rossman et al. (1999).
Scleromyceti Sueciae 303 (Figs 2 a, b, p) comprises a single piece of decorticated wood glued to a piece of cardboard on which is written only “Fries, Scler. Suec. 303. Sphaeria rufa”. Stromata are scattered over the surface of the wood. One is young, semi-effused to pulvinate, light tan, and fibrous. The mature stromata are rufous (ca. 8F8), more or less discoidal, with a deeply wrinkled or convoluted, velutinous surface. Ostiolar openings are not visible. Hyphal hairs arise from the stroma surface. Cells of the stroma surface are angular and brown pigment is deposited unevenly in the cell walls. No asci remain. Discharged part-ascospores are hyaline, spinulose, dimorphic. Subglobose to ovoidal parts, which are assumed to be distal part-ascospores, measure 3.7–4.7(–5.5) × 3.5–4.5(–5.0) μm (n = 30). Ellipsoidal to wedge-shaped parts are assumed to be the proximal part-ascospores and measure (4.0–)4.5–5.0(–5.7) × (3.0–)3.2–3.7(–4.5) μm (n = 30). Although these measurements are somewhat smaller than we have found for about 10 collections, the combined attributes of anamorph and teleomorph that we describe here conform well with Fries's specimen and with the taxonomic identity that was established by Tulasne & Tulasne (1865). We have chosen an epitype for H. rufa, WU 24013, collected in the Czech Republic, that agrees well with the historical concept of H. rufa in teleomorph and anamorph.
Notes: Stromata of H. rufa are usually accompanied by the Trichoderma viride anamorph. Conidia found in nature are dark green, 26F5–8 to 27F4–8, and often citrine- to sulphur-yellow, 4A4–6, hairy patches of mycelium are found. Hypocrea rufa cannot be safely distinguished from the sympatric H. viridescens by the morphology of the teleomorph, and intense yellow cottony patches of the accompanying anamorph are found in both species. The dry stroma colour reported in the description above is difficult to determine due to the small size of stromata, which are often solitary rather than aggregated. Perithecial measurements are only approximated due to indistinct differentiation of the peridium from surrounding tissue. Some pulvinate stromata approach those of H. pachybasioides or H. minutispora in shape and colour when their ostiolar openings are clearly visible, but H. rufa differs from these species in the presence of hairs, especially in young stromata.
While the anamorph, T. viride, is met frequently on wood, but less frequently than T. viridescens, it is difficult to find good teleomorph material. Stromata apparently develop slowly and in a narrow range of ecological conditions, mainly regarding moisture, temperature, and age and degree of decay of substrata. Moreover, they often develop in open habitats that are susceptible to quick desiccation. The frequency of long dry periods has increased in recent years, which may contribute to the fact that teleomorphs are collected rarely. On the other hand, if a habitat is too moist, stromata are soon attacked by hyphomycetes, often seen in specimens as white mould on the stromata. These are obvious reasons why specimens mostly contain immature stromata. Anthropogenic influences, particularly cutting of logs and branches, strongly facilitate development of the teleomorph.
Webster (1964) refuted Bisby's (1939) contention that H. rufa could not be distinguished from H. gelatinosa (Tode: Fr.) Fr. by characterising the anamorphs and teleomorphs of the two species. We have examined several of the specimens collected in the U.K. and cited by Webster (SHU!, now in K). On the basis of the stromata alone we could not identify them as H. rufa or H. viridescens. However, conidia found on 2540, 2611, 2618 and 2621 are globose and coarsely warted; most likely they are H. rufa. One of those, 2621, has conidiophores typical of what we have described for T. viride.
-
Hypocrea viridescens Jaklitsch & Samuels, sp. nov. MycoBank MB494775. Figs 4, 5, 6, 7 g–l, 8 f–h.
Etymology: viridescens, turning green, in reference to the anamorph.
Hypocreae rufae similis sed anamorphosis differt. Ascosporae: pars distalis (3.3–)4.1–5.2(–7.4) × (3.2–)3.8–4.6(–5.4) μm; pars proxima (3.4–)4.4–5.8(–7.9) × (2.7–)3.3–4.0(–5.3) μm. Anamorphosis Trichoderma viridescens (A.S. Horne & H.S. Williamson) Jaklitsch & Samuels. Conidia subglobosa vel ellipsoidea, levia, (2.8–)3.5–4.5(–8.5) × (2.3–)3.0–3.7(–4.7) μm, L/W (0.9–)1.1–1.4(–2.0).
Anamorph: Trichoderma viridescens (A.S. Horne & H.S. Williamson) Jaklitsch & Samuels, comb. nov. MycoBank MB501049.
- ≡ Eidamia viridescens A.S. Horne & H.S. Williamson, Ann. Bot. 37: 396. 1923.
Stromata when fresh (Fig. 4a–g) ca. 0.5–3.5 mm diam, ca. 0.5–1.5 mm thick, single, loosely gregarious or densely aggregated in lines, young downy, covered with yellowish to rust-coloured hairs, first with white inconspicuous margin; later glabrous, pulvinate, centrally attached, margins free, outline circular, angular to irregular, ostioles invisible to inconspicuous or light to dark dots, surface smooth to finely granular by perithecial contours, young light brown 5B5–6 to 6CD5–6, yellow-brown to nearly orange-brown 6CD7-8, intensely orange-rust-reddish brown 8(B)C7–8; mature mostly dark reddish brown, 7DE7–8, 8EF6–8 (to 9F7–8, 10CD7–8, 10F7–8), margin usually dark; rarely rosy-brownish with dark spots to reddish brown 8CD5-6, more rarely 6A6–7 to 7A5–6.
Stromata when dry (Fig. 4h, i) (0.2–)0.6–1.6(–3.6) mm (n = 277) diam, 0.2–0.5(–0.8) mm (n = 33) thick, unchanged or slightly darkened in 3 % KOH; young thin, semi-effuse to effuse, hairy, white to yellowish brown to rust-brown, 4A4, 5BD4–7 to 6–7CD5–8, with white to rust margin; later effluent, discrete and pulvinate with circular, angular to irregular outline; surface uneven, tubercular to wrinkled; ostioles invisible or appearing as light dots with darker marginal rings, not or slightly projecting, convex; colour in the great majority of stromata deep and dark reddish brown 7–9EF5–8.
Stroma anatomy: Cells of stroma surface in face view (Fig. 4 l) glassy, isodiametric to slightly elongated, (2–) 3.5–8.5(–19) × (1.5–)2.5–6(–10) μm (n = 313, 210), walls thickened and pigment not uniformly deposited. Hairs arising from cells of the stroma surface, usually abundant when young, scant on mature stromata, 1–4-celled, thick-walled, (5–)10–24(–47) μm (n = 240) long, (2–)3.2–5.0(–6.5) μm (n = 83) wide, base to 7 μm wide, apically rounded, pale brownish, smooth to slightly verruculose. Ostiolar area of dry stromata (24–)34–64 (–79) μm (n = 33) diam. Cortical region of stroma (Fig. 4k) (15–)18–36(–60) μm (n = 63) thick, around the entire stroma except the point of attachment, reddish brown to yellow-brown, cells in section of textura angularis, (2.0–)3.5–7.5(–17) × (1.7–)2.8–5.0(–8.5) μm (n = 279, 150), with walls thickened in the outer part, thinner towards the interior. Cells immediately below the cortex comprising a mixture of intertwined, (3–)4–6(–6.5) μm wide hyphae (n = 15), vertical and parallel between perithecia, and hyaline, subglobose to angular, mainly isodiametric cells, (3–)5–9.5(–13) μm (n = 30) diam, flanking the ostioles. Perithecia flask-shaped, often somewhat narrowed towards the base, sometimes ellipsoidal to globose, (136–)220–287(–337) μm high, (72–)150–224(–280) μm diam (n = 149); peridium at the base (17–)19–26(–30) μm, laterally (12–)13–20 (–22) μm (n = 15) thick, hyaline. Ostiolar canal (53–) 70–98(–130) μm (n = 138) long, (30–)33–49(–57) μm (n = 15) wide at the opening, the opening formed by a palisade of hyaline, apically elongate narrowly clavate cells, even with the stroma surface or projecting up to 15 μm (n = 15). Subperithecial tissue homogeneous, dense textura angularis to t. epidermoidea, cells (sub-)globose to elongate to subangular or curved, (3.5–) 4.5–15(–39) μm (n = 337) long, (2–)4.5–10 (–17) μm (n = 240) wide, hyaline to pale brownish, with ca. 0.5 μm thick walls, tissue not layered but cells gradually smaller and interspersed with few narrow, ca. 3–4 μm wide hyphae towards the base of the stroma; in the central part of the stroma (point of attachment) stratified by a palisade (Fig. 4n) of oblong refractive glassy cells, ca. 14–31 × 4–9 μm, above the smaller-celled pseudoparenchyma, sometimes irregular brownish pigment in patches incorporated through the whole tissue; basally delimited by the reddish brown cortex. Asci (Fig. 4o) cylindrical, (56–)82–101(–118) × (3–)5–7(–9) μm (n = 314), including a stipe of (4–)6–22(–33) μm (n = 31); apical pore minute. Ascospores hyaline, verruculose to verrucose with verrucae ca. 0.5 μm long and diam, dimorphic; distal part subglobose, oval to wedge-shaped, (3.3–)4.1–5.2(–7.4) × (3.2–) 3.8–4.6(–5.4) μm (n = 411); proximal part oblong to wedge-shaped, lower end broadly rounded, (3.4–)4.4–5.8(–7.9) × (2.7–)3.3–4.0(–5.3) μm (n = 411).
Colony characteristics: Optimal temperature for growth on PDA and SNA 25 °C; after 72 h in darkness colony radius on PDA 32–45 mm, on SNA 24–37 mm; typically slower at 30 °C than at 20 °C; not growing at 35 °C. Colonies grown on PDA for 1 wk at 25 °C with alternating light and darkness (Fig. 7g–j) developing conidia in 3 or 4 distinct concentric rings, but often remaining sterile or producing very few conidia. Conidial masses green (28D–E5–6). Diffusing pigment not noted or reverse only slightly yellowish (3A3–4 to 3B4). Coconut odour typically present. Autolytic activity more pronounced at 15 °C, coilings more frequent at 30 °C.
Colonies grown on CMD (Fig. 7k; 25 °C) filling the Petri plate within 5–6 d, thin, hyaline, regularly circular, hyphae loosely arranged radially, no zonation. Autolytic activity inconspicuous, coilings abundant in some isolates. Aerial hyphae often scarce during fast growth, becoming abundant, particularly towards the margin, broad zone at the margin becoming downy. A diffuse greenish yellow pigment (1B2–6, 2A3–3B4 to 29A2–3) often diffusing through the whole culture. A strong coconut-like odour usually formed. At 15 and 30 °C slower development with less conidiation and less strong coconut-like odour formed, coilings more frequent at 30 °C. Conidia forming within 2–10 d at 25 °C under alternating light, typically effuse, conidial pustules uniformly dispersed throughout the colony, developing in a broad marginal band, or developing in indistinct concentric rings; after ca. 6–7 d (not always) followed by the development of loose, polymorphic tufts (Fig. 5a) of ca. 0.2–1.5 mm diam on thick, thick-walled and verrucose stipes, confluent to 3 × 2 mm, sometimes in up to three concentric rings or more irregularly disposed, never dense or compact; conidial masses light to dark green from 5 d onwards, 28A4–5 to 27F5–8, development slow, final colour often after 14 d; sometimes conidia yellow at first, then becoming green. Inoculation in the middle of the plate often resulting in more regular distribution of tufts. Conidiophores typically visible at the surface of the pustules.
Conidiophores (Fig. 5b–k) dimorphic, representing Types 2 and 3 (described on page 144), the two types typically not forming in the same culture (but see e.g. CBS 333.72 or CBS 119322 with both); conidiophores of both types found on CMD and PDA, Type 2 conidiophores alone found on SNA. Conidiophores of Type 2 (Fig. 6) more or less typical of Trichoderma, conidiophores at the surface of the pustule projecting from the pustule, often with a sterile stipe and a few terminal branches or only few phialides produced at the tip, or conidiophores branched along the length with fertile branches widely spaced; conidiophores forming toward the interior of the pustule tending to have a discernable straight to sinuous main axis with progressively longer fertile branches tending to be paired and arising at right angles to the main axis, branches terminating in 1–3 phialides in a cruciate whorl. Phialides from Type 2 conidiophores lageniform, slightly swollen in the middle when more crowded, straight (Fig. 6d–g), less frequently sinuous or hooked, phialides sometimes appearing to be proliferated percurrently. Conidiophores of Type 3 (Fig. 5) developing in many PDA and CMD cultures, main axis barely or not discernable, branching at acute angles with respect to the parent hypha, branches rebranching or not, each branch terminating in 1 or 2 phialides; phialides appearing to proliferate percurrently (5f–i, k), often resulting in a submoniliform chain of 2–6 cells (Fig. 5f, g), the terminal cell of which is a phialide; phialides and subtending cells distinctive in being swollen in the middle and more or less conspicuously constricted above and below the middle, phialides sometimes with a long, cylindrical neck. Conidiophores often arising from variously distorted and swollen cells in pustules (Fig. 5 j, k); submoniliform chains of proliferated phialides are often conspicuous in older pustules. Phialides measured from both types of conidiophores, the longer phialides found on Type 2 conidiophores, (4.8–)7.2–11.5(–20.5) μm long, (1.5–)2.5–3.5(–5.0) μm at the widest point, (1.0–)1.5–2.5(–4.0) μm wide at the base, L/W (1.4–)2.2–4.5(–10.4) (n = 1125); arising from a (1.1–)2.1–3.3(–5.0) μm wide cell (n = 1064). Conidia (Fig. 6h–m, 8f–h) subglobose to ovoidal to ellipsoidal, warted. Intercalary phialides present but not common on all media (Fig. 5i, 6h). Chlamydospores (Fig. 6n) present or absent, terminal or intercalary and subglobose, (2.5–)8–12(–18) μm diam (n = 390).
On SNA (Fig. 7 l; 25 °C) colonies similar to CMD, thin, hyaline, hyphae loosely arranged radially, no zonation, aerial hyphae and coilings often more pronounced, chlamydospores (mostly intercalary and angular to ellipsoidal) more frequent than on CMD, no odour and no pigment formed. Conidiation (Fig. 6a–g) first effuse on long aerial hyphae in proximal and central parts of the colony, spreading across the whole plate, then condensed into small loose tufts or pustules up to ca. 1 mm diam, confluent to 6 × 4 mm at the proximal margin, becoming green from 5 d onwards, later also in a ring at the margin, more effuse, pustules becoming dark green (27F4–8) after ca. 2 wk. At 30 °C autolytic activity conspicuous and submoniliform terminal branches of conidiophores more frequent.
Habitat: Anamorph isolated from bark, decorticated wood, mushroom compost, paper board, apple core, root of Pseudotsuga menziesii infected with Phellinus weirii, leaf litter; isolated as an endophyte from trunks of Fagus sylvatica. Stromata forming on wood, more rarely on bark of Picea abies and Fagus sylvatica, less commonly on Corylus avellana, Salix caprea, or overgrowing fungi such as resupinate polypores, mostly on cut and usually at least partly decorticated branches and logs 2–100 cm thick, stored in piles, lying on bare ground, in grass, in leaf litter, on acidic or calcareous soils, mostly in open forests, at shady roadsides, associated with Ophiostoma spp., Nectria fuckeliana, Diatrype stigma s.l., Chlorociboria (green wood), Capronia cf. pilosella, Hypocrea lixii, Ascocoryne sarcoides, Corticium roseum, Hypoxylon sp.; usually on little to medium-decomposed, often hard wood.
Distribution: Common, especially as anamorph, in north- and south-temperate regions: Europe (Austria, Czech Republic, France, Germany, England, Northern Ireland, Russia, Sweden) and North America (U.S.A.: Oregon, North Carolina, Georgia; Mexico); Australia, New Zealand; found also in Taiwan and Japan.
Holotype of teleomorph: Austria, Kärnten, Völkermarkt, Eisenkappel-Vellach, Vellacher Kotschna, MTB 9653/1, 46°24`02“N, 14°34`06“E, elev. 970 m, on split and partly decorticated branch of Fagus sylvatica, 7–8 cm thick, on the ground, soc. Corticium roseum, 31 Oct. 2005, H. Voglmayr & W. Jaklitsch, W.J. 2881 (WU 24029; culture CBS 119321 = C.P.K. 2140).
Epitype designated here: C.P.K. 2140 deposited as a dry culture together with the holotype of H. viridescens as WU 24029a.
Neotype of Eidamia viridescens, dried culture of original strain CBS 433.34 (herb. CBS 7868), isolated from rotten apples, U.K.
Additional teleomorphic specimens examined: Australia, Victoria, vic. Healesville, Toolangi State Forest, Myer's Creek Rd., Wirrawalla Rainforest Walk and Myrtle Walking Track in Myrtle Gully, elev. 575 m, with Nothofagus cunninghamii, Eucalyptus regnans and tree ferns, 23 Aug. 1999, on bark of recently dead tree, G.J.S. 8600 (BPI 746813; culture G.J.S. 99-142); between Yarram and Traralgon, Tarra Valley, Tarra-Bulga National Park, Tarra Rainforest Walk, elev. 250 m, with Eucalyptus spp., Nothofagus cunninghamii and tree ferns, 22 Aug. 1999, on Nothofagus cunninghamii infected with Hypoxylon sp., G.J.S. 8568 (BPI 746781; culture G.J.S. 99-175); between Yarram and Traralgon, Tarras Bulga National Park visitor center, Forest Trail, in Nothofagus cunninghamii and Eucalyptus regnans forest, on bark, G.J.S. 8582 (BPI 746793; culture G.J.S. 99-128). Austria, Oberösterreich, Grieskirchen, Natternbach, forest close to Gaisbuchen, MTB 7548/3, 48°24'39” N, 13°41'40” E, elev. 580 m, on branch of Fagus sylvatica on leaf litter in spruce forest, 1 Aug. 2004, H. Voglmayr, W.J. 2553 (WU 24022; culture C.P.K. 2043); Steiermark, Liezen, Kleinsölk, walking path between Schwarzensee and Putzentalalm, MTB 8749/1, 47°17'12” N, 13°52'13” E, elev. 1170 m, on log segment of Picea abies, 100 cm thick, in grass, soc. Nectria fuckeliana, 6. Aug. 2003, H. Voglmayr & W. Jaklitsch, W.J. 2306 (WU 24018; culture CBS 119324 = C.P.K. 942); (Ost-)Tirol: Lienz, Defereggental, Hopfgarten in Defereggen, in Dölsach, at roadside between the current transformer and the beverage depot, MTB 9041/3, 46°55'23” N, 12°32'41” E, elev. 990 m, on stored log of Picea abies, 16 cm thick, in grass, 4. Sep. 2003, W. Jaklitsch, W.J. 2374 (WU 24019; culture C.P.K. 947); Vienna, 23rd district, Maurer Wald, MTB 7863/1, 48°08'57” N 16 14'50” E, elev. 360 m, on decorticated branch of Carpinus betulus on the ground, in mixed deciduous forest, soc. Tubeufia cerea, 3 Oct. 1998, W. Jaklitsch, W.J. 1223 (WU 24009, BPI 747557; culture G.J.S. 98-182 = CBS 120067). France, Haute Garonne, Bord de la Garonne, 31000 Toulouse, on bark of Carpinus sp., 11 Dec. 1994, J.F. Magni, comm. F. Candoussau (BPI 737860; culture G.J.S. 94-118 = IMI 374788); Pyrénées Atlantiques, Isle de Sauveterre de Bearn, elev. 100 m, on bark of recently dead tree, 25 Oct. 1998, G.J.S. & F. Candoussau (BPI 748308; culture G.J.S. 98-129 = CBS 101928). Germany, Baden-Württemberg, Freiburg, Landkreis Schwarzwald-Baar-Kreis, Furtwangen, shortly before Kaltenherberg coming from Gasthof Thurner, MTB 8015/1, 47°59`36“N, 08°10`50“E, elev. 1000 m, on mostly decorticated cut logs of Picea abies, 20–40 cm thick, in a pile in grass at road side, part with white mould, 2 Sep. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2664 (WU 24023; culture C.P.K. 2138); Freiburg, Landkreis Breisgau-Hochschwarzwald, St. Märgen, shortly after Glashütte, coming from Hexenloch, on the right side of the road close to a bridge, MTB 8014/2, 47°59'37” N, 08° 07'32” E, elev. 750 m, on mostly decorticated cut branch of Picea abies, 4 cm thick, on very moist ground, 2 Sep. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2665 (WU 24024; culture C.P.K. 2044); Freiburg, Landkreis Lörrach, Todtnau, at the crossing to St. Blasien, MTB 8113/4, 47°48'11” N, 07°56'01” E, elev. 490 m, on mostly decorticated cut logs of Picea abies, up to 35 cm thick, in pile, wet, in grass, soc. old Ophiostoma sp., white mould, 3 Sep. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2670 (WU 24025; culture CBS 119323 = C.P.K. 2045); Bavaria, Starnberg, Tutzing, Erling, at the Hartschimmel terrain, 47°56'41” N, 11°10'37” E, elev. 700 m, on partly decorticated branch of Fagus sylvatica, 13–15 cm thick, on the ground deep in leaf litter, 3 Sep. 2005, W. Jaklitsch, W.J. 2838 (WU 24028; culture C.P.K. 2139). Sweden, Stockholms Län, Nothamn, mixed forest at the coast, MTB 4179/3, 60°01'42” N, 18°50'46” E, elev. 10 m, on corticated branch of Corylus avellana, 2–2.5 cm thick, in moss in mixed forest of Picea abies, Pinus sylvestris, Betula pendula, Corylus avellana, Sorbus aucuparia, soc. Diatrype stigma s.l., 7 Oct. 2003, W. Jaklitsch, W.J. 2447 (WU 24020; culture C.P.K. 983); Uppsala Län, Sunnersta, forest opposite the virgin forest Vardsätra Naturpark across the main road, MTB 3871/2, 59°47'24” N, 17°37'51” E, elev. 15 m, on cut branch of Salix caprea, 7.5 cm thick, on bare, very moist soil in mixed forest, soc. Capronia cf. pilosella, 8 Oct. 2003, W. Jaklitsch, W.J. 2453 (WU 24021; culture C.P.K. 985). United Kingdom, Devon, Exeter, Stoke Woods, close to the parking place Forest Walks, SX919959, 50°45'10” N, 03°31'54” W, elev. 30 m, on mainly corticated branch of Fagus sylvatica, 4 cm thick, on the ground in leaf litter, 8 Sep. 2004, H. Voglmayr, W. Jaklitsch & J. Webster, W.J. 2686 (WU 24026; culture C.P.K. 2046); North East London, Epping Forest, between Robin Hood Roundabout and Hill Wood, 43–34/1, 51°39'15” N, 00°02'13” E, elev. 40 m, on branch of Fagus sylvatica on the ground in leaf litter, soc. and partly on a resupinate polypore, soc. Hypocrea lixii, Ascocoryne sarcoides, Diatrype decorticata, 16 Sep. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2723 (WU 24027; culture CBS 119322 = C.P.K. 2047). United States, North Carolina, Macon Co., Nantahala National Forest, W of Franklin, Wayah Bald, elev. 5340 ft., 26 Sep. 1989, on decorticated wood, G.J.S., C.T. Rogerson, W.R. Buck, R.C. Harris (BPI 744478, culture G.J.S. 89-142 = CBS 120065).
Anamorphic isolates studied: See Table 1.
Notes: The teleomorph of H. viridescens is usually associated with its anamorph, with dark green conidia and sometimes showing citrine- to sulphur-yellow hairy patches as in H. rufa. Culture CBS 433.34 is the ex-type culture of E. viridescens and a dried specimen of this culture thus becomes neotype (herb. CBS 7868) in the absence of an original specimen. Figs 5 and 6 in the original publication represent the species well.
Separation of H. viridescens and H. rufa can be difficult. These species are sympatric in teleomorph and anamorph, although we have not seen stromata of H. rufa from Australia or New Zealand, where stromata of H. viridescens are common in Nothofagus forests. Many collections of H. viridescens produce distinctly ellipsoidal conidia, a form not found in H. rufa. Phialides of H. rufa are often solitary and hooked to sinuous, and conidiophores lack a discernable main axis, and are also usually distinctly curved to sinuous, whereas conidiophores of T. viridescens observed from SNA, and often also on CMD, tend to be more typical of Trichoderma, with paired branches that increase in length with distance from the tip. Phialides in pustules of H. rufa do not proliferate percurrently, a common and distinctive feature of H. viridescens. A coconut odour is typical of T. viridescens but unusual in T. viride. Conidia of T. viridescens are lighter green and less coarsely tuberculate than those of T. viride. Downy immature stromata are more rusty, fresh colour is less variable and usually darker reddish brown than in H. rufa.
-
Hypocrea vinosa Cooke, Grevillea 8: 65 (Dec 1879) [non Pat., 1881]. MycoBank MB176917. Figs 9, 10, 11, 16. Anamorph: Trichoderma vinosum Samuels, sp. nov. MycoBank MB445737.
Trichodermati viridescenti simile, sed crescens tardius in agaro PDA, conidia uniformiora et ascosporae minores.
Holotypus Hypocrea vinosa: Waitaki 307 (K, herb. Cooke), epitypus designated here: PDD 88476. Holotypus Trichoderma vinosum: Cultus in agaro siccus G.J.S. 8702 (PDD 88476).
Stromata when dry (Fig. 15f-i) 1–2 mm diam, 0.5–1.5 mm thick, unchanged or slightly darkened in 3 % KOH; solitary or in groups of 3 or fewer; tuberculate to pulvinate, broadly attached, often erumpent through bark cankers or lenticels; at first almost white to yellowish brown or light brown, becoming darker brown to reddish brown (7–8D–E8); stroma surface scurfy due to short, rust-coloured hairs, plane to wrinkled or tubercular from perithecial contours; ostiolar openings not visible or less frequently appearing as few viscid dots.
Stroma anatomy: Cells of stroma surface in face view (Fig. 9a) pseudoparenchymatous, (2–)3–8(–10) × 2–6 (–8) μm (n = 90), walls slightly thickened, often with uneven deposit of pigment and surface appearing mottled, cells of stroma surface often obscured by hairs. Hairs (Fig. 9b, e) often abundant, especially on young stromata, cylindrical, unbranched, comprising 2–4 cells, 10–25(–35) μm long, 3–5 μm wide. Cortical region (Fig. 9d-g) of stroma 20–40(–55) μm (n = 10) thick, reddish brown to yellow-brown, around the entire stroma except the point of attachment, cells in section of textura angularis, (2.0–)2.5–6.5(–10.2) × (1.0–)1.7–5.2(–8.5) μm (n = 90), walls thickened. Cells immediately below the cortex (Fig. 9e–g) comprising intertwined hyphae and textura epidermoidea to t. angularis toward the ostiolar canal. Ostiolar canal (Fig. 9d, g) ca. 90 μm long; the ostiolar opening formed by a palisade of narrowly clavate, hyaline filaments even with stroma surface. Perithecia (Fig. 9c–d) elliptical in section, 150–190 μm high, 90–100 μm diam. Subperithecial tissue homogeneous, of textura angularis to t. epidermoidea with some longer hyphal elements, cells (2.7–)4.5–14 (–23.5) μm long, (1.5–)3.7–7.7(–10) μm wide (n = 90), walls slightly thickened. Asci (Fig. 9i) cylindrical, 63–103(–123) × (3.5–)4–6(–8) μm, sessile, apex with an obscure ring. Ascospores (Fig. 9j) hyaline, verruculose; distal part subglobose, (3.7–)5.0–6.5(–8.0) × (3.7–)4.7–6.2(–7.7) μm (n = 90); proximal part wedge-shaped to almost oblong or ellipsoidal, (4.5–)5.0–7.0(–9.0) × (3.7–)4.0–5.0(–8.0) μm (n = 90).
Colony characteristics: Optimal temperature for growth on PDA and SNA 20–25 °C, after 72 h in darkness colony radius on PDA 20–35 mm, on SNA 16–23 mm; at 30 °C < 5 mm; not growing at 35 °C. Colonies grown on PDA for 1 wk at 25 °C under alternating light and darkness producing white mycelium; sterile, conidia forming within 3 wk in compact pustules at the periphery of the colony; no diffusing pigment or distinctive odour noted.
Colonies grown on CMD reaching > 7 cm radius within 10 d at 20–25 °C under alternating light and darkness, no pigment or a pale yellow diffusing pigment formed; typically producing a coconut-like odour; conidia forming in poorly developed to compact, 1–3 mm diam pustules, at the periphery of the colony or in 2 or 3 concentric rings, grey-green (27D4–5) or darker green.
Colonies grown on SNA reaching > 7 cm radius within 10 d at 20–25 °C under alternating light and darkness, no diffusing pigment formed, no distinctive odour detected; conidial production variable, abundant to scant, in 1–2 mm diam, hemispherical to pulvinate pustules formed in a broad band around the margin and adjacent to filter paper; conidia dark green; fertile conidiophores protruding terminally from pustules or not.
Conidiophores dimorphic, representing Types 2 and 3 (described on page 144), the two types typically not forming in the same culture; conidiophores of both types found on CMD (Fig. 10a–g), Type 2 conidiophores alone found on SNA. Conidiophores on SNA (Fig. 11c–i) lacking a well-developed main axis, or main axis short; main axis often nodose, branches tending to be paired and separated by short internodes; branches tending to produce a profusion of phialides directly or at the tip of short secondary branches; branches and phialides tending to arise at right angles with respect to their subtending cells. Protruding conidiophores unbranched or sparingly branched along the length, producing one or few cylindrical phialides at the tip. Phialides narrowly lageniform, more swollen in the middle when crowded than when widely spaced, straight, (6.0–)7.7–11(–14.5) μm long, (2.0–)2.2–3.0(–3.7) μm at the widest point, (1.2–)1.5–2.2(–2.7) μm wide at base, L/W = (0.4–)2.4–4.6(–6.5) (n = 90). Intercalary phialides not common. On CMD Type 3 conidiophores forming in compact pustules, comprising vesiculose cells branching in an irregular fashion, each branch terminating in one or a few phialides; phialides lageniform to greatly swollen, often proliferating percurrently to form a phialide, often submoniliform chains of proliferated phialides (Fig. 10c–g) found in pustules. Conidia subglobose, warted (Fig. 10h–i). Chlamy-dospores not observed.
Habitat: On corticated hardwood trees including Nothofagus menziesii.
Distribution: Australia and New Zealand.
Holotype of teleomorph: New Zealand, Waitaki, [?Berggren] 307 (K, herb. Cooke). Epitype, designated here, PDD 88476. Holotype of anamorph (Trichoderma vinosum): a dried culture of G.J.S. 8702 deposited as PDD 88476.
Additional specimens examined: Australia, New South Wales, Blue Mountains, Morton National Park, vic. Bundnadoon, Fairy Bower Track, on bark of recently dead tree, 19 Aug. 1999, G.J. Samuels 8742, K. Põldmaa, E. Lieckfeldt (BPI 746681; culture G.J.S. 99-156 = ICMP 16293); Blue Mountains National Park, Grand Canyon from Evans Lookout, elev. 850 m, on bark of recently dead tree, 16 Aug. 1999, G.J.S. 8739 & K. Põldmaa (BPI 746677; culture G.J.S. 99-183 = ICMP 16289). New Zealand, South Westland, Jackson River Rd., Martyr River, roadside facing mixed Nothofagus/broad-leaf forest, 44°06' S, 168°32' E, 7 May 2002, S.R. Pennycook (BPI 842436; culture G.J.S. 02-54 = CBS 119086 = ICMP 16295); Westland, Lower Buller River Gorge, “Sinclair's Castle”, at point where Ohikanui River joins the Buller River, along floodplain of Ohikanui River, in Nothofagus and tree ferns, elev. 50 m, 41°51' S, 171°43' E, 6 Sep. 1999, G.J.S. 8702 & S. Dodd (PDD 88476, epitype designated here, isoepitype BPI 746637; culture G.J.S. 99-158 = CBS 119087 = ICMP 16294).
Notes: As was noted by Cooke (1879), H. vinosa differs from H. rufa in having slightly larger ascospores. The type specimen of this species is in poor condition. It consists of three pieces of decorticated wood with stromata on one of them. The stromata are dark reddish brown, discrete, discoidal, 1–2 mm diam, broadly attached to the substratum, with a slight tendency to have free margins; the surface is plane, perithecial contours or ostiolar openings are not visible. The stroma becomes light orange-brown in KOH. Ascospores are hyaline, conspicuously spinulose, dimorphic. Distal part-ascospores (n = 10) are globose to subglobose, 5.0–6.7 × 5.0–5.5 μm; proximal part-ascospores are oblong, 5.7–7.2 × 4.5–5.2 μm.
Webster (1964) attributed a Trichoderma anamorph to a New Zealand collection (PDD 10466) and Rifai (1969) assigned that anamorph to the T. aureoviride aggregate. According to Webster, the specimen that he examined fits the type of H. vinosa well. However our study of PDD 10466 reveals it to be distinct from the type specimen of H. vinosa. PDD 10466 is a poor specimen, with only one old stroma. That stroma is light brown (ca. 6D8). Ascospores are hyaline and dimorphic. Thirty ascospores in asci were measured; they are smaller than in the type collection. The distal parts are (3.7–)4.0–5.0(–6.0) × (3.2–)3.5–4.2(–5.0) μm; the proximal parts are (3.5–)4.0–5.5(–6.3) × (2.7–)3.0–4.0(–4.5) μm. The specimen includes a dry PDA culture. Conidia are green, ellipsoidal to narrowly ovoidal, (3.0–)3.2–3.5(–3.7) × 2.2–2.7 μm, smooth. Conidiophores in the dry culture have collapsed but we did not observe an eidamia-like development. The specimen PDD 10466 was collected in the Auckland region of the North Island of New Zealand, which is biologically distinct from the type locality of H. vinosa. Thus we are convinced that PDD 10466 is not H. vinosa, although we cannot identify it to any known species. The ellipsoidal conidia and collapsed conidiophores are similar to T. koningii, and in New Zealand three species having this morphology are known, viz. H./T. austrokoningii, H./T. dingleyae and H./T. dorotheae. However, conidia of PDD 10466 are smaller than in these species. As far as we are aware, the culture that Webster obtained from that specimen is no longer available. The illustrations provided by Rifai are consistent with many species of Trichoderma in sect. Trichoderma. Smooth conidia were illustrated.
The specimen that we have designated as epitype also agrees well with the type of H. vinosa. This is perhaps not surprising given the relative non-specificity of Hypocrea specimens. The type of H. vinosa was collected in Waitaki, which is located on the east coast of Otago, on the South Island of New Zealand, where Nothofagus is common. It is at least possible that the type specimen was collected on Nothofagus. The epitype and one additional collection were made from N. menziesii in Westland, a region on the east coast of New Zealand that is slightly north of Otago. Thus, the epitype was collected close to the type locality.
-
Trichoderma gamsii Samuels & Druzhinina, sp. nov. MycoBank MB501050. Figs 12, 13, 16 a–d. Teleomorph: None known
Etymology: Named to honour K. Walter Gams, peripatetic mycologist (fan of Sardinia) and trichodermist.
Trichodermati koningii simile, sed conidia majora, (3.2–)4.0–5.0(–6.2) × (2.0–)2.5–3.0(–3.2) μm. Conidiophora dimorphica, conidiophora Trichodermati similia in agaro SNA evoluta; conidiophora ad Eidamiam spectantia in agaro CMD evoluta.
Holotypus: C.P.K. 2074 (dried in BPI 872183; ex-type culture C.P.K. 2074 = G.J.S. 06-09 = CBS120075).
Characteristics in culture: Optimum temperature for growth on PDA and SNA 25–30 °C in intermittent light; after 72 h on PDA colony radius 50–60 mm, on SNA colony radius 40–48 mm; at 35 °C < 7.5 mm on PDA and SNA. Colonies grown on PDA in intermittent light forming conidia sporadically within 1 wk 25 °C. On PDA after 1 wk in light typically a dense white mycelium covering the 9-cm-diam Petri plate; conidial production variable, some colonies remaining sterile and more or less conidia forming in other colonies; conidia tending to form in 3 broad, often obscure, concentric rings; conidia form in a dense, continuous lawn between the inoculum plug and the edge of the Petri plate, typically a shade of yellow (2–3B–D 8).
On SNA at 25 °C in light reaching > 7 cm radius after 1 wk in light; conidia forming abundantly in (2–) 3–4 conspicuous concentric rings, 27E5–8, large, produced in rather broad sheets or in flat pulvinate, often confluent pustules 1–2 mm diam. No pigment diffusing through the agar, no distinctive odour. Conidiophores (Fig. 13a-g) more or less uniformly branched for a short distance, solitary phialides common, internodes between branches typically long, occasionally short and branches crowded; often branches arising from swollen nodes on the conidiophore, occasionally conidiophores terminating in a long extension; extension sterile for a long distance below the tip, a single phialide forming at the tip. Phialides lageniform, at most slightly swollen in the middle, straight; solitary or terminating branches in pairs or in appressed heads of several, (5.2–)8.5–10 (–18.5) μm long, (1.5–)2.2–3.0(–4) μm at the widest point, (1.0–)1.5–2.2(–2.7) μm at the base, L/W = (1.4–) 2.4–4.2(–8.4) (n = 300); arising from a (1.0–)1.8–3.0 (–5.0) μm wide cell. Intercalary phialides common.
On CMD at 25 °C in light reaching > 7 cm radius within 10 d; conidia forming in a broad band of discrete to confluent pustules around the colony margin; pustules densely cottony, individual conidiophores not apparent, 0.5–1.0(–3) mm diam, green (27–28E–F5–8) or yellow in part, conidiophores also forming in the scantily produced aerial mycelium; less frequently colonies remaining sterile; sometimes with a pale yellow diffusing pigment; typically with a more or less strong coconut-like odour. Colonies grown on SNA with filter paper at 25 °C in light > 7 cm radius within 10 d; conidia forming as in darkness, conidial production often heavy and in larger pustules over the filter paper, at first yellow (1A7), then green (27C–F8), pustules cottony to dense and individual conidiophores not seen. Conidiophores (Fig. 12a–h) comprising convoluted and intertwined, 2–3 μm wide hyphae from which phialides arise. Phialides produced laterally from hyphae and solitary, or terminating branches and solitary or held in pairs, typically swollen in the middle and the tip elongated and cylindrical; percurrently proliferated phialides conspicuous, often forming submoniliform chains. Chlamydospores typically abundant, subglobose, terminal on hyphae, (3.0–)8.5–12(–15.7) × (3.0–)8–11.5(–15.5) μm.
Conidia (Figs 12i, 13h) ellipsoidal, oblong, or narrowly ovoidal, smooth.
Habitat: Soil, isolated once as an endophyte of a tree fern and once from the stem of Ricinus communis.
Known distribution: Italy, common in Sardinia, also present in the U.S.A. (Texas), Australia, and Central Russia.
Holotype: Italy, Sardinia, north-east side of the Tyrrhenian island, close to Aglientu, isolated by Q. Migheli from soil under Pistacia lentiscus and Myrtus communis, C.P.K. 2074 (BPI 872183; culture C.P.K. 2074 = G.J.S. 06-09 = CBS 120075).
Additional cultures examined: Australia, A.C.T., Canberra, Australian National Botanic Garden, isolated from xylem of Eucalyptus nitens, Aug. 1992, P.J. Fisher D-12-153 (culture G.J.S. 92-60). Italy, Pisa, isolated from stem of Ricinus communis, Q. Migheli, G. Vanacci 899 (BPI 872184; culture G.J.S. 05-111 = CBS 120072); Sardinia, data as for holotype (cultures C.P.K. 2070 = G.J.S. 06-07 = CBS 120073, C.P.K. 2073 = G.J.S. 06-08 = CBS 120074, C.P.K. 2075, C.P.K. 2076, C.P.K. 2077, C.P.K. 2078, C.P.K. 2079 = G.J.S. 06-13, C.P.K. 2090 = G.J.S. 06-14, C.P.K. 2091, C.P.K. 2092, C.P.K. 2093). Russia, Central Forest State Biosphere Reserve, Tverskaya district (cultures C.P.K. 1010 and C.P.K. 1011) isolated from terric histosol soil type under Piceetum composite forest with Alnus glutinosa. United States, Texas, Gaines County, farm soil, C. Howell TK 77 (culture G.J.S. 04-09).
Notes: The closest morphological comparison of T. gamsii is with T. koningii. The most conspicuous difference between the two species is the production of proliferating phialides in T. gamsii on CMD and PDA. Trichoderma gamsii has the largest conidium length/width ratio that we have yet seen in Trichoderma. Conidiophores of T. gamsii are neither as extensive nor as uniformly branched as they are in T. koningii.
Fisher et al. (1993) recovered T. gamsii (as T. koningii) as an endophyte from xylem of Eucalyptus nitens in Australia in low frequency.
The isolate G.J.S. 04-09 (Howell TK 77) is resistant to the fungicide Bayton (C. Howell, pers. comm.).
-
Trichoderma neokoningii Samuels & Soberanis, sp. nov. MycoBank MB501051. Fig. 14.
Etymology: with reference to a similarity to T. koningii Oudem.
Trichodermati koningii Oudem. simile sed conidia in pustulis compactis in agaro SNA formata, inconspicue crescens, et conidiophora Eidamiae similia in agaro PDA; conidia ellipsoidea, glabra, (3.2–) 3.5–4.0(–4.5) × (2.7–)3.0–3.5(–4.0) μm. Habitat in America australi.
Teleomorph: none known.
Colony characteristics: Optimum temperature for growth on PDA 25 °C, on SNA 30 °C; after 72 h in darkness colony radius on PDA at 25 °C ca. 40 mm and at 30 °C 35 mm, on SNA at 25 °C 30 mm, at 30 °C ca. 35 mm; not growing at 35 °C. Radius of colonies grown on PDA and SNA for 1 wk at 25 °C with alternating light ca. 70 mm. Colonies on PDA cottony, white, on CMD and SNA aerial mycelium scant, colonies nearly invisible; no diffusing pigment or distinctive odour noted. Colonies on PDA (Fig. 14a) producing conidia in 2–3 mm diam hemispherical pustules in the aerial mycelium, especially around the original inoculum, or in continuous lawns around the original inoculum and at the periphery of the colony. Conidiophores on PDA at first of Type 2, but with age becoming Eidamia-like, conidiophores short and irregularly branched, swollen cells developing in pustules (Fig. 14j), phialides abruptly swollen in the middle and the cylindrical neck proliferating conspicuously to form chains of 2 or 3 (Fig. 14i, j). On SNA (Fig. 14b) and CMD large, 2-4 mm diam, pulvinate pustules scattered throughout the colony. Conidia on PDA at first yellow, becoming greyish green (27D–E5), on SNA and CMD deep green to dark green (28E–F8). Pustules formed on SNA (Fig. 14c) and CMD, densely woolly, conidiophores poorly visible at the surface of the pustule. Conidiophores on CMD and SNA exclusively of Type 2 (Fig. 14e), often sterile over a more or less long distance and producing a single terminal phialide or whorl of phialides (Fig. 14g), or tending to be regularly branched, with branches tending to be paired and increasing in length with distance from the tip; branches tending to be widely spaced, each branch terminating in a single phialide or in a whorl of 2 or 3 phialides (Fig. 14e–f). Conidiophores produced toward the interior of the pustule more densely branched with shorter internodes between branches, branches tending to be paired and closely-spaced, terminating in 1 to several phialides. Phialides produced near the tips of conidiophores tending to be solitary, cylindrical or slightly swollen in the middle, often constricted above the middle to form a long cylindrical neck; phialides produced toward the interior of the pustule or on more densely disposed branches (Fig. 14i) shorter than those produced at the tip and conspicuously swollen in the middle, (4.5–)5–10(–13.5) μm long, (2.0–)2.2–3.0 (–3.2) μm at the widest point, (1.2–)1.5–2.0(–2.2) μm at the base, L/W = (1.4–)1.8–4.6(–6.8) (n = 30); arising from a (1.5–)2.0–3.0(–4.0) μm wide cell. Percurrently proliferating phialides common on SNA (Fig. 14h). Intercalary phialides uncommon (Fig. 14f) on SNA or CMD. Conidia (Fig. 14k) oblong to ellipsoidal, smooth, green. Chlamydospores (Fig. 14 l) abundant on SNA, terminal, subglobose, (6.2–)7.5–10.7(–13.7) × (6.0–) 6.7–9.2(–10.7) μm (n = 30).
Habitat: Isolated from the pseudostroma of Moniliophthora roreri infecting a pod of Theobroma cacao.
Known distribution: Peru, known only from the type collection.
Holotype: Peru, Cuzco, Crialo, isolated from Moniliophthora roreri sporulating on cacao pod, date unknown, W. Soberanis `D' (BPI 872182; ex-type culture G.J.S. 04-216 = CBS 120070).
Notes. There is little morphological distinction between T. neokoningii and T. koningii and T. koningiopsis. The latter two species (Samuels et al. 2006a) produce conidia abundantly on most media in the aerial mycelium or in poorly developed pustules, not in conspicuous pustules as they occur in T. neokoningii. Conidiophores of the former two species are also more regularly branched and do not produce conspicuously percurrently proliferating phialides. Trichoderma koningii is a species of north-temperate regions whereas T. koningiopsis is commonly found in tropical soils. Trichoderma koningiopsis grows faster than T. neokoningii on PDA. Although T. koningii and T. koningiopsis are members of Trichoderma sect. Trichoderma, they are phylogenetically distinct from T. neokoningii. Trichoderma gamsii is closely related to, and morphologically very similar to T. neokoningii; conidia of the common temperate T. gamsii are significantly longer than those of T. neokoningii.
-
Trichoderma scalesiae Samuels & H.C. Evans, sp. nov. MycoBank MB501052. Figs 15, 16 e. Teleomorph: none known.
Etymology: With reference to Scalesia pedunculata, the host plant from which T. scalesiae was isolated.
Trichodermati paucisporo simile sed conidia (2.5–)3.0–3.7(–4.0) × (2.2–)2.7–3.2(–3.5) μm et inconspicue crescens; coloniae radius < 15 mm post 96 horas 25 °C in agaro PDA.
Holotypus: BPI 872181
Colony characteristics: Optimal temperature for growth on PDA 30 °C, for growth on SNA 15–30 °C; after 72 h in darkness colony radius on PDA ca. 18 mm, on SNA ca. 10 mm; not growing at 35 °C. Radius of colonies grown on PDA, CMD and SNA for 1 wk at 25 °C with alternating light and darkness 30–40 mm. On PDA aerial mycelium cottony, white to yellow (ca. 2B7); on CMD and SNA aerial mycelium scant and colonies nearly invisible; no diffusing pigment or distinctive odour detected on PDA; no diffusing pigment, a faint coconut-like odour detected on CMD; a faint yellow diffusing pigment but no distinctive odour detected on SNA. Colonies on PDA and CMD sterile, on SNA conidia sparingly produced in cottony aerial mycelium over the filter paper, none elsewhere. Conidiophores (Fig. 15a–g) formed in the aerial mycelium, 25–45 μm long, monophialidic or sparingly to profusely branched, branches arising at or near 90° with respect to the main axis, typically not paired, each branch producing one or a few phialides along the length or rebranching, each secondary and terminal branch terminating in 1–4 phialides, a single watery drop of conidia held at the tip of each phialide. Phialides slightly swollen in the middle or tapering uniformly from base to tip, straight, curved or sinuous, (8.7–)10–15(–17) μm long, 2.0–3.0(–4) μm at the widest point, (1.5–)2.0–2.2(–2.5) μm at the base, L/W = (0.7–)0.8–1.0(–1.3) (n = 30), arising from a (1.7–)2.0(–2.5(–3.0) μm wide cell. Conidia (Fig. 15h) subglobose, (2.5–)3.0–3.7(–4.0) × (2.2–)2.7–3.2(–3.5) μm (n = 30), smooth, pale green. Chlamydospores (Fig. 15i) abundant on SNA, terminal and intercalary, (7.0–)8.0–8.7(–10.0) × (6.5–)7.7–9.5(–10.5) μm (n = 30).
Habitat: Endophytic within woody tissues of Scalesia pedunculata (Asteraceae), the “Daisy Tree” of the Galapagos islands.
Holotype: Ecuador, Galapagos Islands, Santa Cruz, Mt. Cocker, isolated from stem of Scalesia pedunculata (Asteraceae), 19 May 2000, H.C. Evans W2022d (BPI 872181; ex-type culture G.J.S. 03-74 = CBS 120069).
Notes: Trichoderma scalesiae produced conidia only sparingly and then only on SNA to which sterile filter paper squares had been added. In Trichoderma the coconut-like odour is typical of sect. Trichoderma; thus even in the absence of conidia this species was predicted to be a member of that section. Normal Trichoderma species produce conidia abundantly on common mycological media. In the current work we have found that conidial production on CMD and PDA can be unreliable, whereas the carbohydrate-poor SNA can be relied on to stimulate conidial production. In the case of T. scalesiae presumably the cellulose content of filter paper was an absolute requirement for conidium production. We did not attempt to stimulate conidium production on other media by adding filter paper squares to them. Most likely, if this species had been seen in the past it would have been recorded as being sterile. The recently described T. paucisporum Samuels, Suarez & Solis, also a member of sect. Trichoderma, is similar to T. scalesiae in that it produces conidia sparingly on SNA, although it does produce conidia in very low numbers and sporadically on CMD and SNA. Filter paper was not necessary for conidium production on SNA by T. paucisporum. The conidiophores of T. paucisporum are morphologically very similar to those of T. scalesiae (Samuels et al., 2006). Conidiophores in both species are similar to what were described as synanamorphs of some Trichoderma species by (Chaverri et al. 2004). Trichoderma paucisporum was originally isolated from Ecuador, from cacao pods infected with Moniliophthora roreri and lying on the ground.
Scalesia species, which are endemic to the Galapagos Islands, are considered to be the plant equivalents of Darwin's finches. Scalesia pedunculata, a tree, is the largest of the daisy trees. For information concerning S. pedunculata see references found at http://www.arkive.org/species/GES/plants_and_algae/Scalesia_penduculata/more_info.html. In addition to T. scalesiae, we have also identified T. harzianum Rifai as an endophyte in trunks of S. pedunculata (Samuels, unpubl.).
Fig. 7.
Cultures of H. rufa/T. viride and H./T. viridescens after one wk at 25 °C. a–f. H. rufa/T. viride. a. CBS 101526. b. W.J. 2450. c. G.J.S. 05-104. d. Tr 2. e–f. W.J. 2450; all from PDA except e (CMD) and f (SNA). g–l. H./T. viridescens. g. G.J.S. 98-86. h. G.J.S. 99-175. i. G.J.S. 05-185. j. G.J.S. 98-182. k–l. W.J. 2306; all from PDA except k (CMD) and l (SNA).
DISCUSSION
This is the eighth article in our series dealing with members of the phylogenetically and taxonomically complex Trichoderma sect. Trichoderma (Dodd et al. 2002, 2003, Druzhinina et al. 2004, Holmes et al. 2004, Lieckfeldt et al. 1999, Lu & Samuels 2003, Samuels et al. 1999, 2006a, b).
In the introduction to the current work we suggested that reports of T. viride refer to more than one species. We have shown that even the classical morphological concept of T. viride, i.e. a green-conidial species with globose, warted conidia, is paraphyletic. In the present paper we distinguish the two most common species of Trichoderma that have warted conidia and describe a new species that has warted conidia, T. vinosum. We augment the known diversity of morphological expression in Trichoderma to include the peculiar Eidamia morphology. During the course of the study, additional species having warted conidia or that were closely related to T. viride or T. viridescens were revealed to us. In addition we describe a second species, T. scalesiae, that but for DNA sequence analysis and careful examination of cultures on SNA, would be reported as a sterile unknown fungus. Although we present results from sequencing of only a single gene, tef1, the resulting clades conform to phenotypic apomorphies in defining species. Clearly, from Fig. 1 it can be seen that additional species remain to be characterized. These will be discussed in forthcoming publications.
In the present work, as in the accompanying article in this issue that concerns T. koningii (Samuels et al. 2006a), the question that we have had to answer was how to delimit taxonomic species. As we continue to collect new specimens and submit them to DNA sequencing and phylogenetic analysis, we find within clades considerable homoplasy in the few morphological features that are available for analysis. Despite the formation of a teleomorph by several species, this morph is so highly conserved within the viride clade as to be virtually useless in species-level taxonomy while at the same time being diagnostic of the clade. Similarly, while the basic conidiophore morphology in the viride clade, defined here as “Type 2”, signals membership in the clade, it varies only little among the species. For this reason, one cannot fault earlier mycologists for having recognized few species of Trichoderma, or even later mycologists for having failed to recognize the differences between T. viride and T. viridescens. In both the T. viride and T. koningii works our strategy for recognition of taxonomic species was to closely integrate phenetic and phylogenetic characters. These two analyses were undertaken independently in two laboratories, and therefore were unbiased. Species were recognized when the concordance between both approaches were found. Although we clearly understand that the phylogeny of one single locus, in this case tef1, is useful to formulate a hypothesis concerning the phylogeny of the fungus, we also understand that use of this single gene region is not powerful enough to falsify a species hypothesis. Thus, we rely on the concordance between phenotype and genotype. It is also important to note that for some groups the high observed phylogenetic diversity enabled us to recognize a species (e.g. T. gamsii), while in other cases, when the diversity was low and both sets of characters were unclear, we refrained from proposing a new taxonomy (Vd 1–3).
The formation of warted conidia in the viride clade seems to suggest that this character is derived within Trichoderma and that smooth conidia are the primitive state. However, we are not yet in a position to discuss evolution of most phenotypic characters in Trichoderma, and conidium ornamentation is not an exception. Tuberculate conidia are produced by completely unrelated species of Trichoderma, including T. saturnisporum and T. ghanense (both sect. Longibrachiatum, Samuels et al. 1994), and Bissett (1991b) illustrated warted conidia in T. virens (sect. Pachybasium) using scanning electron microscopy. On the other hand the eidamia-like morphology of conidiophores produced on rich media is apomorphic for the large viridescens clade defined here, and the coconut-like odour produced by several members of the entire viride clade.
The level of knowledge of Trichoderma species is perhaps greater than for any other genus of fungi. We acknowledge the difficulty of identifying Trichoderma species on the basis of their phenotype, despite the fact that morphology-based keys to species, and illustrations of at least the most common species is available at www.nt.ars-grin.gov. However, every species is represented in GenBank by sequences of two or more genes and by a multiplicity of correctly identified strains (Samuels 2006). Moreover, all known species of Trichoderma may be identified using molecular markers at www.isth.info. The majority of species can be safely identified by the DNA barcoding based on ITS1 and 2 loci (Kopchinskiy et al. 2005). However, since species from Trichoderma section Trichoderma share the same or very similar alleles of ITS1 and 2, they should be identified by an integration of the barcode method (TrichOKEY) and Trichoderma similarity search TrichoBLAST (Kopchinskiy et al. 2005) as described in Druzhinina et al. (2006). If a researcher has access to a sequencing facility, there is no need to misidentify a species of Trichoderma. We strongly recommend that individuals check the identity of strains that are of interest and we urge editors of journals reporting properties of Trichoderma species to require that the identity of the strains be verified by members of the International Subcommittee on Taxonomy of Hypocrea (ISTH) which can be accessed at www.isth.info.
Acknowledgments
We thank Hermann Voglmayr for the collection of Hypocrea teleomorphs, Svengunnar Ryman and Roland Moberg for support of excursions in Sweden. Drs Moberg and Hennig Knudsen were instrumental in identifying type material of Sphaeria rufa. Cultures were provided by Drs John Bissett (DAOM), Charles Howell (USDA), Toru Okuda (Nippon Roche), Walter Gams (CBS), Willies Soberanis (Tarpoto, Peru), Helgard Nirenberg (BBA, Berlin), and Doustmorad Zafari (Bu Ali SIna University, Hamadan, Iran). Dr. Adnan Ismaiel (BPI) and Mag. Monika Komon-Zelazovska obtained many sequences from Trichoderma cultures. Ms Ellen Bloch (NY) patiently located and expedited the loan specimens of Hypocrea. Mr James Plaskowitz (BPI) and Dr Eric Erbe (USDA, Beltsville), respectively, performed the electron microscopy. A.T. Gräfenhan kindly reviewed an earlier version of this paper. The financial support by the Austrian Science Fund (FWF Project P16465-B03) to W.M.J. is gratefully acknowledged. This study was supported in part by the United States National Science Foundation (PEET) grant 9712308, “Monographic Studies of Hypocrealean Fungi: Hypocrea and Hypomyces” to the Pennsylvania State University, Department of Plant Pathology.
Taxonomic novelties: Hypocrea viridescens Jaklitsch & Samuels sp.nov., Trichoderma viridescens (A.S. Horne & H.S. Williamson) Jaklitsch & Samuels comb.nov., T. gamsii Samuels & Druzhinina sp.nov., T. vinosum Samuels sp.nov., T. neokoningii Samuels & Soberanis sp.nov., T. scalesiae Samuels & H.C. Evans sp.nov.
References
- Baig MM, Baig MLB, Baig MIA, Yasmeen M (2004). Saccharification of banana agro-waste by cellulolytic enzymes. African Journal of Biotechnology 3: 447-450. [Google Scholar]
- Bastos CN (1988). Resultados preliminares sobre a eficácia de Trichoderma viride no controle da vassoura-de-bruxa (Crinipellis perniciosa) do cacaueiro. Fitopatologia Brasiliera 13: 340-342. [Google Scholar]
- Bastos CN (1996a). Effect of water potential on growth and viability of conidia of Trichoderma viride and Crinipellis perniciosa. Summa Phytopathologica 22: 258–261. [Google Scholar]
- Bastos CN (1996b). Mycoparasitic nature of the antagonism between Trichoderma viride and Crinipellis perniciosa. Fitopatologia Brasiliera 21: 50–54. [Google Scholar]
- Bisby GR (1939). Trichoderma viride Pers. ex Fries, and notes on Hypocrea. Transactions of the British Mycological Society 23: 149–168. [Google Scholar]
- Bissett J (1991a). A revision of the genus Trichoderma. II. Infrageneric classification. Canadian Journal of Botany 60: 2357–2372. [Google Scholar]
- Bissett J (1991b) A revision of the genus Trichoderma. III. Section Pachybasium. Canadian Journal of Botany 69 2373–2417. [Google Scholar]
- Brown HL, Bruce A (1999). Assessment of the biocontrol potential of a Trichoderma viride isolate part I: establishment of field and fungal cellar trials. International Biodeterioration and Biodegradation 44: 219–223. [Google Scholar]
- Brown HL, Bruce A, Staines HJ (1999). Assessment of the biocontrol potential of a Trichoderma viride isolate part II: protection against soft rot and basidiomycete decay. International Biodeterioration and Biodegradation 44: 225–231. [Google Scholar]
- Carbone I, Kohn LM (1999). A method for designing primer sests for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Carta LK, Erbe EF, Pooley C, Murphy C, Wergin WP (2003). Chemical fixation/ambient temperature SEM vs cryofixation/low temperature SEM for taxonomic studies of nematodes. Scanning 2: 56. [Google Scholar]
- Celar F, Valic N (2005). Effects of Trichoderma spp. and Gliocladium roseum culture filtrates on seed germination of vegetables and maize. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz 112: 343–350. [Google Scholar]
- Chaverri P, Castlebury LA, Overton BE, Samuels GJ (2004). Hypocrea/Trichoderma: species with conidiophore elongations and green conidia. Mycologia 95: 1100–1140. [PubMed] [Google Scholar]
- Cooke MC (1879). New Zealand fungi. Grevillea 8: 54–68. [Google Scholar]
- Dodd SL, Lieckfeldt E, Chaverri P, Overton BE, Samuels GJ (2002). Taxonomy and phylogenetic relationships of two species of Hypocrea with Trichoderma anamorphs. Mycological Progress 1: 409–428. [Google Scholar]
- Dodd, S.L., E. Lieckfeldt, and G.J. Samuels (2003). Hypocrea atroviridis sp. nov. the teleomorph of Trichoderma atroviride. Mycologia 95: 27–40. [DOI] [PubMed] [Google Scholar]
- Druzhinina IS, Chaverri P, Fallah P, Kubicek CP, Samuels GJ (2004). Hypocrea flaviconidia, a new species from Costa Rica with yellow conidia. Studies in Mycology 50: 401–407. [Google Scholar]
- Druzhinina IS, Kopchinskiy AG, Kubicek CP (2006). The first one hundred of Trichoderma species is characterised by molecular data. Mycoscience 47: 55–64. [Google Scholar]
- Erbe EF, Rango A, Foster J, Josberger E, Pooley C, Wergin WP (2003). Collecting, shipping, storing and imaging snow crystals and ice grains with low temperature scanning electron microscopy. Microscopy Research and Technique 62: 19–32. [DOI] [PubMed] [Google Scholar]
- Evans HC, Holmes KA, Thomas SE (2003). Endophytes and mycoparasites associated with an indigenous forest tree, Theobroma gileri, in Ecuador and a preliminary assessment of their potential as biocontrol agents of cocoa diseases. Mycological Progress 2: 149–160. [Google Scholar]
- Fisher PJ, Petrini O, Sutton BC (1993). A comparative study of fungal endophytes in leaves, xylem and bark of Eucalyptus in Australia and England. Sydowia 45: 338–345. [Google Scholar]
- Fries EM (1822). Systema mycologicum, vol. 2. Gryphiswaldiae.
- Fries EM (1849). Summa Vegetabilium Scandinaviae, Sectio Posterior. Holmiae et Lipsiae.
- Hagn A, Pritsch K, Schloter M, Munch JC (2003). Fungal diversity in agricultural soil under different farming management systems, with special reference to biocontrol strains of Trichoderma spp. Biology and Fertility of Soils 38: 236–244. [Google Scholar]
- Holm L, Nannfeldt JA (1962). Fries's “Scleromycetae Sueciae”. A study on its editorial history with an annotated check-list. Friesia 7: 10–59. [Google Scholar]
- Holmes K, Schroers H-J, Thomas SE, Evans HC, Samuels GJ (2004). Taxonomy and biocontrol potential of a new species of Trichoderma from the Amazon basin of South America. Mycological Progress 3: 199–210. [Google Scholar]
- Huelsenbeck JP, Ronquist F (2001). MrBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 755. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS (2005). Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia 97: 1365–1378. [DOI] [PubMed] [Google Scholar]
- Kopchinskiy AG, Komon M, Kubicek CP, Druzhinina IS (2005). TrichoBLAST: A multiloci database for Trichoderma and Hypocrea identification. Mycological Research 109: 657–660. [DOI] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH (1978). Methuen Handbook of Colour. Third Edition, Sankt Jørgen Tryk, Copenhagen.
- Kullnig-Gradinger CM, Szakács G, Kubicek CP (2002). Phylogeny and evolution of the genus Trichoderma: a multigene approach. Mycological Research 106: 757–767. [Google Scholar]
- Kullnig CM, Krupica T, Woo SL, Mach RL, Rey M, Lorito M, Kubicek CP (2001). Confusion abounds over identities of Trichoderma biocontrol isolates. Mycological Research 105: 770–771. [Google Scholar]
- Leache AD, Reeder TW (2002). Molecular systematics of the Eastern Fence Lizard (Sceloporus undulatus): a comparison of parsimony, likelihood, and Bayesian approaches. Systematic Biology 51: 44–68. [DOI] [PubMed] [Google Scholar]
- Lieckfeldt E, Samuels GJ, Nirenberg HI (1999). A morphological and molecular perspective of Trichoderma viride: is it one or two species? Applied and Environmental Microbiology 65: 2418–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu BS, Samuels GJ (2003). Hypocrea stilbohypoxyli and its Trichoderma koningii-like anamorph: a new species from Puerto Rico on Stilbohypoxylon moelleri. Sydowia 55: 255–266. [Google Scholar]
- Meyer R, Plaskowitz JS (1989). Scanning electron microscopy of conidia and conidial matrix of Trichoderma. Mycologia 81: 312–317. [Google Scholar]
- Mishra SK, Singh RP (2005). Prospects for the bio-management of Trichoderma viride – an organism harmful to button mushroom. Journal of Applied Horticulture Lucknow 7: 38–42. [Google Scholar]
- Nicholas KB, Nicholas HJ Jr (1997). Genedoc: a tool for editing and annotating multiple sequence alignments. www.psc.edu/biomed/genedoc.
- Nirenberg HI (1976). Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 169: i-v + 1–117. [Google Scholar]
- Nobe R, Sakakibara Y, Ogawa K, Suiko M (2004). Cloning and expression of a novel Trichoderma viride laminarinase AI gene (lamAI). Bioscience, Biotechnology and Biochemistry 68: 211–219. [DOI] [PubMed] [Google Scholar]
- Osono T (2005). Colonization and succession of fungi during decomposition of Swida controversa leaf litter. Mycologia 97: 589–597. [DOI] [PubMed] [Google Scholar]
- Persoon CH (1794). Dispositio methodica fungorum. Römer's neues Magazin der Botanik 1: 81–128. [Google Scholar]
- Persoon CH (1801). Synopsis methodica fungorum. Göttingen, Germany.
- Rannala B, Yang Z (2005). Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. Journal of Molecular Evolution 43: 311. [DOI] [PubMed] [Google Scholar]
- Rifai MA (1969). A revision of the genus Trichoderma. Mycological Papers 116: 1–56. [Google Scholar]
- Roiger DJ, Jeffers SN, Caldwell RW (1991). Occurrence of Trichoderma species in apple orchard and woodland soils. Soil Biology and Biochemistry 23: 353–359. [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, Lowen R (1999). Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42: 1–248. [Google Scholar]
- Rudresh DL, Shivaprakash MK, Prasad RD (2005). Tricalcium phosphate solubilizing abilities of Trichoderma spp. in relation to P uptake and growth and yield parameters of chickpea (Cicer arietinum L.). Canadian Journal of Microbiology 51: 217–222. [DOI] [PubMed] [Google Scholar]
- Samuels GJ (2006). Trichoderma: Systematics, the sexual state, and ecology. Phytopathology 96: 195–206. [DOI] [PubMed] [Google Scholar]
- Samuels GJ, Dodd SL, Gams W, Castlebury LA, Petrini O (2002). Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 94: 146–170. [PubMed] [Google Scholar]
- Samuels GJ, Dodd SL, Lu BS, Schroers H-J, Druzhinina I (2006a). The Trichoderma koningii aggregate species. Studies in Mycology 56: 67–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels GJ, Lieckfeldt E, Nirenberg HI (1999). Trichoderma asperellum, a new species with warted conidia, and redescription T. viride. Sydowia 51: 71–88. [Google Scholar]
- Samuels GJ, Pardo-Schultheiss R, Hebbar P, Lumsden RD, Bastos CN, Costa JC, Bezerra JL (2000). Trichoderma stromaticum, sp. nov., a parasite of the cacao witches broom pathogen. Mycological Research 104: 760–764. [Google Scholar]
- Samuels GJ, Petrini O, Kuhls K, Lieckfeldt E, Kubicek CP (1998). The Hypocrea schweinitzii complex and Trichoderma sect. Longibrachiatum. Studies in Mycology 41: 1–54. [Google Scholar]
- Samuels GJ, Suarez C, Solis C, Holmes KA, Thomas SE, Ismaiel A, Evans HC (2006b). Trichoderma theobromicola and T. paucisporum: two new species isolated from cacao in South America. Mycological Research 110: 381–392. [DOI] [PubMed] [Google Scholar]
- Smith WH (1995). Forest occurrence of Trichoderma species: emphasis on potential organochlorine (xenobiotic) degradation. Ecotoxicology and Environmental Safety 32: 179–183. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jenmougin F, Higgins DG (1997). The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 24: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulasne L-R, Tulasne C (1865). Selecta fungorum carpologia. Vol. 3. Paris.
- Webster J (1964). Culture studies on Hypocrea and Trichoderma I. Comparison of perfect and imperfect states of H. gelatinosa, H. rufa and Hypocrea sp. 1. Transactions of the British Mycological Society 47 75–96. [Google Scholar]
- White TJ, Bruns, T, Lee S, Taylor J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for pylogenetics. In: PCR protocols: a guide to methods and applications. Innis MA, Gefland DH, Sninsky JJ, and White TJ, eds. New York: Academic Press: 315–322.