Abstract
Using morphological characters, cultural characters, large subunit and internal transcribed spacer rDNA (ITS) sequences, and provisions of the International Code of Botanical Nomenclature, this paper attempts to resolve the taxonomic and nomenclatural confusion surrounding three species of cladosporium-like hyphomycetes. The type specimen of Hormodendrum resinae, the basis for the use of the epithet resinae for the creosote fungus {either as Hormoconis resinae or Cladosporium resinae) represents the mononematous synanamorph of the synnematous, resinicolous fungus Sorocybe resinae. The phylogenetic relationships of the creosote fungus, which is the anamorph of Amorphotheca resinae, are with the family Myxotrichaceae, whereas S. resinae is related to Capronia (Chaetothyriales, Herpotrichiellaceae). Our data support the segregation of Pycnostysanus azaleae, the cause of bud blast of rhododendrons, in the recently described anamorph genus Seifertia, distinct from Sorocybe; this species is related to the Dothideomycetes but its exact phylogenetic placement is uncertain. To formally stabilize the name of the anamorph of the creosote fungus, conservation of Hormodendrum resinae with a new holotype should be considered. The paraphyly of the family Myxotrichaceae with the Amorphothecaceae suggested by ITS sequences should be confirmed with additional genes.
Keywords: Amorphothecaceae, Cladosporium resinae, creosote fungus, Hormoconis resinae, jet fuel fungus, kerosene fungus, Myxotrichaceae, Pycnostysanus, resinicolous fungi
INTRODUCTION
The ascomycete Amorphotheca resinae Parbery (1969) grows in hydrocarbon-rich substrates such as jet fuel, cosmetics and wood preserved with creosote or coal tar. This fungus is widely known by the anamorph name Hormoconis resinae (Lindau) Arx & G.A. de Vries or its obligate synonym Cladosporium resinae (Lindau) G.A. de Vries. It produces lightly pigmented, warty conidiophores, and branched, acropetally developing chains of lightly pigmented ameroconidia lacking conspicuous scars (Fig. 1B-E). This species is known colloquially as the “creosote fungus”, the “kerosene fungus” or the “jet fuel fungus”; to avoid confusion caused by the many heterotypic names with the epithet “resinae”, in this paper we generally will use the oldest of these informal names, “creosote fungus”, when referring to A. resinae or its anamorph. This fungus grows in jet fuel contaminated with small amounts of water, and the mycelium clogs fuel lines and corrodes metal parts. Consequently, fuel tanks in airports are monitored for this fungus by private companies using various physiological or biochemical tests.
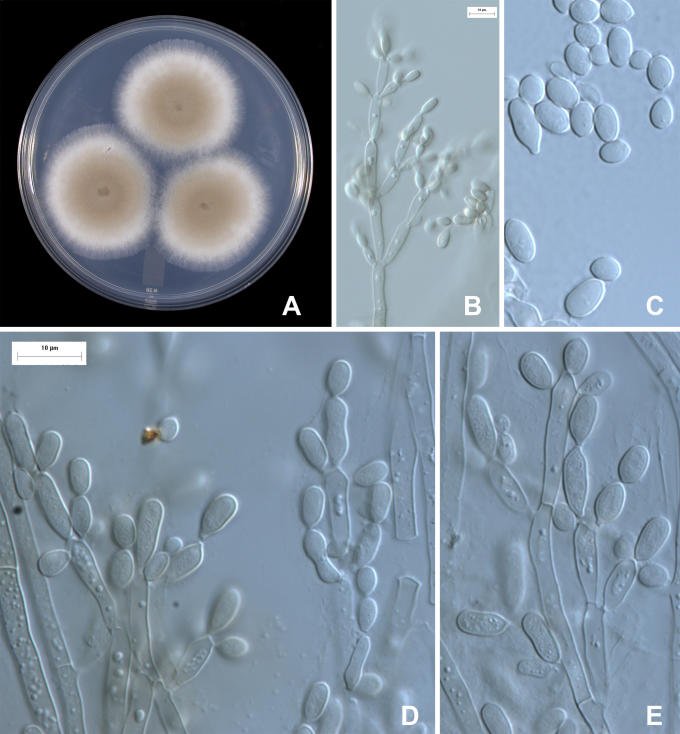
Fig. 1.
Amorphotheca resinae, colony characters and anamorph micromorphology. A. 10-d-old colony on PDA. B, D-E. Micromorphology of conidiophores, showing acropetal conidial chains, ramoconidia, and conidia. C. Conidia. DAOM 170427; for C, E see scale bar in D.
Sorocybe resinae (Fr.) Fr. produces dark black colonies on conifer resin, comprising dark synnemata and an effuse mononematous synanamorph, both with cladosporium-like conidiogenous cells and conidia. Unlike the anamorph of the creosote fungus, the conidia of Sorocybe resinae are dark brown and the lateral walls are conspicuously thicker than the poles (Fig. 2D-G). Colonies with only the mononematous anamorph sometimes occur, and the mononematous anamorph can be sparse on colonies bearing synnemata. However, the conidia of the mononematous anamorph have identical pigmentation and lateral wall thickening to that of the synnematous anamorph. The mononematous anamorph rarely has been referred to by its own binomial name although, as we will show, there is a species epithet available. For the same reasons given above for Amorphotheca Parbery, generally we will refer to Sorocybe resinae herein as “the resin fungus”.
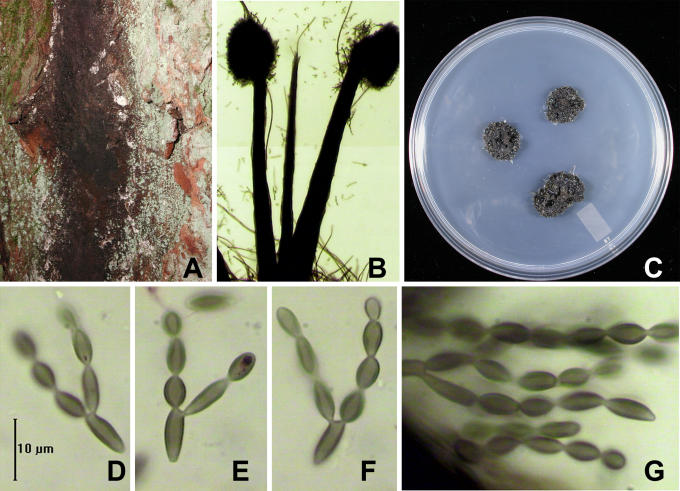
Fig. 2.
Sorocybe resinae, synnematous form. A. Colony on bark of living, standing conifer. B. Synnemata. C. Four-month-old colony on DG18. D-G. Acropetally developing chains of conidia. Note that the lateral walls are conspicuously thickened; compare with Fig. 3. A, C. DAOM 239134. B, D-G. DAOM 11381.
Despite the micromorphological differences noted above, there is disagreement about whether the creosote fungus is conspecific with the mononematous synanamorph of the resin fungus (Parberry 1969). The name for the anamorph of the creosote fungus is based on Hormodendrum resinae Lindau (1906). Christensen et al. (1942) presented a study of a cladosporium-like fungus commonly isolated from wood impregnated with creosote and coal tar and applied Lindau's name without examining its type. A later ecological study by Marsden (1954) employed the same name for the same fungus. An extra dimension was added to the confusion when de Vries (1952, using the name Cladosporium avellaneum G.A. de Vries) described four formae for the creosote fungus (differing in the colours of their conidia, the production of setae, or the total absence of conidia), each based on single conidium isolates made from one parent culture. De Vries (1955) and Parberry (1969) examined the holotype of Hormodendrum resinae and concluded that it represented the creosote fungus. Hughes (1958), prior to the description of Amorphotheca or Hormoconis Arx & G.A. de Vries, examined the same specimen and considered it to be the mononematous synanamorph of the resin fungus. If Hughes (1958) is correct, then neither the species Hormodendrum resinae, nor the genus that it typifies, Hormoconis, can represent the creosote fungus, as intended by Parberry (1969) or von Arx and de Vries (in von Arx 1973).
In this paper, we present micromorphological, cultural and molecular evidence that the resin fungus is a different species from the creosote fungus. Combined with re-examination of the holotype of Hormodendrum resinae, this information is used to provide a revised taxonomy and nomenclature for these two species. A third cladosporium-like fungus, Seifertia azaleae, is also considered in our discussion of generic concepts.
Historical review
The history of the fungus now known as Sorocybe resinae began with Fries (1815), who described Racodium resinae Fr. as follows:
“310. Racodium resinae, expansum molliusculum dense contextum nigrum, filis inaequalibus.
In resina Pini Abietis in silvis Suecia passim.
Habitu et loco natali distinctum. Fila divaricato-ramosa; alia rigidula apice capituli sera, sub miscrosc. Coremio Link similia, Demat. villosum Schleich. huic simile; sed sub microsc. fila maxime differunt.” The comparison with Coremium Link indicates the probability of a synnematous fungus, and an authentic specimen of Fries' fungus, which as the only known authentic material we interpret as the holotype, is preserved in Link's herbarium (see below). It represents the synnematous form of the resin fungus1.
Fries (1832) later transferred his species to Sporocybe Fr. (1825), a genus then used for relatively conspicuous dark hyphomycetes with dry spores (Mason & Ellis 1953). The 1832 description explicitly stated... “capitulo rotundato inaequali, sporidiis seriatis, stipite aequali simplici.” The use of “capitulo” and “stipite” imply what would now be recognised as a synnematous fungus. Fries (1832) further characterised the habit of the fungus as “habitu stipitum Calicii,” a further comparison to a group of black, stipitate lichenized fungi classified in Calicium Pers., which under a hand lens look similar to a dark synnematous fungus.
Fries (1849) next described the genus Sorocybe Fr. for this fungus, as follows:
Sorocybe Fr.
Habitus prioris. sed mycelium floccosum densum, stroma corneo-carbonaceum, sporis moniliformi-concatenatis basi excipulum incompletum praebens. 1. S. resinae. Fr. 1-4. at raro fructif. Klotzsch. exs. C. 2.
Because this description explicitly referred to the Systema, Fries presumably was segregating the fungus, originally described as Racodium resinae, into a new monotypic genus (McNeill et al. 2007; Art. 33.3) and this interpretation of R. resinae as the basionym generally has been followed in subsequent treatments of Sorocybe resinae.
As noted in Table 1, Fries' Racodium resinae was placed in several other hyphomycete genera by eighteenth century authors. These diversions need not be reviewed in detail here because the modern status of these other genera, and their lack of similarity with Sorocybe, is clear.
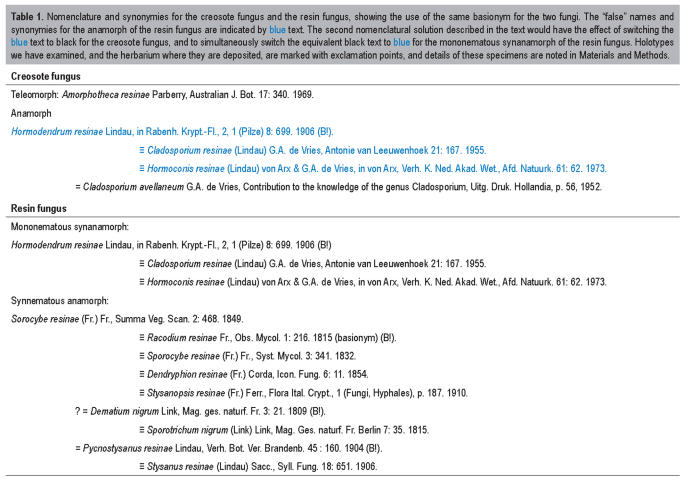
Table 1.
Nomenclature and synonymies for the creosote fungus and the resin fungus, showing the use of the same basionym for the two fungi. The “false” names and synonymies for the anamorph of the resin fungus are indicated by blue text. The second nomenclatural solution described in the text would have the effect of switching the blue text to black for the creosote fungus, and to simultaneously switch the equivalent black text to blue for the mononematous synanamorph of the resin fungus. Holotypes we have examined, and the herbarium where they are deposited, are marked with exclamation points, and details of these specimens are noted in Materials and Methods.
Bonorden (1851) described Hormodendrum Bonord., with four species originally placed in Penicillium Link by Corda (1839); H. olivaceum (Corda) Bonord. (≡ Penicillium olivaceum Corda 1839) was designated as lectotype by Clements & Shear (1931). This genus was frequently, but incorrectly, spelled “Hormodendron”. Bonorden's descriptions and illustrations are of variable quality by modern standards, and his herbarium is unknown (Stafleu et al. 1995). Consequently the actual identities of the species Bonorden placed in Hormodendrum are unknown and Corda's Cladosporium olivaceum (Corda) Bonord. was dismissed in Penicillium monographs because the drawing shows branched conidial chains (Thom 1930), although the specimen has apparently not been re-examined. The generic name was used as a segregate for Cladosporium Link by some authors (e.g. Kendrick 1961), in particular for species with ameroconidia (de Vries 1952). Although it sometimes has been considered a synonym of Cladosporium, it will remain a nomen dubium until the type species is properly typified.
Unaware of the resinicolous fungus described by Fries, Lindau described two species growing on conifer resin, Pycnostysanus resinae Lindau (1904), the type of this anamorph generic name, and Hormodendrum resinae Lindau (1906). The former was clearly illustrated and described as a synnematous species. The protologue of the latter concludes with, “Mit Pycnostysanus resinae hat die Art nichts zu tun.” Clearly, Lindau observed no synnemata on the specimen of the mononematous fungus and he believed it was a different fungus, rather than what would now be called a synanamorph of the synnematous fungus that he had described previously. Lindau (1910) reproduced the 1904 illustration of Pycnostysanus resinae as Stysanus resinae (Fr.) Sacc. (1906), thus accepting its identity with the species originally described as Racodium resinae Fr. Lindau (1910) made no mention of Hormodendrum resinae, indicating he still made no association between the synnematous and mononematous fungi on resin.
De Vries (1952) described a new species, Cladosporium avellaneum G.A. de Vries, isolated from cosmetics. Later, he noted the similarities between his C. avellaneum and the creosote fungus, and suggested that they were the same species (de Vries 1955), replacing the name of one of his previously described formae, i.e. viride, with the forma name resinae. He examined Lindau's type of Hormodendrum resinae and decided that it provided an earlier epithet for C. avellaneum. He transferred the species into Cladosporium as C. resinae (Lindau) G.A. de Vries, and this name was widely used for the creosote fungus until 1973. This binomial is still commonly employed in non-taxonomic literature, especially commercial publications dealing with the creosote fungus.
In his study of type collections of classical hyphomycetes, Hughes (1958) included Pycnostysanus resinae Lindau and Hormodendrum resinae Lindau as facultative synonyms of Sorocybe resinae (Fr.) Fr., with Racodium resinae Fr. and several other nomenclatural variants as obligate synonyms (Table 1). The synnematous Pycnostysanus resinae was cited as “Pycnostysanus state [i.e. synanamorph] of Sorocybe resinae”. Hormodendrum resinae thus remained to represent the mononematous synanamorph of what was interpreted as a single species.
Parberry (1969) described a cleistothecial ascomycete, Amorphotheca resinae, for the teleomorph of the creosote fungus. He also examined the holotype of Hormodendrum resinae and agreed with the conclusions of de Vries (1955). He used the epithet resinae for the teleomorph to correspond with that of the anamorph. He discounted the possibility that the synnematous Sorocybe resinae could be the same fungus as Hormodendrum resinae because synnemata never developed in his cultures of the creosote fungus.
Von Arx and de Vries (in von Arx 1973) described the genus Hormoconis, typified by Hormodendrum resinae, with the new combination Hormoconis resinae (Lindau) Arx & G.A. de Vries. Their intention was to erect an anamorph genus for the anamorph of the creosote fungus, which they suggested was improperly classified in Cladosporium because it lacked darkened, thickened secession scars on the conidia.
A third cladosporium-like fungus is relevant to this story. Seifertia azaleae (Peck) Partridge & Morgan-Jones [until recently known as Pycnostysanus azaleae (Peck) E.W. Mason] is a cosmopolitan fungus causing bud blast and twig blight of azaleas and rhododendrons. This species is morphologically similar to Sorocybe resinae, but the conidia are paler and lack laterally thickened walls. Sorocybe and Pycnostysanus have often been considered taxonomic synonyms (Ellis 1976, Carmichael et al. 1980); as shown above, both are based on the synnematous form of the resin fungus. Partridge and Morgan-Jones (2002) argued that Sorocybe resinae and “Pycnostysanus azaleae” are not congeneric, and described the new genus Seifertia Part. & Morgan-Jones for the Rhododendron fungus. They observed that the connection between conidia in Seifertia azaleae is much narrower than in Sorocybe resinae, and that minute denticles are visible on the conidiogenous cells of the former fungus. The broader connections between conidia of Sorocybe resinae result in broadly protuberant conidiogenous loci on the conidiogenous cells, and more truncate detached conidia.
MATERIALS AND METHODS
Herbarium material and fungal strains
Full details of herbarium material examined are listed below. Cultures and dried herbarium specimens were studied in 90 % lactic acid without stains; preparations of some exsiccate and types were mounted in glycerin jelly. Cultures were grown on potato-dextrose agar (PDA, Difco), oatmeal agar (OA, Samson et al. 2004), Blakeslee's malt extract agar (MEA, Samson et al. 2004) and dichloran-18 % glycerol agar (DG-18, Samson et al. 2004). Colony characters were taken from cultures grown at 25 °C in darkness. Cultures are maintained in the Canadian Collection of Fungal Cultures (DAOM), Agriculture & Agri-Food Canada, Ottawa.
Exsiccati and types
Dematium nigrum [scr. Link]. E. Hbr. Link (23) = Sporocybe resinae III. 341 [scr.?] (herb. Link, B).
Hormodendrum resinae Lindau, n. sp. Fl. v. Hamburg 206, auf Harz an Picea excelsa, Sachsenwald, leg. O. Jaap, 29-4-1906. [scr. Lindau]. (DAOM 41888, slide prepared from the holotype preserved in B.)
Pycnostysanus resinae Lindau nov. gen. et nov. spec., Kabát et Bubák: Fungi imperfecti exsiccati no. 99. Auf erhärteten Fichtenharz an Brockenweg, am Dreieckigen Pfahl in Harz, Deutschland, leg. G. Lindau, 13.VIII. 1903 (holotype, B).
Racodium resinae Fries. E. Hbr. Link, Fries legi, Smol. [scr. Fries]. (DAOM 41890, slide prepared from herb. Link, B). This is the presumed holotype of R. resinae, the basionym for the resin fungus, Sorocybe resinae. The specimen includes dark, decapitated synnemata, brown conidia with laterally thickened walls, and acropetal conidial chains, allowing it to be recognised as the fungus we now know as S. resinae. Fries perhaps sent this fungus to Link to see if it could be differentiated from Coremium Link. The minimal details, that the fungus was collected by Fries, presumably in Småland (a province of Sweden), match the details in the protologue of this species.
Sorocybe resinae. “Fungi Rhenani Fasc. II, 1863, L. Fuckel, no. 129, ad Abietis resinam, raro Hieme, in sylva Hostrichiensi” (as Myxotrichum resinae Fr., DAOM 55543 ex FH). “Flora Suecica, 2956, Ad resinam piceae, Småland: Femsjö, Prostgaidsshogen, 6 Aug. 1929, leg. J.A. Nannfeldt, s.n.” (as Stysanus resinae (Fr.) Sacc., DAOM 41891 ex UPS). “Flora Suecica, 4709, Ad resinam abietinum, Uppland: Bondkysko sin Valsätra, 9 May 1932, leg. J.A. Nannfeldt” (as Hormodendrum resinae Lindau, DAOM 41889 ex UPS). “[on wood scr. Berkeley] J.E. Vize, Hereford 1877” (as Torula pinophila Fr., DAOM 113425 ex K). “Sydow, Mycotheca germanica, 350. Auf Fichtenharz... am Brockenweg 30.9.1904, leg. P. Sydow” (DAOM 41893).
Other material examined
Sorocybe resinae. Canada, British Columbia: Burnaby, Central Park, on resin of Tsuga heterophylla, leg. S. & L. Hughes, 17 Aug. 2000 (DAOM 228572a, 228573a); Cameron Lake, Cathedral Grove, on Pseudotsuga menziesii, leg. isol. S.J. Hughes, 21 Aug. 1957 (DAOM 56088a). Ladysmith, Ivy Green Park, on resinous exudates, leg. R.J. Bandoni no. BC-978, 18 Apr. 1960, det. S.J. Hughes (DAOM 70462). North Vancouver, Lynn Valley Conservation Area, leg. det. S.J. Hughes, 1 Jul. 1975 (DAOM 139385); North Vancouver, Lynn Valley Conservation Area, on bark of living conifer (probably Pseudotsuga menziesii), leg. isol. K.A. Seifert no. 1574, 26 May 2002 (single conidium isolate, culture and specimen DAOM 239134; ITS GenBank EU030275, LSU GenBank EU030277); Terrace, near Kalum, on Tsuga heterophylla, leg. W.G. Ziller no. V-6549, 10 July 1950, det. S.J. Hughes (DAOM 59657); Queen Charlotte Islands, east coast of Moresby Island, north side of Gray Bay, 53°08' N, 131°47' W, on Picea sitchensis, leg. I. Brodo, M.J. Schepanek, W.B. Schofield, 28 Sep. 1973, det. S.J. Hughes (DAOM 144757); Queen Charlotte Islands, Graham Island, Tow Hill area, on resin of Picea sitchensis, leg. S.A. Redhead no. 4440, 20 Sep. 1982, det. G.P. White (DAOM 184025); Revelstoke, Wigwam, on Tsuga heterophylla, leg. W. Ziller V-6567 det. S.J. Hughes, 6 Jun. 1950 (DAOM 59710); Vancouver Island, Cathedral Grove, Cameron Lake, on Pseudotsuga menziesii, leg. det. S.J. Hughes, 21 Aug. 1957 (DAOM 56088a); Vancouver Island, Caycuse, on resin of Pseudotsuga menziesii, leg. det. S.J. Hughes, 17 Jul. 1972 (DAOM 139355); Vancouver Island, Lake Cowichan, Honeymoon Bay, on resin of Pseudotsuga menziesii, leg. J Ginns, det. S.J. Hughes, 29 Oct. 1971 (DAOM 134968); Vancouver Island, Lake Cowichan, Mesachie Lake Forest Experimental Station, leg. det. S.J. Hughes, 5 Jul. 1972 (DAOM 139277a, DAOM 139278) and 6 Jul. 1072 (DAOM 139281). Czechoslovakia, Ještěd near Liberec, leg. det. S.J. Hughes, on resin of Larix europaea, 10 May 1955 (DAOM 51723). United States, Oregon: Andrews' Experimental Forest, Forest Service Rd. no 1553, on resin of Tsuga heterophylla, leg. det. S.J. Hughes, 10 May 1969 (DAOM 134565); Andrews' Experimental Forest, Blue River, on resin of conifer, cut wood, leg. det. K.A. Seifert no. 69, 10 Jul. 1981 (DAOM 228203); Oregon, del Norte Co., J. Smith's State Park, on Tsuga heterophylla, leg. det. S.J. Hughes, 11 May 1069 (DAOM 134614); Devil's Elbow State Park, Cape Perpetus, on Picea sitchensis, leg. det. S.J. Hughes, 6 May 1969 (DAOM 134615); Linn Co., near Cascadia, on Pseudotsuga menziesii, leg. R. Fogel, det. S.J. Hughes, 14 May 1969 (DAOM 127885); U.S. Forest Service Rd. no. 126, North fork Cape Creek, on resin of Abies grandis, leg. det. S.J. Hughes, 7 May 1969 (DAOM 134852,134563); Willamette National Forest, McKenzie Bridge Camp Grounds, leg. det. S.J. Hughes, 10 May 1969 (DAOM 134564). Washington: Kittitas Co., Wanatchee National Forest, Rocky Run, on Abies nobilis, leg. Field Mycology Class 1955, 22 Jul. 1955, det. S.J. Hughes, (mononematous synanamorph only, DAOM 118934 ex WSP 45210, as Helminthosporium sp.); Jefferson Co., Olympic National Forest, 10 mi Camp, Sec. 17, T26N, R3W, on Pseudotsuga mucronata, leg. Field Mycology Class, 22 Jul. 1955 (DAOM 113801 ex WSP 45212, as Helminthosporium); Grays Harbor Co., Twin Harbors Beach State Park, resin of Picea sitchensis, leg. W.B. & V.G. Cooke, 24 Jul. 1951, det. S.J. Hughes (DAOM 118970 ex WSP 28432).
Amorphotheca resinae. Isolated from jet fuel by P. Emonds (culture, DAOM 170427 = ATCC 22711, ITS GenBank EU030278, LSU GenBank EU030280). Canada, British Columbia, source unknown, isol. “Mrs. Volkoff”, Jul. 1969 (culture, DAOM 194228, ITS GenBank EU030279).
Seifertia azaleae. All on flower buds of Rhododendron spp. Canada, British Columbia: Burnaby, Central Park, leg. S.J. Hughes, 17 Aug. 2000 (DAOM 228571); Vancouver, Stanley Park, leg. K.A. Seifert no. 1571, 11 May 2002 (culture and specimen, DAOM 239136, LSU GenBank EU030276). Ireland, Munter, Kerry, near Glenbeigh (ca. N 52° 03' W 9° 54'), leg. K.A. Seifert no. 3197, 26 Sep. 2006 (culture and specimen, DAOM 239135, ITS GenBank EU030273). Netherlands, Gelderland, Kröller-Müller Museum, leg. K.A. Seifert no. 1235, 12 May 2000 (DAOM 227136). United Kingdom, Wales, Hafod Estate (ca. N 52° 22' W 3° 51'), leg. K.A. Seifert no. 3198, 1 Oct. 2006 (culture and specimen, DAOM 239137, ITS GenBank EU030274).
DNA extraction, amplification and sequencing
DNA was isolated using a FastDNA™ Kit and the FastPrep™ FP120 (BIO 101 Inc.) or an UltraClean™ Microbial DNA Isolation Kit (Mo Bio Laboratories, Inc., Solana Beach, CA, U.S.A.) using mycelium removed from agar cultures. PCR and cycle sequencing reactions were performed on a Techne Genius™ thermocycler (Techne Cambridge Ltd.). PCR reactions were performed using Ready-To-Go™ Beads (Amersham Canada Ltd.) in 25 μL volumes, each containing 20-100 ng of genomic DNA, 2.5 units pure Taq DNA Polymerase, 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 1.5 mM MgCl2, 200 μM of each dNTP, 0.2 μL of each primer (50 μM), and stabilizers including bovine serum albumin. The reaction profile included an initial denaturation for 4 min at 94 °C, followed by 30 cycles of 1.5 min denaturation at 95 °C, 1 min annealing at 56 °C, 2 min extension at 72 °C, with a final extension of 10 min at 72 °C. Amplicons were purified by ethanol/sodium acetate precipitation and resuspended as recommended for processing on an ABI PRISM 3100 DNA Analyzer or an ABI 373 Stretch DNA Sequencer (Applied Biosystems, Foster, CA). Amplification products were sequenced using the BigDye v. 2.0™ Terminator Cycle Sequencing Ready Reaction Kit (ABI Prism/Applied Biosystems) following the manufacturer's directions. An approximately 1 000 bp portion of the large subunit (LSU) ribosomal DNA was amplified and sequenced using primers LR0R and LR6, and cycle-sequenced using primers LR0R, LR3R, LR16 and LR6 (Vilgalys & Hester 1990, Rehner & Samuels 1995; www.biology.duke.edu/fungi/mycolab/primers.htm). The complete ITS and 5.8S rRNA genes were amplified and sequenced using the primers ITS5 and ITS4, with ITS2 and ITS3 primers used for cycle sequencing when necessary (White et al. 1990). Some sequences were derived from single PCR amplifications of the ITS5-LR6 region.
Data matrices were subjected to parsimony analysis using heuristic searches in PAUP* v. 4.0b10 (Swofford 2002) with simple stepwise addition of taxa, and tree bisection-reconnection (TBR) branch swapping. Uninformative characters were removed for all analyses. Strict consensus trees were calculated, and the robustness of the phylogenies was tested using full bootstrap analyses (1 000 replications). For all analyses, GenBank accession numbers are given on the tree figures, and the sequences generated in this study are indicated in bold.
The large subunit matrix was assembled from the closest BLAST matches using our sequences for the three fungi of interest, S. resinae, A. resinae and S. azaleae; Golovinomyces cichoracearum was added as an out-group to root the tree. Although these sequences were put into a single matrix, there is no implication that this data set represents the diversity of the Ascomycota. The alignment was calculated using MAFFT (Katoh et al. 2002) and adjusted using Se-Al (Sequence Alignment Program v. 1.d1; http://evolve.zoo.ox.ac.uk/software/Se-Al/main.html) to maximise homology.
The internal transcribed spacers alignment including Sorocybe resinae was derived from an alignment of Capronia and related anamorphs used by Davey & Currah (2007), originally produced using MAFFT. This data set was modified considerably using Se-Al to maximise homology, but still included several areas where the homology of aligned sequences was difficult to evaluate. ITS sequences of Amorphotheca resinae were used to retrieve closely related sequences using a BLAST search of GenBank, and these relevant sequences were added to an alignment of Oidiodendron Robak sequences from the study of Hambleton et al. (1998), and then adjusted using Se-Al.
We attempted direct PCR from two specimens containing only the putative mononematous synanamorph of Sorocybe resinae (DAOM 228772a, 228573a), to allow comparison of sequences obtained from cultures of the synnematous synanamorph. These attempts, using the same methods outlined above, were unsuccessful.
RESULTS
Cultural characters and micromorphology
Most micromorphological characters of the resin fungus Sorocybe resinae (Partridge & Morgan-Jones 2002), the creosote fungus Amorphotheca resinae (Parbery 1969, de Vries 1952, 1955, Ho et al. 1999) and the rhododendron fungus Seifertia azaleae (Ellis 1976, Partridge & Morgan-Jones 2002, Glawe & Hummel 2006) are well-described in the literature and will not be repeated here.
The three species are readily distinguished based on growth rates and overall cultural phenotypes. Agar colonies of Sorocybe resinae are coal-black, wrinkled, and restricted in growth, no matter what agar medium is employed; even after 3 mo, the colonies are rarely more than 2 cm diam (Fig. 2C). Synnemata did not form in our cultures; in vivo, the synnemata produce branched, acropetal chains of conidia with laterally thickened walls (Figs 2D-G). No thickened, refractive or darkened secession scars were evident on individual conidia or ramoconidia. Conidial masses were removed from the mononematous and synnematous parts of a freshly collected specimen (DAOM 56088a) and grown on PDA and sterilised conifer wood. There were no discernable differences between colonies derived from the two types of conidiophores, in all cases yielding restricted black colonies, or in their microscopic characters. Therefore, we conclude that these two types of conidiophores represent synanamorphs of one fungus. An identical conclusion was reached by Partridge & Morgan-Jones (2002). We documented the occurrence of this fungus in California, Oregon, and Washington State, U.S.A. and British Columbia, Canada, on resinous exudates on Abies nobilis, Picea sitchensis, Pseudotsuga menziesii and Tsuga heterophylla.
Microscopic features from the holotype specimen of Hormodendrum resinae Lindau are shown in Fig. 3. Dark, thick-walled conidiophore stipes give rise to branched, acropetally developing conidial chains. The conidia are relatively darkly pigmented, and the lateral walls are more conspicuously thickened and darkened than the polar walls. There are no obvious thickened, refractive or darkened secession scars on any of the cells. Apart from the production of synnemata, the characters of the conidia and conidium ontogeny are identical in Lindau's specimen and the synnematous specimens of Sorocybe resinae examined.
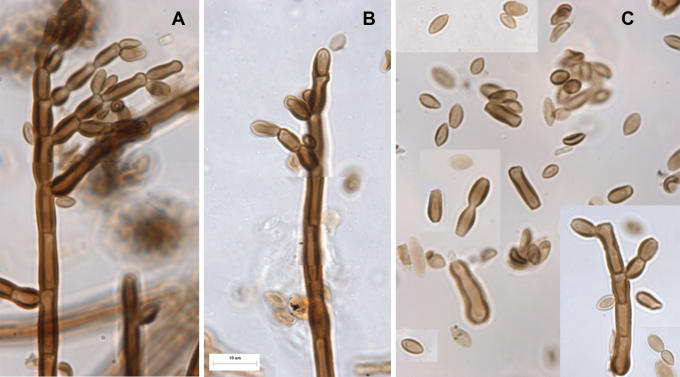
Fig. 3.
Hormodendrum resinae, A-B. Conidiophores and acropetally developing chains of conidia. C. Conidia. Note that the lateral walls are conspicuously thickened compared to the walls at the poles. From a slide (DAOM 41888) prepared from the holotype (B).
In contrast, both the resin fungus and the rhododendron fungus have spreading rather than restricted agar colonies. Cultures of the resin fungus are sandy brown (Kornerup & Wanscher 1989), planar and powdery, growing 4-4.5 cm diam in 10 d on PDA (Fig. 1A). Cultures of the rhododendron fungus are slower, growing 2.5-3.5 cm diam after 21 d on MEA (not shown). They are planar and greyish brown, with an orange-brown reverse. No synnemata were observed in our cultures of the rhododendron fungus on MEA, OA or PDA, but cladosporium-like conidiation occurred in the aerial mycelium.
Phylogeny
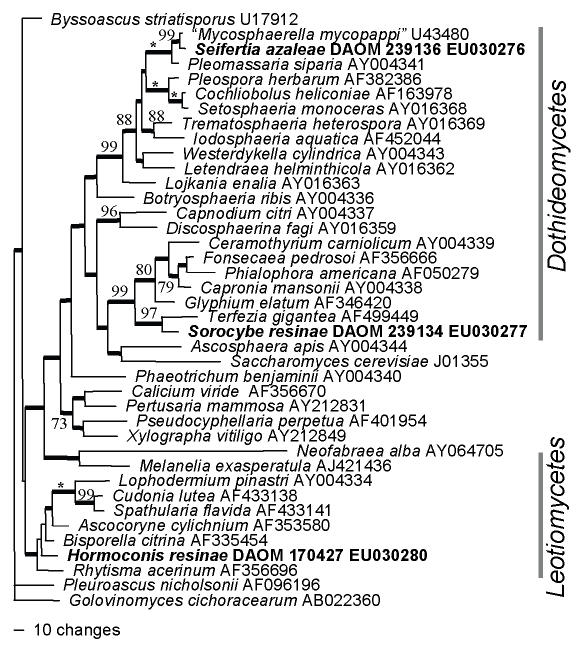
The large subunit analysis (LSU) was used to demonstrate the general phylogenetic relationships of the resin fungus Sorocybe resinae (DAOM 239134), the creosote fungus Amorphotheca resinae (DAOM 170427, 194228) and the rhododendron fungus Seifertia azaleae (DAOM 239136), and subsequent analyses of the internal transcribed spacers were used to estimate more precise affinities. Fig. 4 shows the LSU analysis and demonstrates that Sorocybe resinae appears to be a member of the Herpotrichiellaceae, Chaetothyriales, A. resinae is related to the inoperculate discomycetes (Leotiomycetes) and Seifertia azaleae is most closely related to a sequence labelled Mycosphaerella mycopappi A. Funk & Dorworth, which is unrelated to Mycosphaerella s. str.
Fig. 4.
Parsimony analysis of large subunit sequences, demonstrating the phylogenetic positions of Amorphotheca resinae, Sorocybe resinae and Seifertia azaleae (all shown in bold) in the Ascomycota. One of 12 equally parsimonious trees (1 888 steps, CI = 0.390, RI = 0.554, RC = 0.216, HI = 0.610) with Golovinomyces cichoracearum as the out-group. Bootstrap values above 70 % are shown at the relevant nodes, with an asterisk representing 100 % bootstrap support; branches with thick lines occurred in all equally parsimonious trees.
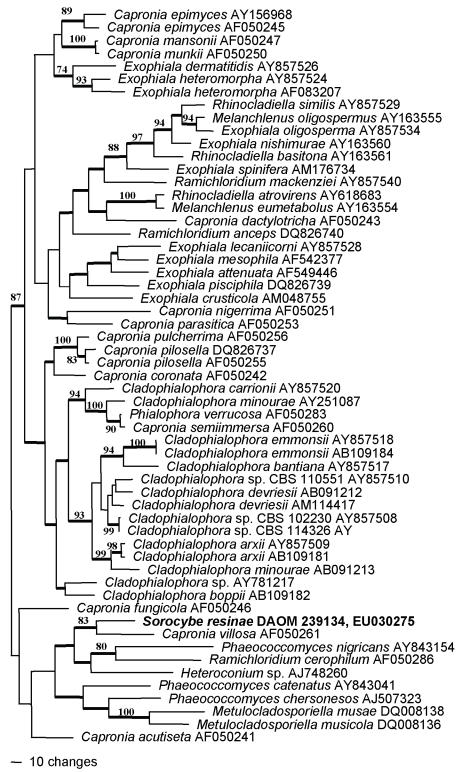
For the ITS alignment of Sorocybe resinae, two preliminary parsimony analyses were conducted, one with informative characters from the full alignment, the second with a subset with 179 characters excluded from seven ambiguously aligned regions. The consistency indices (full 0.301, partial 0.324), tree topologies, and bootstrap supports for the two analyses were relatively similar. Therefore, the complete alignment was used for the tree presented here (Fig. 5). The data matrix included 57 taxa, with 352 of 752 characters phylogenetically informative. Sorocybe resinae clearly is related to Capronia and allied anamorph genera, as suggested by the LSU analysis. In the ITS analysis (Fig. 6) it forms a well-supported clade with C. villosa Samuels, that is a well-supported sister group to species now in three different anamorph genera, Phaeococcomyces nigricans (M.A. Rich & A.M. Stern) de Hoog, Ramichloridium cerophilum, and an undescribed species of Heteroconium Petr.
Fig. 5.
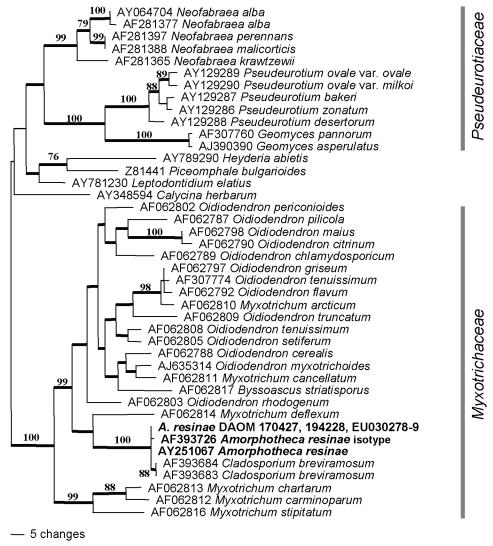
Parsimony analysis of internal transcribed spacers sequences, demonstrating the position of Amorphotheca resinae (shown in bold) in the ascomycete family Myxotrichaceae. One of 44 equally parsimonious trees (645 steps, CI = 0.460, RI = 0.758, RC = 0.349, HI = 0.540) with mid-point rooting. Bootstrap values above 70 % are shown at the relevant nodes; branches with thick lines occurred in all equally parsimonious trees.
Fig. 6.
Parsimony analysis of internal transcribed spacers sequences, demonstrating the position of Sorocybe resinae (shown in bold) among species of Capronia (Herpotrichiellaceae, Chaetothyriales) and its associated anamorph genera. One of 34 equally parsimonious trees (2 607 steps, CI = 0.301, RI = 0.506, RC = 0.153, HI = 0.699), with mid-point rooting. Bootstrap values above 70 % are shown at the relevant nodes; branches with thick lines occurred in all equally parsimonious trees.
The ITS matrix for A. resinae included 42 taxa, with 171 phylogenetically informative characters in the 530 base alignment. The phylogenetic analysis confirmed the relationship of this species with the Leotiomycetes, and provided a more precise hypothesis of its family-level relationships (Fig. 6). Amorphotheca resinae DAOM 170427 and 194228 had identical ITS sequences to another strain of the same species reported in GenBank (AY251067, from Braun et al. 2003), and one bp substitution from a second strain (AF393726 based on the isotype ATCC 200942 = CBS 406.68). These four sequences formed a sister group to two sequences of “Cladosporium” breviramosum Morgan-Jones (AF393683, AF393684). The well-supported clade of A. resinae and C. breviramosum, which represent the proposed family Amorphothecaceae, was previously noted by Braun et al. (2003). The nesting of this clade within two well-supported clades of Myxotrichum spp. and the associated anamorph genus Oidiodendron, which comprise the family Myxotrichaceae, has not been documented previously.
The ITS sequences of two strains of Seifertia azaleae were 474 bp and differed by one bp. BLAST searches with these sequences revealed significant homologies only with unidentified fungi, and lower probability matches with various members of the Dothideomycetes. Therefore, no taxonomically meaningful phylogenetic analysis can be presented with these ITS sequences. The species does seem to have affinities with the Dothideomycetes, but the putative relationship with Mycosphaerella, suggested by the LSU analysis, could not be confirmed with the ITS analysis.
DISCUSSION
Micromorphological comparisons, differences in culture characters, and phylogenetic analysis all support the conclusion that the mononematous synanamorph of Sorocybe resinae, the resin fungus, is different from the anamorph of Amorphotheca resinae, the creosote fungus. Based on ribosomal DNA sequences, the creosote fungus is related to the family Myxotrichaceae, the genus Myxotrichum and its Oidiodendron anamorphs (Fig. 5). In this gene tree, Myxotrichum and the Myxotrichaceae are paraphyletic, with Amorphotheca and the Amorphothecaceae nested within them. Sorocybe appears to be an additional anamorph genus phylogenetically associated with Capronia (Herpotrichiellaceae, Chaetothyriales, Fig. 6). The genetic connection between the synnematous and mononematous morphs of S. resinae was verified by morphological comparison of polyspore isolates derived from the two synanamorphs. However, the living cultures are no longer available and the connection was not confirmed with single conidium isolations. The type specimen of Hormodendrum resinae (Fig. 3) is the basis for the application of the most frequently used anamorph epithet for the creosote fungus. This specimen represents the mononematous synanamorph of Sorocybe resinae, not the anamorph of Amorphotheca resinae.
It is difficult to understand how these two fungi were confused when their micromorphologies are so different. The conidia are of the same general size and shape, but in both morphs of Sorocybe resinae (Figs 2D-G, 3C), the lateral walls are conspicuously thickened, a condition not present in the creosote fungus (Fig. 1C), and the conidia are much darker. In his monograph of Cladosporium, de Vries (1952) noted that single conidium isolates of C. avellaneum gave rise to four different colony types. In 1955, he extended these observations and decided that the much darker resin fungus was the same as one of his mutant forms of the creosote fungus, despite never having isolated such a dark spored form from any of his cultures. Parbery (1969) implied that the demonstrated ability of the creosote fungus to grow on a diversity of hydrocarbon-rich substrates favoured the thought that it would be able to grow on conifer resin. If cultures of the true Sorocybe resinae had been available, it is unlikely that this confusion would have persisted for so long. In vitro, the creosote fungus and the resin fungus are so different (Figs 1A, 2C) that it would difficult to defend the idea that they were mutants of the same fungus. These differences in the cultures are reflected by the disparate phylogenetic affinities of what now are clearly demonstrated to be two different species.
Unfortunately, the name Hormodendrum resinae has been misapplied to the creosote fungus, a species of economic importance. Also unfortunately, this species is the type of Hormoconis, a generic name that the community concerned with this fungus has been slow to adapt to in the 30 years since its introduction. There are several possible solutions to this problem. The conventional solution would be to apply names based strictly on the type specimens and accept Hormoconis as a synonym of Sorocybe, or to use it as a generic name for the mononematous synanamorph of the resin fungus. A new anamorph genus would then be described for the creosote fungus, making Cladosporium avellaneum G.A. de Vries the basionym for its type. However, the resulting binomial would be unfamiliar to those concerned with the creosote fungus, and the earlier literature citing H. resinae would be misleading.
A more parsimonious solution is possible. Article 14.9 of the International Code of Botanical Nomenclature (McNeil et al. 2006) allows for conservation of a name with a different type from that designated by the authors. The name Hormodendrum resinae is not otherwise needed because the mononematous synanamorph of the resin fungus is rarely referred to by a Latin binomial, and because Sorocybe resinae is based on a different type. Therefore, a new type specimen could be proposed and conserved for Hormodendrum resinae Lindau, preferably the holotype of A. resinae (MELU 7130). This would make the anamorph-teleomorph connection unequivocal, maintain current species epithets and taxonomic authorities, and ensure that most of the historical literature can be interpreted easily without the need to consult complicated nomenclators (Table 1). However, by perpetuating the use of the epithet “resinae”, this change would also perpetuate the misunderstanding that resin is a possible substrate for the creosote fungus. In any case, the use of this epithet for the teleomorph of the creosote fungus, Amorphotheca resinae, is legitimate and valid, and unlikely ever to be changed.
A third option would be an intermediate one. The application of the name Cladosporium avellaneum G.A. de Vries has never been in doubt, and it would be possible to conserve this species as the type of Hormoconis. This has the advantage of maintaining the familiar generic name Hormoconis, in combination with a species epithet that has been consistently applied. Furthermore, this solution would allow the confusion about the application and correct author citation around the epithet “resinae” for the anamorph of creosote fungus to recede.
The second and third solutions require formal taxonomic proposals to be published in Taxon. We will argue the merits of these possible solutions at more length in that venue.
The phylogenetic position of A. resinae raises additional taxonomic problems. This fungus typifies the monotypic family Amorphothecaceae, which has been considered incertae sedis since its description by Parbery (1969). Our phylogenetic analysis suggests that this family sits within the Myxotrichaceae. Amorphothecaceae (1969) is the older name, but Myxotrichaceae (1985) is well-entrenched in the mycological literature. As a consequence, the Myxotrichaceae are paraphyletic with respect to the Amorphothecaceae. The peridium of A. resinae, the only species presently placed in this family, lacks the thick-walled appendages that characterise most species of the Myxotrichaceae. Furthermore, the acropetal-blastic features of the anamorph of A. resinae differ from the thallic-arthric conidiogenesis of the other anamorphs associated with the Myxotrichaceae, principally Oidiodendron. These morphological differences explain why the affinity of A. resinae with the Myxotrichaceae was not noted before. A formal proposal to conserve Myxotrichaceae as the name for this family might be prudent eventually, but this should await analysis of additional genes to confirm the phylogenetic relationship.
Whether Cladosporium breviramosum, originally isolated from discoloured wallpaper, is actually a distinct species from A. resinae requires further study. It is clear that this species, if it is distinct, would be a member of Hormoconis rather than Cladosporium. Apart from the study of additional specimens, it might be fruitful to attempt to induce an Amorphotheca-like teleomorph in the two available cultures of C. breviramosum, and to compare the morphology with that of A. resinae. According to Parbery (1969), A. resinae includes both homothallic and heterothallic strains.
Unfortunately, the phylogenetic affinities of Seifertia azaleae were not established with certainty in this study. Its closest relative in the LSU analysis is a sequence identified as Mycosphaerella mycopappi Funk & Dorworth (U43480, based on the apparent type culture ATCC 64711), but this sequence does not cluster with others representing the family Mycosphaerellaceae (data not shown). Similarly, the ITS sequences of the rhododendron fungus did not cluster with the many ITS sequences of Mycosphaerella available. Presently, it seems that Seifertia azaleae fungus is allied with the Dothideomycetes, but its precise affinities are uncertain. It is clear that this fungus should not be classified in Pycnostysanus (a taxonomic synonym of Sorocybe), and continued recognition of the monotypic genus Seifertia seems justified.
Acknowledgments
We are grateful to Sarah Hambleton and Marie Davey for providing ITS alignments that served as the basis for analyses in this paper. Walter Gams and Scott Redhead provided expert nomenclatural advice and helpful reviews of this manuscript. We thank Uwe Braun for proposing the third option for resolving the name of the creosote fungus, outlined in the text. The Canadian Collection of Fungal Cultures (DAOM) provided several of the strains for this study. The curators of DAOM and B kindly provided access to critical type specimens.
Footnotes
References
- Arx JA von (1973). Centraalbureau voor Schimmelcultures Baarn and Delft. Progress Reports 1972. Verhandelingen der Koninklijke Nederlandsche Akademie van Wetenschappen, Afdeeling Natuurkunde 61: 59-81. [Google Scholar]
- Bonorden HF (1851). Handbuch der allgemeinen Mykologie. Stuttgart.
- Braun U, Crous PW, Dugan FM, Groenewald JZ, Hoog GS de (2003). Phylogeny and taxonomy of cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s. str. Mycological Progress 2: 3-18. [Google Scholar]
- Carmichael JW, Kendrick WB, Connors IL, Sigler L (1980). Genera of hyphomycetes. University of Alberta Press, Edmonton.
- Christensen CM, Kaufert FH, Schmitz H, Allison JL (1942). Hormodendrum resinae (Lindau), an inhabitant of wood impregnated with creosote and coal tar. American Journal of Botany 29: 552-558. [Google Scholar]
- Clements FC, Shear CL (1931). The genera of Fungi. H. W. Wilson, New York.
- Corda, ACJ (1839). Icones fungorum hucusque cognitorum, vol. 3. Prague.
- Davey ML, Currah RS (2007). A new species of Cladophialophora (hyphomycetes) from boreal and montane bryophytes. Mycological Research 111: 106-116. [DOI] [PubMed] [Google Scholar]
- Ellis MB (1976). More Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew.
- Fries EM (1815). Observationes mycologicae, vol. 1, p. 216. [Google Scholar]
- Fries EM (1832). Systema Mycologicum, vol. 3(2). E. Moritz, Greifswald.
- Fries EM (1849). Summa vegatabilium Scandinaviae,vol. 2: 468. [Google Scholar]
- Glawe DA, Hummell RL (2006). New North American host records for Seife rtia azaleae. Pacific Northwest Fungi 1(5): 1-6. [Google Scholar]
- Hambleton S, Egger KN, Currah RS (1998). The genus Oidiodendron: species delimitation and phylogenetic relationships based on nuclear ribosomal DNA analysis. Mycologia 90: 854-869. [Google Scholar]
- Ho MH-M, Castañeda RF, Dugan FM, Jong, SC (1999). Cladosporium and Cladophialophora in culture: descriptions and an expanded key. Mycotaxon 72: 115-157. [Google Scholar]
- Hughes SJ (1958). Revisiones hyphomycetum aliquot cum appendice de nominibus rejiciendis. Canadian Journal of Botany 36: 727-836. [Google Scholar]
- Hughes SJ (1968). Strigopodia. Canadian Journal of Botany 46: 1099-1107. [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick WB (1961). Hyphomycetes of conifer leaf litter. Hormodendrum staurophorum sp. nov. Canadian Journal of Botany 39: 833-835. [Google Scholar]
- Kornerup A, Wanscher JH (1989). Methuen Handbook of Colour, 3rd edn. Methuen, London.
- Lindau G (1904). Beiträge zur Pilzflora des Harzes. Verhandlungen des Botanischen Vereins der Provinz Brandenburg 45: 148-161 ('1903'). [Google Scholar]
- Lindau G (1906). Dr. L. Rabenhorst's Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz. Zweite Auflage. Erster Band: Die Pilze Deutschlands, Österreichs und der Schweiz. VIII. Abteilung: Fungi imperfecti: Hyphomycetes (erste Hälfte), Lief. 102: 641-704. [Google Scholar]
- Lindau G (1910). Dr. L. Rabenhorst's Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz. Zweite Auflage. Erster Band: Die Pilze Deutschlands, Österreichs und der Schweiz. IX. Abteilung: Fungi imperfecti: Hyphomycetes (zweite Hälfte), Dematiaceae (Phaeophragmiae bis Phaeostaurosporae), Stilbaceae, Tuberculariaceae, sowie Nachträge, Nährpflanzenverzeichnis und Register. Leipzig.
- Marsden DH (1954). Studies of the creosote fungus, Hormodendrum resinae. Mycologia 46: 161-183. [Google Scholar]
- Mason EW, Ellis MB (1953). British species of Periconia. Mycological Papers 56: 1-127. [Google Scholar]
- McNeill J, Barrie FR, Burdet HM, Demoulin V, Hawksworth DL, Marhold K, Nicolson DH, Prado J, Silva PC, Skog JE, Wiersema JH, Turland NJ (eds) (2007). International Code of Botanical Nomenclature (Vienna Code). Regnum Vegetabile 146: 1-568. [Google Scholar]
- Parbery DG (1969). Amorphotheca resinae gen. nov., sp. nov.: The perfect state of Cladosporium resinae. Australian Journal of Botany 17: 331-357. [Google Scholar]
- Partridge EC, Morgan-Jones G (2002). Notes on hyphomycetes, LXXXVIII. New genera in which to classify Alysidium resinae and Pycnostysanus azaleae, with a consideration of Sorocybe. Mycotaxon 83: 335-352. [Google Scholar]
- Persoon CH (1822). Mycologia europaea. Sectio Prima. Erlangae.
- Rehner SA, Samuels GJ (1995). Molecular systematics of the Hypocreales: a teleomorph gene phylogeny and the status of their anamorphs. Canadian Journal of Botany 73 (Suppl 1): S816-S823. [Google Scholar]
- Samson RA, Hoekstra ES, Frisvad JC (2004). Introduction to food- and airborne fungi. 7th ed. Centraalbureau voor Schimmelcultures, Utrecht.
- Stafleu FA, Frans A, Mennega EA (1995). Taxonomic Literature. A selective guide to botanical publications and collections with dates, commentaries and types. Suppl. III (Br - Ca). Regnum Vegetabile 132: 1-550. [Google Scholar]
- Swofford DL (2002). PAUP*: Phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland, Massachusetts.
- Thom C (1930). The Penicillia. Williams & Wilkins, Baltimore.
- Vilgalys R, Hester M (1990). Rapid identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vries GA de (1952). Contribution to the knowledge of the genus Cladosporium Link ex Fries. Thesis, University of Utrecht.
- Vries GA de (1955). Cladosporium avellaneum de Vries, a synonym of Hormodendrum resinae Lindau. Antonie van Leeuwenhoek 21: 166-168. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA sequences for phylogenetics. In: PCR Protocols: a guide to methods and applications. (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds.) Academic Press, San Diego, CA: 315-322.